At two years of existence, ALSUntangled (www.alsuntangled.org) has 363 twitter followers. Within our NING there are 77 ALS clinician scientists from across eight countries currently participating in more than 30 active discussions. New discussions include Cold Comfort, marijuana, Clinics of Drs. Warren Levin and Joseph Jemsek, and Fry Labs. We have now published nine investigations on 10 different alternative and off-label treatment options and continue to collaborate with Quackwatch (www.quack watch.org), Patients Like Me (www.patientslikeme.com) and ALS Worldwide (www.alsworldwide.org). Here, at the request of PALS, we investigate luteolin as a treatment for ALS.
Why might this be useful in ALS?
Luteolin, 3,4,5,7-tetrahydroxyflavone, is a natural flavinoid that occurs in its glycosylated form in certain vegetables. It can be neuroprotective in ischemic brain injury models (Citation1), can inhibit microglial expression of cytokines (Citation2), CD40 (Citation3), TNF-α and IL-6, and can activate the p53 system, promoting apoptosis of cancer cells (Citation4). It is said to protect human skin from UVB-induced damage by a combination of UV-absorbing, DNA-protective, antioxidant, and anti-inflammatory properties (Citation5). Luteolin is combined with rutin, another bioflavinoid, and a number of vitamins and minerals and marketed as Lutimax by ImmunoBiotics Inc., a Southern California based nutriceutical company, for “the treatment and prevention of neurodegenerative, autoimmune, and metabolic diseases including autism, ALS, Parkinson's disease and fibromyalgia” (Citation6). Lutimax, according to the company's website, “is an effective treatment for the common underlying mechanism for all neurodegenerative and autoimmune diseases” (Citation6).
Since oxidative stress, neuroinflammation, and DNA dysregulation may play roles in ALS progression, it is at least theoretically possible that Lutimax might be useful in slowing progression. However, the cause of most ALS is unknown; thus, it is not clear how Lutimax could prevent this condition. Also, we know of no data supporting significant overlap in the mechanisms of autism, ALS, Parkinson's and fibromyalgia and thus it is difficult to imagine that all these diseases could be effectively treated by the same compound.
Are there any data to support its use in ALS?
As part of the promotion for this supplement, ImmunoBiotics has created a series of videos that are available for review on youtube.org or filmannex.com. These show patients with a variety of diseases (ALS, autism, lupus) who are taped just prior to and after taking Lutimax. In one of them an ALS patient is filmed before Lutimax is given to him. He reports fasciculations and inability to move his arms. After 45 min of taking Lutimax he “shows great improvement” with ability to lift his arms (http://www.filmannex.com/movie/immunobioticslutimax-als-patient-2/17134). Unfortunately, there is no way to confirm this patient's diagnosis, no blinding, and no objective assessments or standardized testing shown. It is difficult to understand why Lutimax with its proposed mechanisms would restore motor function, and it does not seem physiologically possible that such restoration could be this dramatic or rapid.
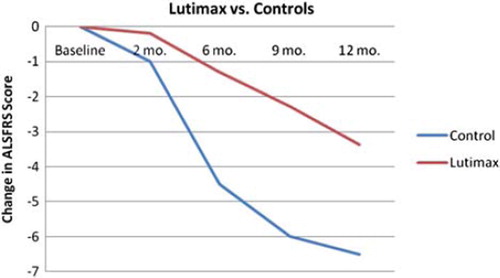
The company website references an unpublished study by Elijah Stommel at Dartmouth-Hitchcock Medical Center in Lebanon, New Hampshire. Stommel was kind enough to provide us with details via personal communications. He recruited 25 adult patients with ALS, disease duration less than five years, FVC greater than 65% predicted, for an open-label unblinded study of Lutimax 800 mg daily. The 40-point version of the ALSFRS was measured at baseline and then every three months for one year; a slope of decline was calculated and compared to that of the placebo group of a published clinical trial (topiramate). The Lutimax study group wound up consisting of nine females and 16 males, with average age 60 years, and with only two of them taking riluzole during the study. By the two month time-point, 13 of 25 subjects had dropped out of the study, and by the end 17 had dropped out. Analyses did not include intention to treat or any other plan for dealing with drop-outs. The slope of ALSFRS decline in the Lutimax group appeared significantly slower than that of the comparison group (). Unfortunately, there are several problems with this study that preclude any definite conclusion, including the lack of randomization, lack of blinding, large drop-out rate and lack of intention-to-treat analysis.
Finally, within the online community Patients Like Me (PLM), eight patients with ALS report taking Lutimax. Of these, four remain on the supplement and there are four detailed reports. These four are taking between 1600 and 3200 mg daily. Only one of the four reports any significant efficacy; this patient had been on Lutimax for 1.5 weeks when she reported subjective improvements in balance and hyperreflexia.
Are there any potential harms?
Lutimax is promoted to be most effective when taken with a carbohydrate-restricted diet. As a result, patients with ALS may stop taking carbohydrates in their diet, some to the extent of going on the Atkins diet, resulting in significant weight loss. Most experts believe that weight loss should be avoided in patients with ALS, as it is correlated with accelerated ALS progression. One of the patients who stopped taking Lutimax for ALS in the PLM cohort did so because the required dietary changes were intolerable. Another discontinued because they felt that their disease was progressing faster than it had before they started Lutimax. The Federal Drug Administration (FDA), after reviewing the original submission for registration of the supplement Lutimax, raised its own concerns. The FDA letter from 16 January 2003 states “your notification does not meet the requirements establishing history of use or other evidence of safety when used as recommended or suggested as required by 21 CFR 190.6(b)(Citation4)… Therefore, your product may be adulterated under 21 U. S.C. 342(f)(l)(B) as a dietary supplement that contains a new dietary ingredient for which there is inadequate information to provide reasonable assurance that such ingredient does not present a significant or unreasonable risk of illness or injury” (Citation7).
The PLM cohort reports costs ranging from less than $25 to $199 per month of treatment.
Conclusions
In summary, luteolin is an interesting naturally occurring bioflavinoid that has been shown to have a myriad of functions in various models that could potentially be useful in slowing progression in patients with ALS. However, convincing data to support any positive effect on human ALS do not yet exist. Furthermore, there are legitimate reasons to be concerned about safety in patients with ALS including the need for a concomitant carbohydrate-deficient diet which might induce unwanted weight loss, and an anecdotal report of accelerated progression on this supplement. Until carefully controlled, well-designed human efficacy and safety studies are performed, ALSUntangled does not support the use of luteolin or any luteolin-containing products in patients with ALS.
The ALSUntangled Group currently consists of the following members: Tahseen Mozaffar, Richard Bedlack, Orla Hardiman, Peter Andersen, Jeff Dietz, Josep Gamez, Mazen Dimachkie, Yunxia Wang, Paul Wicks, James Heywood, Steven Novella, L.P. Rowland, Eric Pioro, Lisa Kinsley, Kathy Mitchell, Jonathan Glass, Sith Sathornsumetee, Hubert Kwiecinski, Jon Baker, Nazem Atassi, Dallas Forshew, John Ravits, Robin Conwit, Carlayne Jackson, Alex Sherman, Kate Dalton, Katherine Tindall, Ginna Gonzalez, Janice Robertson, Larry Phillips, Michael Benatar, Eric Sorenson, Christen Shoesmith, Steven Nash, Nicholas Marigakis, Dan Moore, James Caress, Kevin Boylan, Carmel Armon, Megan Grosso, Bonnie Gerecke, Jim Wymer, Bjorn Oskarsson, Robert Bowser, Vivian Drory, Jeremy Shefner, Terry Heiman-Patterson, Noah Lechtzin, Melanie Leitner, Robert Miller, Hiroshi Mitsumoto, Todd Levine, James Russell, Khema Sharma, David Saperstein, Leo McClusky, Daniel MacGowan, Jonathan Licht, Ashok Verma, Michael Strong, Catherine Lomen-Hoerth, Rup Tandan, Michael Rivner, Steve Kolb, Meraida Polak, Stacy Rudnicki, Pamela Kittrell, Muddasir Quereshi, George Sachs, Gary Pattee, Michael Weiss, John Kissel, Jonathan Goldstein, Jeffrey Rothstein, Dan Pastula.
Note: this paper represents a consensus of those weighing in. The opinions expressed in this paper are not necessarily shared by every investigator in this group.
Acknowledgement
ALSUntangled is sponsored by the Packard Center and the Virginia Gentlemen Foundation.
References
- Zhao G, Zang S-Y, Jiang Z-H, Chen YY, Ji XH, Lu BF, . Post-ischemic administration of liposome-encapsulated luteolin prevents against ischemia reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2010; doi:10.1016/j.jnutbio. 2010.07.014.
- Kao T-K, Ou Y-C, Lin S-Y, Pan HC, Song PJ, Raung SL, . Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. J Nutr Biochem. 2010; doi:10.1016/j.jnutbio.2010.01.011
- Rezai-Zadeh K, Ehrhart J, Bai Y, Sanberg PR, Bickford P, Tan J, . Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J Neuroinflammation. 2008;5:41.
- Amin ARMR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, . Enhanced anti-tumor activity by the combination of the natural compounds (‚àí)-Epigallocatechin-3-gallate and luteolin. J Biol Chem. 2010;285:34557–65.
- Wölfle U, Esser PR, Simon-Haarhaus B, Martin SF, Lademann Jr, Schempp CM. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic Biol Med. 2011; doi:10.1016/j.freeradbiomed.2011.01.027
- http://www.immunobiotics.com/
- www.fda.gov/ohrms/dockets/DOCKETS/.../95s-0316-rpt0150-01-vol108.pdf