Abstract
Background and purpose — The value of core decrompression for treatment of osteonecrosis of the femoral head (ONFH) is unclear. We investigated by a literature review whether implantation of autologous bone marrow aspirate, containing high concentrations of pluripotent mesenchymal stem cells, into the core decompression track would improve the clinical and radiological results compared with the classical method of core decompression alone. The primary outcomes of interest were structural failure (collapse) of the femoral head and conversion to total hip replacement (THR).
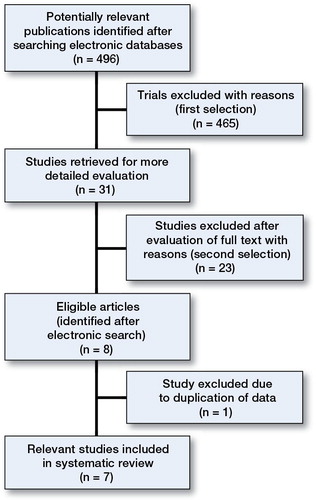
Patients and methods — All randomized and non-randomized control trials comparing simple core decompression with autologous bone marrow cell implantation into the femoral head for the treatment of ONFH were considered eligible for inclusion. The methodological quality of the studies included was assessed independently by 2 reviewers using the Cochrane Collaboration tool for assessing risk of bias in randomized studies. Of 496 relevant citations identified, 7 studies formed the basis of this review.
Results — The pooled estimate of effect size for structural failure of the femoral head favored the cell therapy group, as, in this treatment group, the odds of progression of the femoral head to the collapse stage were reduced by a factor of 5 compared to the CD group (odds ratio (OR) = 0.2, 95% CI: 0.08–0.6; p = 0.02). The respective summarized estimate of effect size yielded halved odds for conversion to THR in the cell therapy group compared to CD group (OR = 0.6, 95% CI: 0.3–1.02; p = 0.06).
Interpretation — Our findings suggest that implantation of autologous mesenchymal stem cells (MSCs) into the core decompression track, particularly when employed at early (pre-collapse) stages of ONFH, would improve the survivorship of femoral heads and reduce the need for hip arthroplasty.
Osteonecrosis of the femoral head (ONFH) is a progressive disease caused by a critical reduction in the blood supply to the femoral head and elevation of intraosseous pressure. Although its pathogenesis is poorly understood, it is generally accepted that various traumatic and non-traumatic insults compromise the already precarious circulation of the femoral head, leading to bone marrow and osteocyte death—and eventually collapse of the necrotic segment (CitationMont and Hungerford 1995). It mostly affects young adults, causing considerable morbidity (CitationSlobogean et al. 2015). The annual incidence of ONFH in the USA is estimated to be 15,000–20,000 cases (CitationVail and Covington 1997). Most cases without any treatment progress to femoral head collapse and joint destruction, with total hip arthroplasty being the only treatment option (CitationLieberman et al., 2003). Magnetic resonance imaging (MRI) has contributed to early (pre-collapse) detection of the disease, providing an opportunity for timely intervention in order to avoid femoral head collapse and subsequent joint destruction.
Various nonoperative and operative treatment modalities have been used to prevent—or at least delay—the progress of the disease towards femoral head collapse. Core decompression is a commonly used procedure, particularly in pre-collapse stages, but its effectiveness remains controversial (CitationFicat 1985, CitationLearmonth et al. 1990, CitationMarkel et al. 1996, CitationSaito et al. 1988, CitationYoon et al. 2001). Current research has focused on clarifying the molecular mechanisms involved in the pathogenesis of ONFH (CitationGangji and Hauzeur 2009, CitationKasten et al. 2008, CitationLee et al. 2009). Particular attention has been paid to multipotent mesenchymal stem cells (MSCs) and their ability to maintain mitotic multiplication while being capable of differentiating into various cellular types, such as osteoblasts, osteocytes, chondrocytes, and adipocytes (CitationBaksh et al. 2004). Experimentally, MSCs have been shown to enhance tissue regeneration when transplanted in areas of necrotic bone (CitationYan et al. 2009). Various researchers have pioneered the clinical application of cell-based methods for the treatment of ONFH (CitationHernigou and Beaujean 2002, CitationGangji and Hauzeur 2005, CitationCalori et al. 2014). Their technique was used in conjunction with the classical core decompression procedure and involved harvesting of autologous bone marrow aspirate, isolation of its mononuclear cell fraction, and injection of it into the necrotic zone of the femoral head through the canal of the preceding core decompression. This treatment strategy was based on the hypothesis that multiipotent MSCs in the bone marrow aspirate could repopulate the trabeculae of the necrotic zone within the femoral head, enhancing regeneration and remodeling of the necrotic bone (CitationHernigou et al. 2004).
We performed a meta-analysis to investigate whether implantation of autologous bone marrow aspirate, containing MSCs, into the core decompression track would improve the clinical and radiological results of ONFH compared to the classical method of core decompression alone. The primary outcomes of interest were structural failure (collapse) of the femoral head and conversion to total hip replacement (THR).
Material and methods
Our systematic review of the literature adhered to the PRISMA guidelines (CitationLiberati et al. 2009, CitationMoher et al. 2009).
Eligibility criteria
All full-text articles describing randomized and non-randomized control trials comparing simple core decompression with autologous bone marrow cell implantation into the femoral head for the treatment of ONFH were considered eligible for inclusion. Experimental or animal studies, case reports, editorials, letters to editors, and studies with less than10 subjects were excluded. No language restrictions were imposed.
Literature search and data extraction
An electronic search of the MedLine database via the PubMed search machine was initially undertaken using the following terms and Boolean operators: (“osteonecrosis of femoral head” OR “avascular necrosis of femoral head”) AND (“core decompression” OR “cell therapy”) The search was further extended to the Ovid MEDLINE, CINAHL, AMED, EMBASE, Cochrane Library, and Scopus databases. The reference lists of all potentially eligible studies and review papers were carefully scrutinized for additional eligible papers. The reviewers independently assessed the titles and abstracts of the articles retrieved. For all potentially eligible articles, the full text was obtained and evaluated against the eligibility criteria. Any disagreement between reviewers was resolved by discussion. Specific demographic data, baseline characteristics, follow-up data, and outcome data were extracted from each eligible article and tabulated.
Assessment of risk of bias (ROB) in the studies included
The methodological quality of the studies included was assessed independently by 2 reviewers (CP and TT) using the Cochrane Collaboration tool for assessing ROB in randomized studies (CitationHiggins et al. 2011). Any discrepancy between them was resolved through discussion.
Statistics
Binary outcomes were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Heterogeneity was assessed using Cochran chi-square test and Higgins I2 statistic (CitationCochran 1954; CitationHiggins et al. 2003). For the former, heterogeneity was considered significant at p-values < 0.1. For the latter, an I2-value of greater than 50% was taken to represent significant heterogeneity. Pooling of data was performed using the Mantel-Haenszel (M-H) statistical method, with either a fixed-effects model or a random-effects model based on the degree of statistical heterogeneity present. The results of each primary study and the combined estimate of effect size are presented graphically as forest plots. Funnel plots were used to detect the presence of publication bias. RevMan 5.2 software (Review Manager, the Nordic Cochrane Center, Copenhagen, Denmark) was used to present the study findings, to produce pooled estimates of effect size, to test the presence of statistical heterogeneity, and to generate forest and funnel plots.
Subgroup analysis
We decided a priori to explore the effect of stage of ONFH on the final outcome. Thus, we intended to separately analyze the subgroups, including hips at pre-collapse stage.
Sensitivity analysis
Where appropriate, we planned sensitivity analyses to investigate the effect of various components of the ROB tool (such as allocation concealment, detection bias, and attrition bias) on the final outcomes.
Results
Search process
Using the initial search strategy, we identified 496 citations. After application of eligibility criteria, 8 eligible reports remained for final analysis (Table and ). 1 of these reports was excluded to avoid duplication of data (CitationGangji et al. 2004)
Descriptive characteristics of included studies
Descriptive characteristics of included studies continued
All component studies compared 2 treatment groups: (1) a group of patients suffering from ONFH who were treated with a combination of core decompression (CD) and local instillation of a bone marrow concentrate (bmc) containing osteoprogenitor cells/MSCs (the cell therapy group), and (2) a group of patients with ONFH who were treated with CD, serving as the control group. 3 of the studies were randomized control trials (RCTs) (CitationSen et al. 2012, CitationZhao et al. 2012, CitationMa et al. 2014), 1 was a prospective control study (CitationGangji et al. 2011), and the remaining 3 were retrospective case-control studies (CitationYamasaki et al. 2010, CitationLim et al. 2013, CitationLiu et al. 2013).
Critical appraisal and assessment of ROB in the studies included
Our systematic review of the literature was based on a lucidly stated research question, appropriately defined inclusion and exclusion criteria, a thorough search of the literature, and clearly defined outcome measures. However, almost half of the studies included were retrospective in nature (CitationYamasaki et al. 2010, CitationLim et al. 2013, CitationLiu et al. 2013) and most of them possibly suffered from selection bias (due to lack of random generation and concealment of the allocation sequence), performance bias and detection bias (due to poor blinding of participants, personnel, or outcome assessors), and attrition bias (i.e. in most studies, follow-up losses were not taken into account in an intention-to-treat analysis) (Figure 2, see Supplementary data).
Publication bias
Publication bias was assessed by generating funnel plots for the primary outcomes of interest (structural failure, conversion to THR). The distributions of data points in funnel plots were symmetrical, indicating avoidance of publication bias (Figure 3, see Supplementary data).
Primary outcome measures
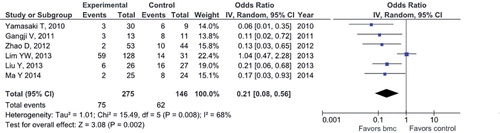
Structural failure (collapse) of the femoral head (FH). 6 studies (with 421 participants) provided relevant data (CitationYamasaki et al. 2010, CitationGangji et al. 2011, CitationZhao et al. 2012, CitationLim et al. 2013, CitationLiu et al. 2013, CitationMa et al. 2014). The pooled estimate of effect size for structural failure of the FH favored the cell therapy group, as, in this treatment group, the odds of progression of the femoral head to the collapse stage were shown to be decreased by 5 times compared to the control (CD) group (OR = 0.2, 95% CI: 0.08–0.6; p = 0.02). However, this result should be interpreted with caution due to the presence of significant statistical heterogeneity (I2 = 68%, Q-test = 16, df = 5, and p = 0.008) ().
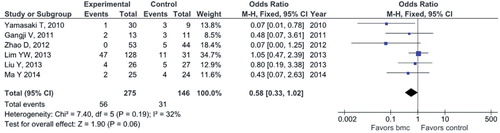
Conversion to THR. Relevant data were obtained from the same 6 studies (with 421 participants) (CitationYamasaki et al. 2010, CitationGangji et al. 2011, CitationZhao et al. 2012, CitationLim et al. 2013, CitationLiu et al. 2013, CitationMa et al. 2014). The respective summarized estimate of effect size almost reached borderline levels of statistical significance and yielded halved odds for conversion to THR in the cell therapy group compared to the control (CD) group (OR = 0.58, 95% CI: 0.33–1.02; p = 0.06) in the absence of significant statistical heterogeneity (I2 = 32%, Q-test = 7.40, df = 5, and p = 0.19) ().
Secondary outcome measures
Functional outcomes. 4 studies provided relevant data, but not in a consistent way to allow us to obtain a summarized estimate of effect size of any functional outcome (CitationGangji et al. 2011, CitationSen et al. 2012, CitationLiu et al. 2013, CitationMa et al. 2014).
Gandji et al. (2011) reported an overall decrease in the level of pain between the cell therapy group and the controls at 5-year follow-up.
CitationSen et al. (2012) found an improvement in Harris hip score (HHS) in the cell therapy group compared to the control group at 12 months postoperatively (p < 0.02). However, this difference became reduced to statistically insignificant levels at 24 months (p = 0.09).
CitationLiu et al. (2013) found an improvement in both HHS and visual analog scale (VAS) in the cell therapy group compared to the controls at the end of follow-up (p < 0.0001 for both comparisons).
CitationMa et al. (2014) reported a statistically significant improvement (p < 0.001) in the cell therapy group at final follow-up compared to the baseline situation, with respect to the level of pain and joint symptoms.
Subgroup analysis
We re-analyzed the primary outcomes of interest, including only studies reporting on early-stage (pre-collapse) ONFH.
Structural failure of the FH (failure to collapse). Relevant data were obtained from 5 studies (with 252 participants) (CitationYamasaki et al. 2010, CitationGangji et al. 2011, CitationZhao et al. 2012, CitationLiu et al. 2013, CitationMa et al. 2014). The pooled estimate of effect size for structural failure of FH indicated an 8-fold decrease in the odds of collapse of the FH in the cell therapy group compared to the CD group (OR = 0.12, 95% CI: 0.06–0.26; p < 0.001) in the absence of statistical heterogeneity (Q-test = 1.81, df = 4, p = 0.77, and I2 = 0%) (Figure 6A, see Supplementary data).
Conversion to THR. 4 studies (with 208 participants) provided relevant data (CitationYamasaki et al. 2010, CitationGangji et al. 2011, CitationZhao et al. 2012, CitationLiu et al. 2013). The overall estimate of effect size for conversion to THR favored the cell therapy group, although it reached only borderline significance levels (OR = 0.30, 95% CI: 0.08–1.06; p = 0.06) in the presence of a moderate degree of statistical heterogeneity (Q-test = 4.39, df = 3, p = 0.22, and I2 = 32%), (Figure 6B, see Supplementary data).
Some component studies of our review presented hybrid treatment protocols. In 2 of them, CD and cell therapy were combined with autologous bone graft (CitationLim et al. 2013, CitationMa et al. 2014), while in 2 other studies they had been combined with hydroxyapatite (HA) fillers (CitationYamasaki et al. 2010, CitationLiu et al. 2013). After exclusion of the above studies, we created another subgroup consisting of reports comparing CD alone with CD plus application of autologous bone marrow concentrate (cell therapy) (CitationGangji et al. 2011, CitationSen et al. 2012, CitationZhao et al. 2012). Only 2 studies provided relevant data for the primary outcome measures (CitationGangji et al. 2011, CitationZhao et al. 2012) (Figure 7, see Supplementary data).
Sensitivity analysis
We excluded all retrospective case-control studies, as they were considered to be more vulnerable to risk of bias, and repeated the analyses for the primary outcomes of interest, including 2 RCTs (CitationZhao et al. 2012, CitationMa et al. 2014) and one prospective cohort study (CitationGangji et al. 2011). This process did not produce substantially different results compared with the original ones (Figure 8, see Supplementary data).
Discussion
Our findings indicate that the application of autologous bone marrow concentrate (autologous cell therapy) in combination with core decompression in osteonecrotic femoral heads is superior to core decompression treatment, as it was found to markedly decelerate the progression of the disease to the stage of femoral head collapse, and also limit the need for total hip arthroplasty. Some component studies also reported on clinical results, demonstrating that autologous cell therapy in addition to core decompression for the treatment of ONFH resulted in reduction of painful joint symptoms and improvement in Harris hip score compared to core decompression technique alone. However, the presentation of functional and clinical results across component studies was not consistent with producing a summarized estimate of effect size for any clinical outcome measure used, and we have had to resort to narrative reporting of these results.
Core decompression—originally described by CitationFicat (1985) as a method of acquiring biopsy specimens in order to establish the diagnosis of osteonecrosis—is the most widely used treatment method for ONFH at pre-collapse stage. It is generally believed that core decompression works by reducing elevated intraosseous pressure and restoring vascularity of the femoral head (CitationLieberman et al. 2003). However, the results of core decompression alone usually deteriorate with more advanced lesions. Thus, while CD was effective as a definitive procedure in more than 80% of cases in Steinberg stage-I disease, with more advanced stages (Steinberg stage-II and -III disease), the need for further reconstructive intervention has been documented (in 37% and 71% of cases, respectively) (CitationMcGrory et al. 2007).
Recent research has focused on the role of MSCs in the pathogenesis of osteonecrosis. Such cells were found to be reduced in number and activity in osteonecrotic femoral heads (CitationGangji et al. 2003). On the other hand, the capillaries within the necrotic femoral head serving as conduits for stem cell and osseous cell delivery in the bone remodeling unit are believed to be occluded by emboli or thrombosis (CitationJones 1985, CitationMont and Hungerford 1995). These findings prompted researchers to develop a new approach for the treatment of ONFH, based on implantation into the necrotic zone of the femoral head of a concentrated bone marrow preparation, containing endothelial progenitor cells (promoting angiogenesis) and MSCs (promoting osteogenesis) (CitationGangji et al. 2004, CitationHernigou and Beaujean 2002) .
The effectiveness of autologous cell therapy is highly related to the stage of the disease and also to the number of MSCs transplanted. Hernigou (CitationHernigou & Beaujean, 2002) showed that when patients were operated upon before collapse of the FH ensued and when they received a greater number of MSCs in the autologous bone marrow concentrate injected into the necrotic lesion, a more favorable outcome could be expected. These observations are confirmed by our findings, as the results of subgroup analysis, including those studies with early-stage disease, showed a clear superiority of autologous cell therapy over CD with a complete absence of statistical heterogeneity. In addition, the bone marrow preparations described in component studies constituted a highly concentrated autologous bone marrow aspirate containing a large number of MSCs (ranging from 1,160 per mL to 4,900 per mL) (CitationHernigou et al. 2009). However, the exact number of MSCs that is required to induce remodeling and repair of the osteonecrotic zone is still unknown (CitationGangji et al. 2011) .
Limitations of the analysis
Our systematic review had some limitations that could have affected the validity of the results. These mainly arose from the presence of both clinical and methodological diversity across component studies, and from the fact that power calculations were not justified from the sample sizes, predisposing to occurrence of type-II error. Sources of clinical heterogeneity included age, sex, etiology of ONFH, stage of the disease, and surgical intervention. Some sources of clinical diversity (type of surgical intervention and stage of the disease) were addressed by appropriate subgroup analysis. The presence of methodological diversity predisposed to various forms of bias. The principal sources of methodological heterogeneity were biased allocation to interventions (predisposing to selection bias), poor blinding of participants, poor blinding of personnel (performance bias), poor blinding of outcome assessors (detection bias), and inappropriate handling of incomplete outcome data (attrition bias). The presence of methodological diversity was addressed with sensitivity analysis, which failed to generate substantially different results (compared to those from the initially recruited studies). We are therefore confident that our results have not been essentially distorted by the presence of methodological heterogeneity.
Conclusion
Our findings would suggest that implantation of autologous mesenchymal stem cells into the core decompression track, particularly when employed at early (pre-collapse) stages of ONFH, would improve the survivorship of femoral heads and reduce the need for hip arthroplasty. However, better designed RCTs with adequate sample sizes, based on power calculations, are required to further determine the exact role of cytotherapy in the management of ONFH.
Supplementary data
Figures 2, 3, 6, 7, and 8 are available at the Acta Orthopaedica website, www.actaorthop.org, identification number 8929.
IORT_A_1077418_SM9621.pdf
Download PDF (1.2 MB)All the authors participated in conduction of the study and contributed to the final manuscript. Design of the study: PVG and CP. Data analysis and writing: CP, THT, EJ, and PVG. Statistical analysis: CP. Critical assessment and improvement: PVG.
No competing interests declared.
- Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 2004; 8(3): 301–16.
- Calori GM, Mazza E, Colombo M, Mazzola S, Mineo GV, Giannoudis PV. Treatment of AVN using the induction chamber technique and a biological-based approach: indications and clinical results. Injury 2014; 45(2): 369–73.
- Cochran W. The combination of estimates from different experiments. Biometrics 1954; 10: 101–29.
- Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 1985; 67(1): 3–9.
- Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am 2005; 87 Suppl 1 (Pt 1): 106–12.
- Gangji V, Hauzeur JP. Cellular-based therapy for osteonecrosis. Orthop Clin North Am 2009; 40(2): 213–21.
- Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 2011; 49(5):1005–9.
- Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am 2004; 86-A(6): 1153–60.
- Gangji V, Hauzeur JP, Schoutens A, Hinsenkamp M, Appelboom T, Egrise D. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol 2003; 30(2): 348–51.
- Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 2002; (405): 14–23.
- Hernigou P, Manicom O, Poignard A, Nogier A, Filippini P, De Abreu L. Core decompression with marrow stem cells. Operative Tech Orthop 2004; 14(2): 68–74.
- Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop 2009; 43(1): 40–45.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–560.
- Jones JP, Jr. Fat embolism and osteonecrosis. Orthop Clin North Am 1985; 16(4): 595–633.
- Kasten P, Beyen I, Egermann M, Suda AJ, Moghaddam AA, Zimmermann G, Luginbuhl R. Instant stem cell therapy: characterization and concentration of human mesenchymal stem cells in vitro. Eur Cell Mater 2008; 16: 47–55.
- Learmonth ID, Maloon S, Dall G. Core decompression for early atraumatic osteonecrosis of the femoral head. J Bone Joint Surg Br 1990; 72(3): 387–90.
- Lee K, Chan CK, Patil N, Goodman SB. Cell therapy for bone regeneration--bench to bedside. J Biomed Mater Res B Appl Biomater 2009; 89(1): 252–63.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700.
- Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, Urbaniak JR. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect 2003; 52: 337–55.
- Lim YW, Kim YS, Lee JW, Kwon SY. Stem cell implantation for osteonecrosis of the femoral head. Exp Mol Med 2013; 45: e61.
- Liu Y, Liu S, Su X. Core decompression and implantation of bone marrow mononuclear cells with porous hydroxylapatite composite filler for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg 2013; 133(1): 125–33.
- Ma Y, Wang T, Liao J, Gu H, Lin X, Jiang Q, Bulsara MK, Zheng M, Zheng Q. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: a prospective, double-blinded, randomized, controlled study. Stem Cell Res Ther 2014; 5(5):115.
- Markel DC, Miskovsky C, Sculco TP, Pellicci PM, Salvati EA. Core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res 1996; (323): 226–33.
- McGrory BJ, York SC, Iorio R, Macaulay W, Pelker RR, Parsley BS, Teeny SM. Current practices of AAHKS members in the treatment of adult osteonecrosis of the femoral head.. J Bone Joint Surg Am 2007; 89(6): 1194–204.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535.
- Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am 1995; 77(3): 459–74.
- Saito S, Ohzono K, Ono K. Joint-preserving operations for idiopathic avascular necrosis of the femoral head. Results of core decompression, grafting and osteotomy. J Bone Joint Surg Br 1988; 70(1): 78–84.
- Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty 2012; 27(5): 679–86.
- Slobogean GP, Sprague SA, Scott T, Bhandari M. Complications following young femoral neck fractures. Injury 2015; 46(3): 484–91.
- Vail TP, Covington DB. The incidence of osteonecrosis. In: (Urbaniak JR, Jones JR, eds.) Osteonecrosis: Etiology, Diagnosis, Treatment. Rosemont, IL: American Academy of Orthopedic Surgeons 1997: 43–49.
- Yamasaki T, Yasunaga Y, Ishikawa M, Hamaki T, Ochi M. Bone-marrow-derived mononuclear cells with a porous hydroxyapatite scaffold for the treatment of osteonecrosis of the femoral head: a preliminary study. J Bone Joint Surg Br 2010; 92(3): 337–41.
- Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res 2009; 27(4): 442–46.
- Yoon TR, Song EK, Rowe SM, Park CH. Failure after core decompression in osteonecrosis of the femoral head. Int Orthop 2001; 24(6): 316–8.
- Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, Liu B, Yu X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012; 50(1): 325–30.