Abstract
Background and purpose — Hydroxyapatite has been used for a long time as an adjunct to enhance cementless fixation. The benefit of this is still debated, but new methods of hydroxyapatite deposition have emerged, offering possible gains. In order to investigate this further, we compared the migration pattern and periprosthetic bone remodeling in a cementless femoral stem with either electrochemically deposited hydroxyapatite—called Bonemaster (BM)—or a conventional plasma-sprayed hydroxyapatite (HA) coating.
Patients and methods — 55 hips were randomized to either BM or HA cementless femoral stems. Patients were followed with radiostereometry (RSA), dual-energy X-ray absorptiometry (DXA), radiographic measurements, and hip questionnaires for 5 years.
Results — For both stems, migration occurred mainly as subsidence and retroversion during the first 3 months. The BM group had a higher retroversion rate of 0.17° per month during this period, as compared to 0.06° per month for the HA group (p = 0.006). Thereafter, there was almost no movement in any direction for both stem types. Bone resorption occurred mainly during the first year, and subsequently decreased to a rate close to what is seen in normal ageing. The greatest total decrease occurred in Gruen zones 1 and 7, similar in the groups at 5 years. There was a slightly higher resorption rate in Gruen zone 7 from 2 to 5 years in the BM group (1.3% per year; p = 0.04), but in a magnitude that would scarcely affect stem stability or survival.
Interpretation — There were no clinically relevant differences between the 2 stems regarding stability or periprosthetic bone loss at 5 years. Electrochemically deposited HA does not appear to affect fixation or bone remodeling when compared to conventional plasma spraying at 5 years. Thus, at this point, Bonemaster appears to be safe.
The survival of cementless femoral stems relies on a durable fixation between implant and bone. To obtain this, some kind of integration between the 2 surfaces has to occur. The initial stability is provided by a tight fit and friction between implant and bone, relying on both the surgical technique and the properties of the implant (Dalton et al. Citation1995, Kuiper and Huiskes Citation1996, Viceconti et al. Citation2004). The temporary initial fixation will in turn buy time for osseointegration to occur, which ensures lasting “secondary” stability. The much desired osseointegration can be facilitated by modifications such as making the surfaces porous (Kienapfel et al. Citation1999) and adding biomaterials that induce bone formation. Being a natural constituent of human bone, hydroxyapatite ought to be promising for this purpose.
The Taperloc cementless femoral stem with proximal porous titanium alloy coating (without HA) has shown excellent clinical results, with a 10-year survival for aseptic loosening of the stem of 98.2% and also a stable migration pattern measured by RSA (Wykman and Lundberg Citation1992, Ciccotti et al. Citation1994, Hallan et al. Citation2007). A plasma-sprayed HA coating was then added in pursuit of even better results, but regretfully without any detectable benefit. The mechanical stability and osteoconductive potency of the HA layer depends on the coating method (Narayanan et al. Citation2008), which could explain the lack of effect. Taperloc HA-coated by a different method (electrochemical deposition) was therefore introduced, hoping to put right what was wrong. The Taperloc trial was initiated in 2003, in which Taperloc stems with either electrochemically deposited hydroxyapatite (Bonemaster; BM) or plasma-spray hydroxyapatite (HA) coating were compared with RSA and DXA measurements. The 2-year results published in 2011 revealed statistically significantly less bone resorption in Gruen zone 1 in the BM group (13% vs. 21%), but no significant differences in stability as measured by RSA (Boe et al. Citation2011). Our hypothesis was that there would be no detectable differences in medium-term stability and periprosthetic bone remodeling between the 2 stems.
Patients and methods
Patients eligible for primary total hip arthroplasty were considered for participation according to the following inclusion criteria: age below 80, skeletal maturity, ability to follow instructions, good general health for age, and willingness to return for follow-up examinations. The patient was excluded when there was infection, revision surgery, marked bone loss, lack of cooperativity, Parkinson’s disease, vascular insufficiency of the affected limb, severe impairment of surrounding soft tissues, and pregnancy. There were 31 women and 19 men, with a mean age of 63 (27–81) years.
Surgery
The Taperloc cementless stem with either BM or HA proximal coating was implanted (Biomet UK Healthcare, Bridgend, UK). According to the manufacturer, the HA coating applied by plasma spraying is 50 μm thick and has a crystallinity of 62%. On the other hand, the BM coating has a thickness of 5 μm and a crystallinity of 70–72%. The stems were implanted through a modified Hardinge approach, along with 28-mm CoCr femoral head and SHP cemented polyethylene cup (Biomet, Warsaw, IN) using Palacos bone cement with gentamicin (Schering-Plough, Welwyn Garden City, UK). 7–9 tantalum markers (1 mm) were implanted into the proximal femur, while the femoral stems were supplied with 3 markers by the manufacturer. The operations were performed by 4 consultant orthopedic surgeons at 2 hospitals.
Outcomes
The primary outcome variable was the periprosthetic changes in bone mineral density (BMD) between the immediate postoperative scan and subsequent measurements. The secondary variables were stem migration measured by RSA, signs of radiographic loosening on conventional radiographs, and the results of hip questionnaires.
DXA
DXA scans were performed at 3, 6, 12, 24 and 60 months postoperatively. 3 different scanners were used: Lunar Expert (Lunar, Madison, WI) was used in 1 hospital (hospital A) until January 2005, but was then replaced by Hologic QDR (Hologic Inc., Bedford, MA). In order to make allowances for this change, all measurements after the exchange were adjusted using a formula calculated using 5 double examinations on both densitometers. Lunar Prodigy was used during the whole trial at the other hospital (hospital B). At 2 years, separate analyses were performed on patients from hospital B only, considering the possible bias introduced by using different densitometers. No difference was found compared to analyses on all patients. A foot support was used during scanning to ensure standard rotation at the hip. BMD was measured in 7 regions of interest (ROIs) corresponding to the Gruen zones. Precision—expressed as coefficient of variation (CV%)—was calculated for each densitometer using 130 double examinations, with repositioning between the scans.
RSA
RSA images were taken at the same intervals as DXA in both hospitals. Analysis was done at one RSA laboratory. Images were analyzed with UmRSA (Digital Measurement 6.0; RSA Biomedical, Umeå, Sweden). Those with condition number > 150 and ME > 0.3 were excluded. Precision was determined with 83 double RSA examinations, and calculated as the mean of differences +2 SD expressed in absolute values. Results are presented as early (0- to 3-month) and late (24- to 60-month) monthly migration rates calculated from a linear mixed model.
Other evaluations
Standard radiographs were also performed up to 5 years, and evaluated for signs of instability and osseointegration (change in alignment, gross subsidence, calcar atrophy, periprosthetic radiolucency, pedestal formation, and trabecular remodeling). Periprosthetic radiolucent lines were defined as being >1 mm thick and spanning ≥50% of the Gruen zone. Harris hip score (HHS) and Oxford hip score (OHS) were used to evaluate clinical results. A single surgeon (BF) evaluated the radiographs. For further methodological issues, we refer the reader to the initial 2-year publication (Boe et al. Citation2011).
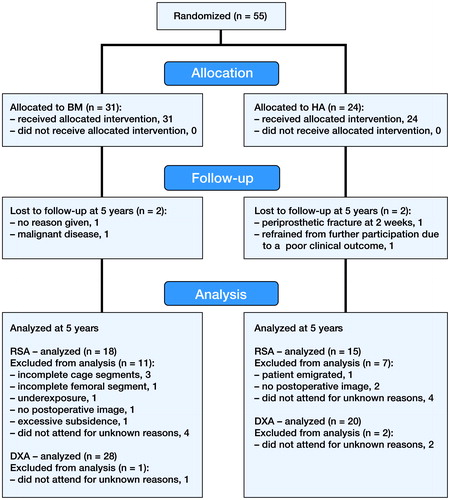
Patient flow ()
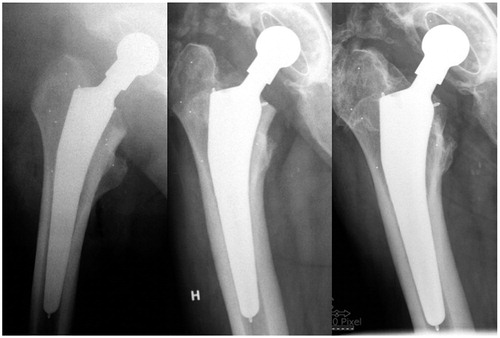
55 hips in 50 patients were included. 1 patient was excluded due to a periprosthetic fracture after 6 weeks, and 3 patients were lost to follow-up due to concomitant disease or withdrawal from the trial, leaving 29 BM and 22 HA hips at 5 years. 2 hips in the BM group underwent cup revision, but remained in the trial. 1 patient, who contracted early postoperative infection and was operated at 5 weeks with soft tissue revision and exchange of the femoral head, also remained in the trial. 1 stem in the BM group subsided excessively (10.4 mm) during the first 3 months. However, it did not subside any further after that; nor was there movement in any other direction. This stem appeared to be undersized when we were evaluating postoperative radiographs, and it was excluded from the overall subsidence rate calculation since we considered it to be a confounding factor (). This patient was also excluded at the 2-year analysis, due to a condition number (CN) expressing the quality of marker segments above the threshold accepted at that time (CN > 100).
Figure 2. The postoperative, 3-month and 5-year radiographs from the patient that was excluded from RSA analysis. The stem seems to be undersized in the postoperative image. There is macroscopic subsidence from postoperatively to 3 months, but no visible changes thereafter, consistent with the RSA data.
Statistics
We initially planned to include 50 hips in each group, but a delayed sample size calculation was performed during the trial, which led to a reduction in the initially planned sample size from 100 to 55. Assuming a difference in BMD of 10% (SD 10), 0.6 mm stem subsidence (SD 0.6), and 0.7° y-rotation of the stem (SD 0.7) represents a clinically significant difference, 17 patients would be needed to detect a potential effect with a statistical power of 80% and a significance level of 5%. To take account of possible dropouts, at least 24 patients would be needed in each group. The inclusion was completed after random allocation (with sealed envelopes) to 31 in the BM group and 24 in the HA group; hence the unequal sample sizes. Baseline demographic data are presented as mean value and range. Due to the repetitive, correlated nature of the data, HHS, OHS, RSA, and DXA were analyzed with multilevel modeling techniques (Matthews et al. Citation1990, Molenberghs Citation2001). A linear spline mixed model with a common knot was applied to fit the data and estimate the slopes for the line segments. Differences between the 2 groups were tested by the interactions between corresponding time segments and group variables in the same model. For the random part of the mixed model, patient ID, time, and unstructured variance-covariance were used to allow each patient to have their own intercept and slope over time. Patients from all time points were included in the overall analysis; 49 patients were included in the RSA analysis and 54 were included in the DXA analysis. Results are presented as mean values with corresponding 95% confidence intervals (CIs). Fisher’s exact test was used to evaluate differences in periprosthetic radiolucency.
All analyses were performed with IBM SPSS Statistics version 20 and STATA version 13.1.
Ethics and registration
The trial was planned and executed according to the WMA Declaration of Helsinki. Approval from the regional ethics committee was granted on November 10, 2003 (project number 03162), and the trial started in December 2003. Inclusion was complete in June 2005, and 5-year follow-up by 2008. After completion, the trial was registered at Clinicaltrials.gov (identifier NCT02321683).
Results
DXA
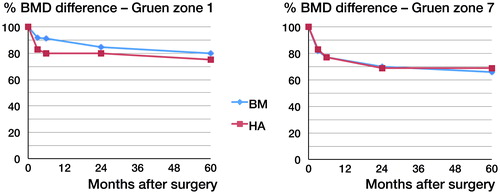
Precision, measured for all densitometers in all ROIs, ranged from 0.5 to 5.8 (CV) (Boe et al. Citation2011). Periprosthetic BMD declined in all Gruen zones. The greater part of this bone loss occurred during the first year. At 2 years, we reported a uniform bone loss in Gruen zones 2–7 for both groups, which ranged from 8% to 14% in zones 2–6 and approximately 30% in Gruen zone 7. A difference in statistical significance was, however, found in Gruen zone 1, being 13% in the BM group as compared to 21% in the HA group. From 2 to 5 years, the bone remodeling rate decreased to a rate close to what we see in normal ageing (Hannan et al. Citation2000). The greatest decrease occurred in Gruen zone 7 (34% for BM and 31% for HA) and 1 (20% for BM and 25% for HA) ( and ). There were no statistically significant differences between groups regarding the total decrease in any zone at 5 years, but we did find a statistically significant difference in remodeling rate between 2 and 5 years in Gruen zone 7. BMD in the BM group decreased by 0.11% per month (CI: −0.19 to −0.04) whereas in the HA group it increased by 0.01% per month (CI: −0.08 to 0.1; p = 0.04) ( and ).
Figure 3. BMD difference from the postoperative scans up to 5 years in all Gruen zones for each group.
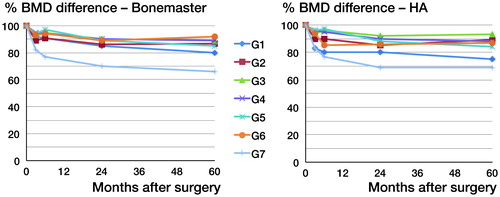
Table 1. Percentage BMD changes (g/cm2) from the postoperative scans in BM and HA Taperloc stems. The upper half shows the bone remodelling rates per month, and the lower shows the total 5-year reduction
RSA
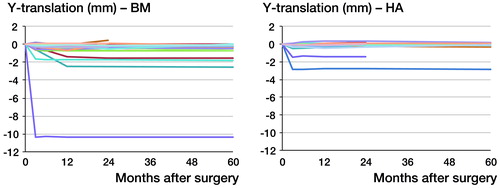
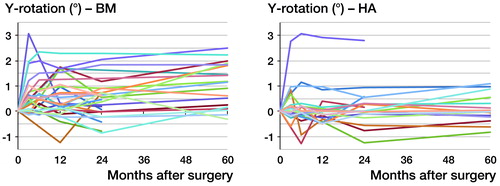
Both stems mainly migrated by subsidence and retroversion. Migrations in other directions were minuscule and below the respective precision limit. Subsidence occurred almost exclusively during the first 3 months, with equal slopes in both groups (p = 0.6). After that, there was hardly any subsidence, with no statistically significant differences between groups (p = 0.4) ( and ). 1 stem in the BM group showed excessive subsidence of 10.4 mm during the first 3 months, but it remained completely stable during the remaining observation period. The same pattern of movement was seen for retroversion, but the rate was higher during the first 3 months in the BM group: 0.17° per month (CI: 0.11–0.23) as compared to 0.06° per month (CI: −0.01 to 0.12) in the HA group (p = 0.006). From then on, both groups appeared to remain stable, without any differences in the slope between 2 and 5 years (p = 0.3) ( and ).
Table 2. Monthly rates for y-translation (subsidence), y-rotation (retroversion) and z-rotation (coronal rotation) during the early (0–3 months) and late (2–5 years) migration period
Other evaluations
HHS at 5 years was 91 (87–95) in the BM group and 89 (83–95) in the HA group, which was a total increase of 37 (32–41) and 35 (30–39) for the BM and HA groups, respectively (p = 0.3). OHS at 5 years was 42 (39–44) in the BM group and 39 (34–43) in the HA group, which was a total increase of 21 (18–25) and 21 (18–24) for the BM and HA groups, respectively (p = 0.6).
At 5 years, periprosthetic radiolucency was seen in 9 of 26 patients in the BM group and 8 of 20 patients in the HA group (p = 0.8). This was exclusively seen in Gruen zones 3 and 5 around the smooth, uncoated part of the stem. All stems except 1 showed no signs of instability such as gross migration, change in alignment, or pedestal formation. 1 stem in the BM group migrated 1.2 mm when measured on the AP radiographs at 3 months. No further subsidence could be measured after that. 3 stems in the HA group and 1 stem in the BM group showed trabecular remodeling as a sign of osseointegration (p = 0.3, Fisher’s exact test).
Discussion
A durable cementless fixation can only happen in the presence of 2 main events. First, an initial mechanical fixation should provide enough stability to allow a secondary biological fixation to occur through bony ingrowth. In many cases, a layer of hydroxyapatite has been added to the surface of the femoral stem in the hope of improving such osseointegration. This modification was encouraged by findings from several studies showing the promising osteoconductive properties of hydroxyapatite (Cook et al. Citation1988,Citation1991, Soballe et al. Citation1992, Daugaard et al. Citation2010). However, later clinical trials have not been able to prove any differences in coated stems of identical design, with or without HA coating (Lazarinis et al. Citation2011, Kim et al. Citation2012, Li et al. Citation2013). The obvious question that arises is why we should see this discrepancy between laboratory and clinical findings. One possible explanation is that the properties of hydroxyapatite coatings can be highly variable depending on their morphological, chemical, and physical characteristics. Coating thickness affects the integrity of the layer, with a thicker layer being prone to fractures and delamination. This creates debris products that can cause abrasive third-body wear (Narayanan et al. Citation2008) and inflammatory osteolysis (Nordsletten et al. Citation1996). Crystallinity: crystalline hydroxyapatite is less soluble than amorphous hydroxyapatite—possibly being positive in the sense of mechanical stability but negative in terms of osteoinduction.
These characteristics can be altered by the mode of application (among other things). Plasma spraying has been used in the majority of cementless stems, and usually creates an HA coating of heterogeneous structure and uneven thickness, necessitating a thick layer to ensure complete coating. This unfavorable characteristic of the hydroxyapatite coating may outweigh the possible advantages and explain the absence of effect in clinical trials. Electrochemical deposition, on the other hand, creates a thin, uniform HA coating with a more biocompatible composition and structure (Rossler et al. Citation2003). The crystallinity is also higher, being about 70%—as compared to 60% in the plasma-spray coating. Some experimental studies have shown that the BM coating has osteoconductive properties, but no obvious superiority over plasma-sprayed coatings (Wang et al. Citation2006, Daugaard et al. Citation2010). Others have even shown decreased bone formation when hydroxyapatite is added to a porous titanium surface, either by plasma spraying or electrochemical deposition (Boe et al. Citation2012).
We have compared both types of hydroxyapatite coating in a clinical setting and have not found any obvious differences that would affect clinical performance. Even though there was a statistically significantly higher degree of retroversion in the BM stems during the initial period, both groups remained completely stable during the late period. Both coatings appear to provide the necessary stability to obtain secondary fixation through bone in-growth or on-growth. The only difference we could find regarding bone remodeling was a slightly higher decrease in BMD in Gruen zone 7 in the BM group from 2 to 5 years. However, this rate (1.3% per year) barely exceeds the average reduction in BMD by age alone, and should hardly have an impact on later fixation. Otherwise, BMD reduction was in the range usually seen with cementless stems 2–5 years after implantation, for both groups (Kiratli et al. Citation1992, Nishii et al. Citation1997). Supporting the findings from these indirect parameters, no significant differences in clinical or plain radiographic results were found between the BM and HA groups at 5 years.
In conclusion, electrochemical deposition does not appear to offer any advantage over conventional plasma-spray technique in terms of medium-term stability and periprosthetic bone remodeling. The void between the theoretical benefit of hydroxyapatite in femoral stem fixation and the absence of any clinical effect is yet to be filled.
BF did the DXA and RSA data extraction and analysis, and wrote the manuscript. SMR assisted in RSA analysis and revision of the manuscript. BB performed clinical follow-up and assisted in revision of the manuscript. LN designed the trial, operated the patients, and assisted in revision of the manuscript.
We thank our colleagues at the radiology departments of the participating hospitals, and our statistical adviser Lien Diep. Biomet Europe contributed with funding of the trial, but did not participate in any other way.
No competing interests declared.
- Boe B G, Rohrl S M, Heier T, Snorrason F, Nordsletten L. A prospective randomized study comparing electrochemically deposited hydroxyapatite and plasma-sprayed hydroxyapatite on titanium stems. Acta Orthop 2011; 82 (1): 13-9.
- Boe B G, Stoen R O, Solberg L B, Reinholt F P, Ellingsen J E, Nordsletten L. Coating of titanium with hydroxyapatite leads to decreased bone formation: A study in rabbits. Bone Joint Res 2012; 1 (6): 125-30.
- Ciccotti M G, Rothman R H, Hozack W J, Moriarty L. Clinical and roentgenographic evaluation of hydroxyapatite-augmented and nonaugmented porous total hip arthroplasty. J Arthroplasty 1994; 9 (6): 631-9.
- Cook S D, Thomas K A, Kay J F, Jarcho M. Hydroxyapatite-coated titanium for orthopedic implant applications. Clin Orthop Relat Res 1988; (232): 225-43.
- Cook S D, Thomas K A, Dalton J E, Kay J F. Enhanced bone ingrowth and fixation strength with hydroxyapatite-coated porous implants. Semin Arthroplasty 1991; 2 (4): 268-79.
- Dalton J E, Cook S D, Thomas K A, Kay J F. The effect of operative fit and hydroxyapatite coating on the mechanical and biological response to porous implants. J Bone Joint Surg Am 1995; 77 (1): 97-110.
- Daugaard H, Elmengaard B, Bechtold J E, Jensen T, Soballe K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J Biomed Mater Res A 2010; 92 (3): 913-21.
- Hallan G, Lie S A, Furnes O, Engesaeter L B, Vollset S E, Havelin L I. Medium- and long-term performance of 11,516 uncemented primary femoral stems from the Norwegian arthroplasty register. J Bone Joint Surg Br 2007; 89 (12): 1574-80.
- Hannan M T, Felson D T, Dawson-Hughes B, Tucker K L, Cupples L A, Wilson P W, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000; 15 (4): 710-20.
- Kienapfel H, Sprey C, Wilke A, Griss P. Implant fixation by bone ingrowth. J Arthroplasty 1999; 14 (3): 355-68.
- Kim Y H, Kim J S, Joo J H, Park J W. Is hydroxyapatite coating necessary to improve survivorship of porous-coated titanium femoral stem? J Arthroplasty 2012; 27 (4): 559-63.
- Kiratli B J, Heiner J P, McBeath A A, Wilson M A. Determination of bone mineral density by dual x-ray absorptiometry in patients with uncemented total hip arthroplasty. J Orthop Res 1992; 10 (6): 836-44.
- Kuiper J H, Huiskes R. Friction and stem stiffness affect dynamic interface motion in total hip replacement. J Orthop Res 1996; 14 (1): 36-43.
- Lazarinis S, Karrholm J, Hailer N P. Effects of hydroxyapatite coating on survival of an uncemented femoral stem. A Swedish Hip Arthroplasty Register study on 4,772 hips. Acta Orthop 2011; 82 (4): 399-404.
- Li S, Huang B, Chen Y, Gao H, Fan Q, Zhao J, et al. Hydroxyapatite-coated femoral stems in primary total hip arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg 2013; 11 (6): 477-82.
- Matthews J N, Altman D G, Campbell M J, Royston P. Analysis of serial measurements in medical research. BMJ 1990; 300 (6719): 230-5.
- Molenberghs G. A review on linear mixed models for longitudinal data, possibly subject to dropout. Statistical modelling 2001; (1): 235-69.
- Narayanan R, Seshadri S K, Kwon T Y, Kim K H. Calcium phosphate-based coatings on titanium and its alloys. J Biomed Mater Res B Appl Biomater 2008; 85 (1): 279-99.
- Nishii T, Sugano N, Masuhara K, Shibuya T, Ochi T, Tamura S. Longitudinal evaluation of time related bone remodeling after cementless total hip arthroplasty. Clin Orthop Relat Res 1997; (339): 121-31.
- Nordsletten L, Hogasen A K, Konttinen Y T, Santavirta S, Aspenberg P, Aasen A O. Human monocytes stimulation by particles of hydroxyapatite, silicon carbide and diamond: in vitro studies of new prosthesis coatings. Biomaterials 1996; 17 (15): 1521-7.
- Rossler S, Sewing A, Stolzel M, Born R, Scharnweber D, Dard M, et al. Electrochemically assisted deposition of thin calcium phosphate coatings at near-physiological pH and temperature. J Biomed Mater Res A 2003; 64 (4): 655-63.
- Soballe K, Hansen E S, H B R, Jorgensen P H, Bunger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10 (2): 285-99.
- Viceconti M, Pancanti A, Dotti M, Traina F, Cristofolini L. Effect of the initial implant fitting on the predicted secondary stability of a cementless stem. Med Biol Eng Comput 2004; 42 (2): 222-9.
- Wang H, Eliaz N, Xiang Z, Hsu H P, Spector M, Hobbs L W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006; 27 (23): 4192-203.
- Wykman A, Lundberg A. Subsidence of porous coated noncemented femoral components in total hip arthroplasty. A roentgen stereophotogrammetric analysis. J Arthroplasty 1992; 7 (2): 197-200.