Abstract
Osmotic stress results in the accumulation of osmolytes in tissues. Synthesis of these osmolytes is mediated by the transcription factor NFAT5/TonEBP in the human kidney. We tested for the presence of NFAT5 mRNA and protein in the human and ovine placenta and confirmed sorbitol and inositol osmolyte concentrations in these tissues. To determine NFAT5 protein, human and ovine placenta were tested for inositol, sorbitol and glucose using high performance liquid chromatography (HPLC). Additionally, RNA was extracted and cDNA was made from these tissues. PCR was performed and products were sequenced. Western blotting was used to assess the expression of the NFAT5 protein. Human and ovine placenta demonstrated: 1) high concentrations of sorbitol and inositol, 2) presence of NFAT5 mRNA, 3) confirmation by NFAT5 sequence identity, and 4) presence of NFAT5 protein. NFAT5 is present in the ovine and human placenta at the RNA and protein levels that suggest a role for this protein in the induction of these osmolytes. Further trophoblast studies of osmotic stress effects on osmolytes are planned.
| Abbreviations | ||
| HPLC: | = | high performance liquid chromatography |
| SMIT: | = | sodium-myo-inositol cotransporter |
| AR: | = | aldose reductase |
| NFAT5: | = | nuclear factor of activated T cells 5 |
INTRODUCTION
Without an adaptive response, cells subjected to hypertonic stress lose intracellular water leading to an increase in intracellular electrolyte concentration [Ko et al. Citation2002; Miyakawa et al. Citation1998]. Cells adapt to this stress by replacing excess electrolytes with an accumulation of organic osmolytes such as myo-inositol and sorbitol [Ko et al. Citation2002; Miller et al. Citation2000; Miyakawa et al. Citation1998], which reestablishes osmotic equilibrium and intracellular ionic strength via osmotic replacement [Handler and Kwon Citation2001; Woo et al. Citation2002a; Woo and Kwon Citation2002]. The accumulation of some organic osmolytes is regulated by activation of the nuclear factor of activated T cells 5 (NFAT5) or tonicity-responsive enhancer binding protein (TonEBP) [Ko et al. Citation2002; Miyakawa et al. Citation1998; Woo et al. Citation2002a,Citationb; Zhou et al. Citation2006].
NFAT5 is activated when the tonicity is raised in the cells. Activation of this transcription factor involves three processes: nuclear translocation, upregulation of transcriptional activity and increased NFAT5 synthesis at the RNA and protein levels [Aramburu et al. Citation2006; Woo et al. Citation2000]. Once activated, NFAT5 stimulates the genes encoding for both transporters which plays an important role in the cellular adaptation to osmotic stress such as sodium-myo-inositol cotransporter (SMIT) and the aldose reductase (AR) enzyme [Go et al. Citation2004; Han et al. Citation2004; Miyakawa et al. Citation1998; Woo et al. Citation2002a].
The increase in SMIT transporter is responsible for the accumulation of inositol while AR catalyzes the production of sorbitol from glucose [Lopez-Rodriguez et al. Citation2004]. Studies have shown that mice lacking NFAT5 had several renal defects associated with loss of cells in the renal medulla, cells that did not express SMIT and AR, and underwent apoptosis. These results suggest an important role for NFAT5 in the control of hypertonic stress. Interestingly, reports have shown that a higher concentration of inositol and sorbitol is found in the amnionic fluid as compared to the maternal serum, while others have shown that hyperosmolality caused by increased sorbitol induces apoptosis in cultured trophoblast stem cells suggesting that osmolyte control by NFAT5 is tightly regulated [Jauniaux et al. Citation2005; Liu et al. Citation2009].
Previous studies in our laboratory have shown that, in ovine pregnancy, there is a significant umbilical uptake of inositol and sorbitol [Teng et al. Citation2002]. Furthermore, in human pregnancy there is a significant uptake of sorbitol [Brusati et al. Citation2005]. Although NFAT5 was identified in the kidney and other tissues, its presence in the placenta of any species has not been previously shown. The present study had two goals: 1) to determine the concentration of inositol, sorbitol and other carbohydrates in the human and ovine placenta, and 2) to assess the presence of NFAT5 protein and RNA expression in the human and ovine placenta.
RESULTS
Inositol, sorbitol, glucose and other carbohydrates were identified by HPLC. compares the HPLC chromatograms for all the carbohydrates detected in the sugar standard and in a human placental sample.
FIGURE 1 HPLC chromatograms depicting the peaks for various sugars and polyols in the human placenta. INO-OL, myo-inositol; GLY-OL, glycerol; ERY-OL, erythritol; XYL-OI, xylitol; ARA-OL, arabitol; SOR-OL, sorbitol; RIB-OL, ribitol; MAN-OL, mannitol; MAN, mannose; GLU, glucose; GAL, galactose; FRU, fructose.
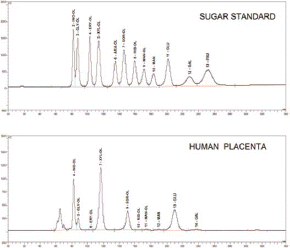
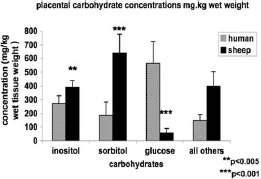
The carbohydrate concentrations are presented in . In the human placenta there was a higher concentration of glucose (565.7 mg/kg wet tissue weight) compared to inositol (272.7 mg/kg wet tissue weight) and to sorbitol (185.5 mg/kg wet tissue weight). In contrast, the ovine placenta had higher levels of sorbitol (641.0 mg/kg wet tissue weight) than inositol (388.9 mg/kg wet tissue weight) and very low glucose concentrations (56.3 mg/kg wet tissue weight).
FIGURE 2 Sugar and polyol concentrations in the human and ovine placenta. Number of observations: human (n=8), sheep (n=6). Statistically significant differences: **p <0.005, ***p <0.001. Concentrations are expressed as mg/kg wet tissue weight.
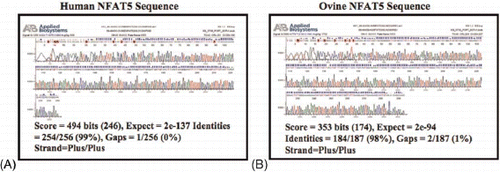
To determine if NFAT5 mRNA was present in the placental tissues, specific primers were designed. RNA was extracted from the placental tissues and reversed transcribed to cDNA. shows a representative 2% agarose gel of the PCR product. cDNA from 293 FT cells (Invitrogen, Carlsbad, CA) was used as the positive control. The primers for human NFAT5 were designed to produce a 300 bp product while ovine primers were designed to produce a 260 bp product. For negative controls, primers were mixed with water in place of DNA. To confirm identity of these PCR products, samples were sequenced. presents a representative chromatogram of the PCR product sequence from human placenta and from ovine cotyledon tissues. The human PCR product was 99% identical to the published NFAT5 product (NM_173215.1). The ovine PCR product was 98% identical to the published ovine NFAT5 sequence (DQ152983).
FIGURE 3 NFAT5 mRNA in the human and ovine placenta. Specific primers produced a 308bp PCR product for the human placenta and a 260bp product for the ovine placenta. mRNA from 293 FT cells was detected and used as positive control. For negative controls, cDNA was omitted from each reaction. A molecular weight standard ranging from 2,645 to 51 bp was used to identify the sizes of the PCR products.
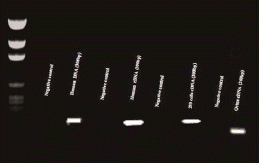
FIGURE 4 Sequence chromatograms from the human and ovine placenta PCR products. (A) The published human NFAT5 sequence shared 99% identity with the human placenta PCR product. (B) The published ovine NFAT5 sequence shared 98% identity with the ovine cotyledon PCR product.
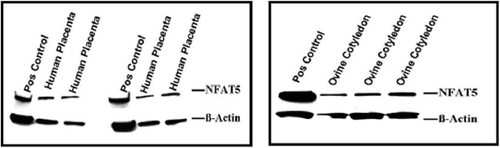
Western blot analysis was used to determine if the NFAT5 protein was also present in these tissues. Protein lysates from 293 FT cells were used as the positive control. presents Western blots for NFAT5 in the human and ovine placenta. NFAT5 protein was present in both the human and ovine placental tissues.
DISCUSSION
The in vivo studies of the umbilical uptake of sorbitol and inositol in the ovine pregnancies and in human pregnancies led us to assay for the presence of NFAT5 at the level of mRNA and protein in the human and ovine placenta [Brusati et al. Citation2005; Teng et al. Citation2002]. The present study demonstrates for the first time the presence of NFAT5 mRNA and protein in the human and sheep placenta. This was coupled with the presence of a high concentration of inositol, sorbitol and glucose in the human placenta. The concentration of inositol in human tissue was higher than sorbitol. The concentration of glucose in ovine placenta was very low in comparison to the high concentration of sorbitol and inositol. Previous in vivo studies in ovine pregnancies had shown a large placental uptake of glucose from the maternal circulation despite the relative low maternal plasma glucose concentration. Thus, there was an ample supply of glucose for inositol and/or sorbitol production within the placenta.
Osmotic stress causes several adaptive mechanisms to restore tonicity across the cell. One such mechanism is the accumulation of organic osmolytes in the cell. As previously mentioned, two of the osmolytes accumulated during stress are inositol and sorbitol. The molecules responsible for the transport or production of these osmolytes are the transporter SMIT for inositol and the AR enzyme for sorbitol [Burg et al. Citation1997; Kwon et al. Citation1995]. Expressions of these proteins are regulated at the level of transcription by the transcription factor NFAT5. NFAT5 stimulates genes coding for these transporters and enzymes [Ko et al. Citation2002; Rim et al. Citation1998; Woo et al. Citation2000; Woo et al. Citation2002a; Woo and Kwon Citation2002]. In vitro studies have shown that cells from mice lacking NFAT5 fail to adapt to hypertonicity and this was due to a decrease in expression of SMIT and AR [Go et al. Citation2004; Lopez-Rodriguez et al. Citation2004]. In our study, the presence of NFAT5 in the placenta suggests an involvement of this protein in the induction of osmolytes, such as inositol and sorbitol, and a role for these organic osmolytes during hypertonic stress in the placenta.
NFAT5 belongs to the NF-kappaB (NF-kB) family of transcription factors [Aramburu et al. Citation2006]. Besides its adaptive role in hypertonic stress, this protein can regulate other processes in mammals. It had been shown to induce inflammatory cytokines in vivo [Shapiro and Dinarello Citation1995]. In mice lacking the NFAT5 gene, Lopez-Rodriguez et al. [2004] showed an increase in embryonic and perinatal lethality associated with this mutation. This suggests an important role for this protein during placental and fetal development. The role of NFAT5 during normal and abnormal placental development has not yet been characterized. To our knowledge, this is the first report showing the presence of this transcription factor in the human placenta. The present study suggests a role for NFAT5 in maintaining osmotic homeostasis in the placenta for both human and ovine pregnancies. Further studies are needed to determine the mechanism and role of NFAT5 during normal and abnormal placentation. More specifically, given the high concentrations of placental inositol and sorbitol and the presence of placental NFAT5, we addressed the effects of varying degrees of osmotic stress on NFAT5 in the placenta.
MATERIALS AND METHODS
Placental Tissue Collection
This study was approved by the Colorado Multiple Institutional Review Board at the University of Colorado at Denver Health Sciences Center. For high performance liquid chromatography (HPLC) studies, placenta were collected from five human and six ovine pregnancies. For the molecular studies (protein and mRNA), placenta were obtained from two human and three ovine pregnancies. All human placenta were from normal full-term uncomplicated pregnancies and all sheep placenta were from normal near term (135 days; 147 days ¼ term) pregnancies. Samples obtained for studies from the human placenta were full thickness from maternal to fetal surface near the umbilical cord insertion site. Three cotyledons per sheep and two placental full-thickness (maternal to fetal surface) tissue samples per human placenta were used for western analysis. All the samples were analyzed in duplicate. Ovine placentomes were dissected into caruncle (maternal) and cotyledon (fetal) components. All tissue samples were collected and placed into liquid nitrogen within 10 min of placental removal from the uterus.
HPLC Analysis
HPLC analyses were performed in the Perinatal Research Facility on the university campus [Teng et al. Citation2002]. Briefly, whole human placenta tissues (n=5) and ovine placentomes (n=6) were collected. Placentomes were separated into caruncle (maternal) and cotyledon (fetal) components. Human placenta and cotyledon tissues were homogenized and sonicated in distilled water at 41C to obtain extra intracellular concentrations. After centrifugation, the tissue supernatant was deproteiinized and analyzed. A Dionex HPLC analyzer equipped with a CarboPac MA1 anion-exchange column was used for separation of the hexoses and polyols (Dionex, Sunnyvale, CA). The analysis was run isocratically with 500 mM sodium hydroxide for 25 min, followed by a step change to 400 mM sodium hydroxide for 20 min at ambient temperature. The flow rate was 0.4 ml/hr. The sodium hydroxide solution was prepared with degassed, deionized water. All the peaks were quantified using a pulse amperometric detector with a gold working electrode. The Dionex PeakNet software was used for instrument operation and data analysis. Xylitol was used as internal standard to correct for instrument variance.
RNA Extraction
Total RNA was extracted from the collected tissue using the TRI REAGENT method. Briefly, 100 mg of tissue was homogenized in 1 mL of TRI REAGENT (Sigma, Saint Louis, MO). After homogenization, samples were centrifuged at 12,000×g for 10min. The supernatants were transferred to a fresh tube and 0.2 ml of chloroform was added to each sample. After centrifugation the aqueous phase was transferred and RNA was precipitated with 0.5 ml of cold isopropanol followed by centrifugation at 12,000×g for 10min. The RNA pellet was washed in 1 ml of cold 75% DEPC treated ethanol. After centrifugation, ethanol was removed, and pellet dried at room temperature. Pellets were resuspended in 50 uL of DEPC treated water. The extracted RNA was then subjected to RNA clean-up using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). To quantify the RNA, sample absorbance was measured at 260, and 280 nm using a GE Healthcare Ultrospec 4300 Pro UV-VIS spectrophotometer (GE Healthcare, Piscataway, NJ).
cDNA Synthesis
cDNA was produced through reverse transcription using the First-Strand cDNA Synthesis protocol from the SuperScript III kit (Invitrogen, Carlsbad, CA). Briefly, 5 μg of total RNA from human or ovine placenta were mixed with 50 μM of oligo(dT) primers, 10 mM diNTP mix and DEPC-treated water. Following this, samples were incubated at 65°C for 5 min. 10 μl of cDNA Synthesis mix (10×RT buffer, 25 mM MgCl2, 0.1 M DTT, RNase out and SuperScript III RT) was added to each sample and incubation at 50°C followed for 50 min. Reactions were terminated by incubation at 85°C for 5 min. RNase H (1 μl) was added to each sample and samples were stored at —20°C until needed.
Reverse Transcriptase-PCR and Sequencing of NFAT5
RT-PCR was performed using MJ Research PTC-200 Pelier Thermal Gradient Cycler (Bio-Rad, Hercules, CA). Tissue specific cDNA was used with either human NFAT5 Forward (5′-GCT TTC TCA GCT TAC CAC GG-3′ and Reverse (5′-TCA CTC GTC CAG AGT CGT TG-3′ primers or ovine NFAT5 Forward (5′ -TTC CAC GGA GAT GGA GAA GAG ACT-3′ and Reverse (5′-TCC TGC TGG GTC TGT GAA TGA GAA-3′ primers at an annealing temperature of 60.0°C during RT-PCR. The NFAT5 PCR product was purified using QIAquick PCR Purification kit (Qiagen, Valencia, CA) and sequenced. The human placental product showed sequence identity of 99% to the human NFAT5 sequence while cotyledon tissue showed a 98% identity to the ovine NFAT5 sequence.
Western Blot Analysis
Ovine cotyledon and human placental tissues were homogenized in protein lysis buffer containing 10mM of PMSF, 10mM of Na3VO4, 1×triton TX-100, 150 mM NaCl, 20 mM Tris Base, 5 μM of AEBSF, 5 μM of EDTA, 10 nM of E-64, 10 nm of Leupeptin and 10ng/ml of Aprotinin. Protein tissue lysates containing 50 μg were separated on 10% SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were incubated with an antibody against mouse NFAT5 (at a dilution of 1:500 for human placenta and 1:100 for ovine placenta) (Affinity BioReagents, Golden, CO). A secondary anti-mouse IgG-HRP antibody (dilution 1:10,000) (Upstate Cell Signaling solutions, Lake Placid, NY) was incubated for 1 h at room temperature. The membranes were incubated with chemiluminescent substrate (Pierce, Rockford, IL) for 5 min, developed with chemiluminescent reagent and exposed to X-ray film. To determine loading consistencies, each membrane was stripped of antibodies and reprobed utilizing antibody against mouse beta-actin (dilution 1:4,000) (MP Biomedicals, Aurora, Ohio) to determine the amount of total protein present in each lane.
Statistical Analysis
Bar graph data are shown as mean±SE. They-f-test was used to assess equality of variance. Differences in p values between groups were determined using student's t-test for paired observations withp <0.05 considered significant.
ACKNOWLEDGMENTS
This study was supported by NIH grant R01 HDO34837. We thank Bradley Ziebell for his technical assistance.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Aramburu, J., Drews-Elger, K., Estrada-Gelonch, A., Minguillon, J., Morancho, B., Santiago, V. and Lopez-Rodriguez, C. (2006) Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol 72:1597–1604.
- Brusati, V., Jozwik, M., Jozwik, M., Teng, C., Paolini, C., Marconi, A. M. and Battaglia, F. C. (2005) Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res 58:700–704.
- Burg, M. B., Kwon, E. D. and Kultz, D. (1997) Regulation of gene expression by hypertonicity. Annu Rev Physiol 59:437–455.
- Go, W. Y., Liu, X., Roti, M. A., Liu, F. and Ho, S. N. (2004) NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101:10673–10678.
- Han, K. H., Woo, S. K., Kim, W. Y., Park, S. H., Cha, J. H., Kim, J. and Kwon, H. M. (2004) Maturation of TonEBP expression in developing rat kidney. Am J Physiol Renal Physiol 287:F878–F885.
- Handler, J. S. and Kwon, H. M. (2001) Cell and molecular biology of organic osmolyte accumulation in hypertonic renal cells. Nephron 87:106–110.
- Jauniaux, E., Hempstock, J., Teng, C., Battaglia, F. C. and Burton, G. J. (2005) Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: Maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab 90(2):1171–1175.
- Ko, B. C., Lam, A. K., Kapusi, A., Fan, L., Chung, S. K. and Chung, S. S. (2002) Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP). J Biol Chem 277:46085–46092.
- Kwon, H. M., Itoh, T., Rim, J. S. and Handler, J. S. (1995) The MAP kinase cascade is not essential for transcriptional stimulation of osmolyte transporter genes. Biochem Biophys Res Commun 213:975–979.
- Liu, J., Xu, W., Sun, T., Wang, F., Puscheck, E., Brigstock, D., Wang, Q. T., Davis, R. and Rappolee, D. A. (2009) Hyperosmolar stress induces global mRNA responses in placental trophoblast stem cells that emulate early post-implantation differentiation. Placenta 30:66–73.
- Lopez-Rodriguez, C., Antos, C. L., Shelton, J. M., Richardson, J. A., Lin, F., Novobrantseva, T. I., Bronson, R. T., Igarashi, P., Rao, A. and Olson, E. N. (2004) Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA 101:2392–2397.
- Miller, T. J., Hanson, R. D. and Yancey, P. H. (2000) Developmental changes in organic osmolytes in prenatal and postnatal rat tissues. Comp Biochem Physiol A Mol Integr Physiol 125:45–56.
- Miyakawa, H., Woo, S. K., Chen, C. P., Dahl, S. C., Handler, J. S. and Kwon, H. M. (1998) Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am J Physiol 274:F753–F761.
- Rim, J. S., Atta, M. G., Dahl, S. C., Berry, G. T., Handler, J. S. and Kwon, H. M. (1998) Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 50 -flanking region. J Biol Chem 273:20615–20621.
- Shapiro, L. and Dinarello, C. A. (1995) Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA 92:12230–12234.
- Teng, C. C., Tjoa, S., Fennessey, P. V., Wilkening, R. B. and Battaglia, F. C. (2002) Transplacental carbohydrate and sugar alcohol concentrations and their uptakes in ovine pregnancy. Exp Biol Med (Maywood) 227:189–195.
- Woo, S. K., Dahl, S. C., Handler, J. S. and Kwon, H. M. (2000) Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. Am J Physiol Renal Physiol 278:F1006–F1012.
- Woo, S. K. and Kwon, H. M. (2002) Adaptation of kidney medulla to hypertonicity: Role of the transcription factor TonEBP. Int Rev Cytol 215:189–202.
- Woo, S. K., Lee, S. D. and Kwon, H. M. (2002a) TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch 444:579–585.
- Woo, S. K., Lee, S. D., Na, K. Y., Park, W. K. and Kwon, H. M. (2002b) TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol 22:5753–5760.
- Zhou, X., Ferraris, J. D. and Burg, M. B. (2006) Mitochondrial reactive oxygen species contribute to high NaCl-induced activation of the transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol 290:F1169–F1176.