Abstract
Environmental chemicals that alter steroid production could interfere with male reproductive development and function. Three agricultural antifungal triazoles that are known to modulate expression of cytochrome P450 (CYP) genes and enzymatic activities were tested for effects on steroidogenesis using rat in vivo (triadimefon), rat in vitro (myclobutanil and triadimefon), and human in vitro (myclobutanil, propiconazole, and triadimefon) model systems. Hormone production was measured in testis organ cultures from untreated adult and neonatal rats, following in vitro exposure to 1, 10, or 100 μM of myclobutanil or triadimefon. Myclobutanil and triadimefon reduced media levels of testosterone by 40–68% in the adult and neonatal testis culture, and altered steroid production in a manner that indicated CYP17-hydroxylase/17,20 lyase (CYP17A1) inhibition at the highest concentration tested. Rat to human comparison was explored using the H295R (human adrenal adenocarcinoma) cell line. Following 48 h exposure to myclobutanil, propiconazole, or triadimefon at 1, 3, 10, 30, or 100 μM, there was an overall decrease in estradiol, progesterone, and testosterone by all three triazoles. These data indicate that myclobutanil, propiconazole, and triadimefon are weak inhibitors of testosterone production in vitro. However, in vivo exposure of rats to triazoles resulted in increased serum and intra-testicular testosterone levels. This discordance could be due to higher concentrations of triazoles tested in vitro, and differences within an in vitro model system lacking hepatic metabolism and neuroendocrine control.
| Abbreviations | ||
| CYP: | = | cytochrome P450 |
| LH: | = | luteinizing hormone |
| HPG: | = | hypothalamic-pituitary-gonadal |
| AR: | = | androgen receptor |
| PND: | = | postnatal day |
| hCG: | = | human chorionic gonadotropin |
| PRL: | = | prolactin |
| LDH: | = | lactate dehydrogenase |
| CoV: | = | coefficient of variation |
INTRODUCTION
Conazoles are triazole fungicides used for crop protection and pharmaceutical treatment of fungal infections. They inhibit cytochrome P450 (CYP) 51 by competitively binding to the heme component of the CYP enzyme [Ghannoum and Rice Citation1999]. In fungal cells this binding depletes ergosterol, which leads to a buildup of precursor sterols in the cellular membrane, disrupting turgor pressure and triggering cytotoxicity. Conazoles disrupt several CYPs, including hepatic CYPs (CYP1A1, CYP2B2, CYP3A1, CYP3A2, CYP4A1) and steroidogenic CYPs (CYP17A1, CYP19A1) and consequently there is concern that inadvertent exposure to agricultural conazoles may inhibit steroidogenesis and adversely affect reproduction in humans and other mammalian species [Zarn et al. Citation2003].
It has been shown that some triazole conazoles inhibit aromatase-mediated conversion of testosterone to estrogen [Andersen et al. Citation2002; Trösken et al. Citation2004; Vinggaard et al. Citation2000]. In addition, there are reports that have investigated the effects of agricultural triazoles on testosterone synthesis. The triazoles hexaconazole and flusilazole inhibited testosterone synthesis in Leydig cell culture and increased the incidence of Leydig cell tumors in rats [Inchem IPCS Citation1990; Citation1995]. In contrast, the triazoles myclobutanil and triadimefon, did not cause Leydig cell tumors in rats, but did increase serum testosterone levels in adult male rats after 14 days. This occurred without affecting serum luteinizing hormone (LH), estradiol, or stimulating Leydig cell hyperplasia [Tully et al. Citation2006].
The current study was designed to investigate the effect of myclobutanil, propiconazole, and triadimefon on testis testosterone synthesis in vivo or in vitro, and to determine whether and how exposure to these triazoles alters testis testosterone levels in conjunction with increased serum testosterone levels. In the first experiment, the effects of triadimefon following exposure in vivo on serum and testis testosterone levels were measured in rats to test the hypothesis that increased testis testosterone production contributes to the reported elevated serum testosterone levels. We selected triadimefon because it had caused the most robust increase in serum testosterone in previous rat experiments [Goetz et al. Citation2007; Tully et al. Citation2006]. Serum LH was measured to determine if it contributed to altered testosterone production, and serum estradiol to test if inhibition of aromatase occurred in vivo. In the second experiment, rat in vivo to in vitro comparisons were explored by measuring intra-testicular testosterone production in organ cultures of neonatal and adult rat testes exposed to varying concentrations of either myclobutanil or triadimefon to assess in vitro testis testosterone production. In the third experiment, rat to human comparisons were explored by measuring hormone production (testosterone, estradiol, and progesterone) in the H295R cell line following exposure to myclobutanil, propiconazole, or triadimefon.
RESULTS
In Vivo Triadimefon Effects
The mean dose received was 126 mg triadimefon/kg body weight/day. Feed intake of treated males was on average 10% less than the control males over the course of dosing. During the first week of treatment, body weights were significantly decreased 8–10% at 1800 ppm triadimefon compared to the controls, however, the rate of body weight gain was similar between treatment groups throughout the remainder of the study (). Four individuals (one control and three treated) were removed from the study due to factors not related to treatment (dehydration).
FIGURE 1 Body weight of control and triadimefon treated rats over the course of dosing. Solid line, control, dashed line, triadimefon 1800ppm treatment. Error bars represent standard error mean. **p < 0.01, ***p < 0.001.
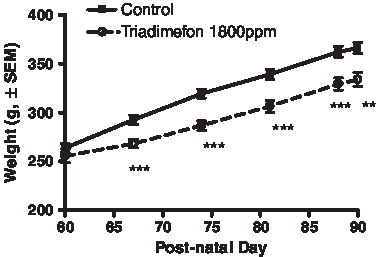
Liver weights were increased (27% absolute, 37% adjusted for body weight) after 30 days exposure (). Absolute pituitary and paired epididymal weights were decreased (9.8% and 5.2%, respectively) at necropsy. There were no treatment effects on the androgen-dependent tissues; ventral prostate or seminal vesicle. Serum testosterone levels were unaffected following 2 weeks exposure, however they were increased after 4 weeks exposure along with intra-testicular testosterone levels (). Serum levels of estradiol, LH, and prolactin were elevated, but not statistically significant.
TABLE 1 Weight and Hormone Measurements (± SEM) from Control and Treated Animals Following 30 days Dietary Exposure to Triadimefon.
Rat In Vitro Testis Cultures
Testosterone production remained fairly constant (<11% change) in the positive control adult testis tissue after each successive time point (0.5, 1.5, and 2.5 h; human chorionic gonadotropin (hCG) stimulated) (). Testosterone levels increased ∼88% between the 0.5 and 1.5 h time point in the positive control neonatal testis, and remained constant during the 1.5 and 2.5 h time point (). Accounting for incubation time, testosterone production decreased slowly after each successive time point in the negative control adult and neonatal testis cultures that were not administered hCG and triazole treatment. The control data suggests hCG continued to stimulate testosterone production in both adult and neonatal testis cultures; and testosterone production decreased over time without hCG in the testis cultures.
FIGURE 2 In vitro testosterone production. Adult (A) and neonatal (B) testis testosterone production after varied exposure to myclobutanil (Myc) and triadimefon (Tri). Error bars represent standard error mean. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01, and ***p < 0.001) between the treatment group and the control group ((+), hCG with no chemical) at each time point. (−) refers to tissue not stimulated by hCG and without chemical treatment.
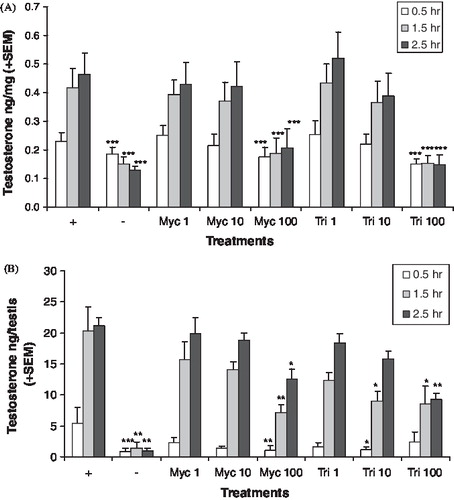
Following administration of myclobutanil or triadimefon in the adult testis cultures, testosterone levels were reduced by a statistically significant amount only at the highest concentration tested (). Testosterone levels were reduced by myclobutanil or triadimefon by less than 50% following 100 μM treatment compared to the respective control at 0.5 h with hCG. At 1.5 and 2.5 h, testosterone levels decreased 63–68% following 100 μM triadimefon treatment and ∼50% following 100 μM myclobutanil treatment (). In the neonatal testis culture, testosterone levels were reduced following myclobutanil (100 μM ) and triadimefon (10 and 100 μM) treatment, which suggests that triadimefon might be the stronger inhibitor of the two (). Inhibition of neonatal testosterone production was more pronounced at the 0.5 h time point by myclobutanil and triadimefon compared to the 1.5 and 2.5 h time points. This may be due to testosterone levels increasing over time, as was seen in the control groups containing hCG. Neonatal testosterone production was reduced ∼57% by triadimefon (10 and 100 μM) at 1.5 and 2.5 h. The 100 μM myclobutanil treatment reduced neonatal testosterone production by 65% and 40% at 1.5 and 2.5 h, respectively.
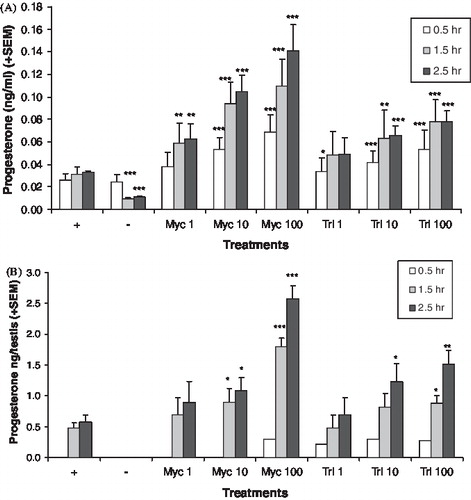
The hormone pattern after chemical treatment suggests CYP17A1 was inhibited. Media levels of androstenedione were reduced by both chemicals in the adult () and neonatal () testis cultures, but not at all concentrations and time points that reduced testosterone levels. In the adult testis cultures, the levels of 17alphahydroxyprogesterone () and progesterone () were increased by myclobutanil and triadimefon at lower concentration levels than those that affected the androgens. In the neonatal testis cultures, the levels of 17alphahydroxyprogesterone () and progesterone () were also increased. The progesterones were not detected in the media of the neonatal testis cultures at all time points. LDH levels were variable in the adult testis culture, which made cytotoxicity evaluation difficult. Analysis of the log transformed LDH data found no significant differences among the treatment groups, with the exception of 100 μM triadimefon at the 2.5 h time point. At this concentration and time point, LDH levels were significantly lower than the control (p < 0.011, data not shown). Cytotoxicity in the neonatal testis cultures could not be evaluated since LDH was not detected in these cultures.
FIGURE 3 In vitro androstenedione production. A adult (A) and neonatal (B) testis androstenedione production after varied exposure to myclobutanil (Myc) and triadimefon (Tri). Error bars represent standard error mean. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01, and ***p < 0.001) between the treatment group and control group ((+), hCG with no chemical) at each time point. (−) refers to tissue not stimulated by hCG and without chemical treatment.
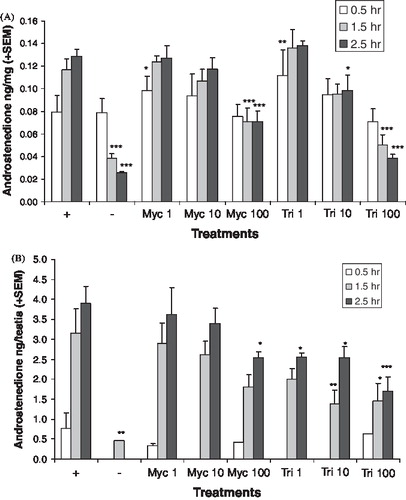
FIGURE 4 In vitro 17alpha-hydroxyprogesterone production. Adult (A) and neonatal (B) testis 17alpha-hydroxyprogesterone production after exposure to various levels of myclobutanil (Myc) and triadimefon (Tri). Error bars represent standard error mean. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01, and ***p < 0.001) between the treatment group and control group ((+), hCG with no chemical) at each time point. (−) refers to tissue not stimulated by hCG and without chemical treatment.
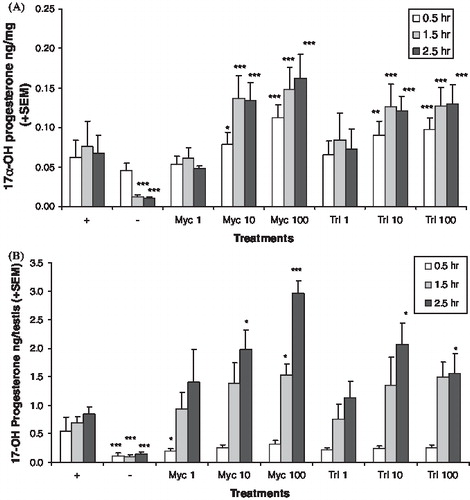
FIGURE 5 In vitro progesterone production. Adult (A) and neonatal (B) testis progesterone production after exposure to various levels of myclobutanil (Myc) and triadimefon (Tri). Error bars represent standard error mean. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01, and ***p < 0.001) between the treatment group and control group ((+), hCG with no chemical) at each time point. (−) refers to tissue not stimulated by hCG and without chemical treatment.
H295R Cell Viability and H295R Hormone Assays
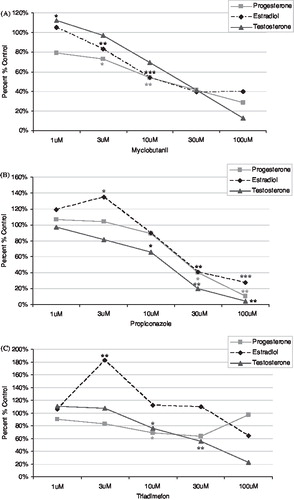
No adverse effects on cell growth or viability were observed following triazole treatments (data not shown). Relative change in estradiol, progesterone, and testosterone levels by myclobutanil, propiconazole, or triadimefon in H295R cells are shown in . Myclobutanil reduced the levels of estradiol in a concentration-dependent manner. Propiconazole and triadimefon produced an increase in estradiol at the lower doses of 1 and 3 μM but a decrease as the dose increased. All three triazoles decreased levels of progesterone at all doses assessed, however progesterone levels returned to control levels at the highest dose of triadimefon. Testosterone levels were decreased by all three triazoles in a consistent manner.
FIGURE 6 Hormone levels from H295R cell media following exposure. The levels of progesterone, estradiol, and testosterone were assessed in H295R cell media following 48 h exposure to triazoles (A) myclobutanil, (B) propiconazole, and (C) triadimefon. Each panel presents progesterone, estradiol, and testosterone changes. Error bars omitted for clarity. All data presented as percent change relative to controls. *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
Toxicology studies using animals and in vitro cellular or tissue preparations have been used to study the toxic effects and mechanism of action of chemicals, to determine the effective and safe dose of drugs in humans and the risk of toxicity from chemical exposures. The current approach to risk assessment includes uncertainty factors associated with interspecies (human relevance of laboratory animal studies) and intraspecies extrapolation (susceptible populations); and extrapolation from high dose levels down to lower environmental exposures. This uncertainty can be informed through the use of mechanistic and mode of action data from in vitro biological models, like H295R cells or cultured testis, that provide concentration-response of relevant end-points in the target species [Hecker and Giesy Citation2008; U.S. EPA Citation2009]. In this case in vitro testing allows for a specific evaluation of a chemical's ability to alter the synthesis of steroids; however the absorption, distribution, metabolism, and elimination (ADME) of the chemical is not accounted for in such testing [Gray et al. Citation1997]. In order to completely understand the dose-responsive effects of a chemical in vivo, dose-response modeling of perturbations of pathway functions will need to be explored in further detail.
The ability of each triazole antifungal to affect testosterone production was examined in this study, which may be helpful in understanding the mode of action for the reproductive effects observed in toxicology studies with some conazoles. This series of experiments was designed to address the hypothesis that the triazoles myclobutanil and triadimefon elicit their reproductive effects through disruption of testosterone production in the testis.
Results from this set of experiments demonstrate that all three triazoles were weak inhibitors of testosterone production in vitro and suggest that, at least for myclobutanil and triadimefon, inhibition of CYP17A1 occurs in vitro. Inhibition of CYP17A1 has been reported with several imidazole compounds [Ayub and Levell Citation1987; Engelhardt et al. Citation1991], and triazole compounds such as hexaconazole [Lloyd Citation1991] and flusilazole [Inchem IPCS Citation1995]. Inhibition of the LH signal transduction pathway, as stimulated by hCG, may have contributed to lowered testosterone production. Although this hypothesis was not tested in our study design, the increased progestin levels in the cultures treated with myclobutanil or triadimefon suggest that hCG stimulation of the LH pathway was not altered.
The data suggest that changes in hormone levels were not due to direct cytotoxicity. Indirect evidence from the progestin hormone data suggests an absence of cytotoxicity; levels of these hormones continued to rise with increasing concentrations of the triazoles. This effect was also observed in the adult testis culture, where no significant cytotoxicity was observed.
Inhibition of testosterone synthesis was the hypothesis used to rationalize the increased incidence of Leydig cell tumors observed by the triazoles hexaconazole and flusilazole. A similar hypothesis was proposed that ketoconazole would induce similar tumors by the same mechanism if tested under the U.S. EPA guideline criteria (i.e., maximum tolerated dose and length of exposure [Cook et al. Citation1999]). Triadimefon and myclobutanil appear to inhibit CYP17A1 in vitro, decrease testosterone levels in vitro, but increase serum testosterone levels in vivo and do not induce Leydig cell tumors. The difference across the triazole and imidazoles may be a result of the strength with which different conazoles bind and inhibit metabolizing and/or steroidogenic CYPs. Additionally, the effect of liver metabolism and clearance of the conazoles needs to be considered [Goetz and Dix Citation2009]. This marked reduction in testosterone production by some conazoles in vivo is likely due to a threshold dose response as part of the mode of action for the production of Leydig cell tumors in rats.
In the dietary exposure experiment, triadimefon increased serum testosterone levels similar to previous reports [Goetz et al. Citation2007; Inchem IPCS Citation1985; Tully et al. Citation2006]. Serum testosterone levels were not increased after the first two weeks of dosing suggesting this effect developed over time. The increased intra-testicular level of testosterone by triadimefon suggests increased testis testosterone production contributes to the increase observed in serum testosterone. In addition treatment related hepatic adaptive response, presumably causing a decrease in liver testosterone metabolism and clearance, also likely contributed to the increased circulating levels of testosterone [Goetz et al. Citation2007; Goetz and Dix Citation2009].
The in vitro data demonstrated the opposite effect on testosterone production, producing a discrepancy between the in vitro and in vivo results. Several triazole compounds have been examined in both in vivo and in vitro studies, such as fluconazole [Hanger et al. Citation1988] and R76713 and its enantiomers [Wouters et al. Citation1990]. In both cases, the inhibitory effects observed in vitro on androgen synthesis and the concomitant increase in the precursor progestins [Wouters et al. Citation1990], which is indicative for some effect on the CYP17A1 enzyme, were not repeated in the in vivo experiments. As noted in these other studies, it is likely that the in vitro triadimefon concentrations which significantly inhibit testosterone production (100 μM) are not achieved through dietary exposure in the adult rat, and another cellular response pathway is stimulating the increased serum testosterone levels at lower concentrations of triadimefon.
A similar situation occurs with the triazoles tebuconazole and epoxiconazole [Taxvig et al. Citation2007; Citation2008]. Reproductive toxicity studies with tebuconazole and epoxiconazole demonstrated female virilization (increased anogenital distance) by both triazoles, fetal male feminization following exposure to tebuconazole with a concomitant decrease in the level of fetal testosterone, and increased testosterone levels in the dams following epoxiconazole which was observed following myclobutanil exposure to pregnant dams as well [Goetz et al. Citation2007]. Although the overall results from these reproductive toxicity studies show that many of the azole fungicides behave similarly following gestational exposure, the profile of action in vivo varies. The route of exposure (oral); method of exposure, whether dietary or by gavage with a vehicle such as corn oil, and duration of exposure will all certainly have a significant impact on the outcome of the studies. In addition it is important to define and characterize the effects observed at the different dose levels to better inform the relative potency of individual conazoles.
Other factors to consider in the disparity of testosterone levels between the different experiments are the strain of rat (Wistar Han v. Sprague Dawley), use of an adrenocortical carcinoma cell line (H295R) vs. testis tissue, metabolism in vivo vs. in vitro, dose levels used, and the duration of exposure in each experiment. The H295R adrenocortical carcinoma cell lines have been characterized in detail and appear to be useful tools for the study of steroidogenesis [Hecker and Giesy Citation2008]. However, H295R cell expression of key enzymes necessary for steroidogenesis differs from in vivo expression of these genes and enzymes in tissue and developmental stage-specific combinations, under neuroendocrine control [Hecker and Giesy Citation2008]. Thus the steroidogenesis environment is different between the H295R cells and the testis, making it possible that the changes in progesterone levels are due to differences in enzyme levels, feedback loops, and signal transduction pathways between the two systems.
In terms of the actual chemical moiety and dose level reaching tissues and cells, it is possible that triadimenol, a major metabolite of triadimefon [Roberts and Hutson Citation1999] is reaching the testis in vivo, whereas effects observed in vitro are likely solely due to triadimefon. Actual dose metrics for the levels of triadimefon, or triadimenol, were not determined in the in vivo studies and testis, making direct dosimetric comparisons impossible. In addition, the duration of exposure was very different in some of the in vivo vs in vitro comparisons. Following in vivo exposures to these triazoles serum testosterone levels increase at six and 24 h of exposure, decreased at 4 days, and returned to control levels at day 14 [Goetz Citation2007; Martin et al. Citation2007; Tully et al. Citation2006]. Thus, the decreased testosterone levels seen in vitro, in both testis and H295R, appear most similar to decreases observed in vivo following 96 h exposure.
The mechanism of action responsible for the increased serum and testis testosterone levels is not clear. The elevated level of testosterone would be expected to reduce LH levels through a negative feedback loop. However, triadimefon exposure did not significantly alter serum LH suggesting that the hypothalamic-pituitary-gonadal (HPG) axis may be altered. Triadimefon could be acting as an androgen receptor (AR) antagonist to stimulate increased testosterone production, but it is unlikely since high levels of triadimefon/triadimenol are required to inhibit AR function [Okubo et al. Citation2004]. In addition, the androgen-dependent ventral prostate and seminal vesicle weights were unaffected in this study, and other studies do not show much evidence of AR antagonism in androgen-dependent tissues [Goetz et al. Citation2007; Tully et al. Citation2006]. Triadimefon has been reported to be an aromatase inhibitor in vitro, but this inhibition was not evident in serum estradiol within our study and others [Goetz et al. Citation2007; Tully et al. Citation2006] which suggests that an altered HPG regulation from reduced estradiol levels did not occur.
One explanation for a disrupted HPG axis that has not been explored is by means of altered neurotransmitters within the hypothalamus. Triadimefon exposure has been shown to affect behavior presumably by altering neurotransmitters within the brain [Crofton et al. Citation1988; Reeves et al. Citation2004a,Citationb; Walker and Mailman Citation1996]. If triadimefon is affecting neurotransmitters within the hypothalamus, this mechanism could disrupt the HPG axis. This hypothesis was investigated by measuring serum PRL levels as a proxy of altered dopamine levels within the hypothalamus [Waeber et al. Citation1983], but there was no effect. The mechanism by which triadimefon increases testis testosterone production requires further investigation and should include examination of hypothalamic-pituitary axis regulation.
In summary, triadimefon and myclobutanil show weak inhibition of testosterone production in vitro in rat testis cultures, and the data suggests inhibition of CYP17A1. However, following a longer exposures in vivo, triadimefon increased rat testis testosterone production and serum testosterone levels. The mechanism of action for increased testis testosterone levels in vivo is unresolved, but may possibly involve a disruption of the HPG axis. Further studies into the effects of triadimefon and other triazoles on the pituitary, hypothalamus, testis, and steroidogenesis will be needed to fully elucidate the dose-response of mechanisms relevant to human health risk assessment.
MATERIALS AND METHODS
Animal Husbandry
All animal procedures were approved by the U.S. Environmental Protection Agency's National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee. All animals were purchased from Charles River Laboratories (Raleigh, NC) and housed in an Association for Assessment and Accreditation for Laboratory Animal Care-International accredited facility. Animals were individually housed in polypropylene boxes containing Alpha-Dri® bedding (Shepherd Specialty Papers, Watertown, TN), and subjected to a 12 h:12 hight:dark cycle under controlled temperature (22 ± 2°C) and humidity (40−60%). Animals were provided unlimited access to LabDiet 5002 Rodent Diet (PMI LabDiet, Richmond, IN) and water. Feed was prepared by Bayer CropScience (Kansas City, MO) as part of a Materials Cooperative Research and Development Agreement between the USEPA and the US Triazole Task Force. Control animals were fed 5002 Certified Rodent Diet. The triadimefon treated group received feed containing 1800 ppm triadimefon.
In Vivo Dosing and Sample Collection
The treated animals started dietary exposure on postnatal day (PND) 60. Male Wistar Han rats (n = 15) were fed rat chow 5002 containing 1800 ppm triadimefon. This dose level was selected to match the highest dose level used in regulatory studies for registering triadimefon with the U.S. EPA, and for its significant effect on serum testosterone levels in previous studies [Goetz et al. Citation2007]. Treatment lasted 30 days, body weight and feed were weighed on a weekly basis to determine dose. Animals were tail bled 14 days into dosing (PND74) for testosterone measurements. On day 30 (PND 90) animals were decapitated between 08:30 and 10:30 and then necropsied. Trunk blood was collected for serum measurements of testosterone, estradiol, luteinizing hormone, and prolactin. Liver, epididymis, ventral prostate, seminal vesicle, and pituitary were collected and weighed. Testes were weighed and snap frozen in liquid nitrogen and stored at −80°C until analysis. The frozen right testis was homogenized in cold Dulbecco's PBS (Gibco, Grand Island, NY) with an Ultra-Turrax T25 homogenizer (Janke-Kunkel IKA, Boutersem, Belgium), and centrifuged at 4°C for 10 min at 4,000 rcf using a Beckman J2-21 M centrifuge. Supernatant was then centrifuged with a 5417R centrifuge (Eppendorf, Westbury, NY) at 20,000 rcf for 10 min at 4°C. Supernatant was collected and stored at −80°C until analysis of intratesticular testosterone measurements.
Rat In Vitro Testis Culture
Testes from adult (PND 90–100, n = 5–8) and neonatal (PND 1, n = 5 litters) Sprague Dawley rats were used in the in vitro study. Testis parenchyma was sliced into ∼100 mg pieces for each adult testis and neonatal testes were left intact (expected mass of 2–4 mg; [Goetz et al. Citation2007]). Testis tissue was incubated in 1.5 ml of M199 media (Gibco, Grand Island, NY) supplemented with 0.2% bovine serum albumin (Sigma, St. Louis, MO) and 10% charcoal/dextran treated fetal bovine serum (Hyclone, Logan, UT). Human chorionic gonadotropin (hCG), an LH receptor agonist, was used to stimulate testosterone production. Human chorionic gonadotropin (Sigma, St. Louis, MO) was added at 100 mU/mL to all treatment groups except the negative controls. Technical grade (>95% purity) myclobutanil (LKT Laboratories Inc., St. Paul, MN) or triadimefon (Bayer CropScience, Kansas City, KS) was added to a final concentration of 1, 10, or 100 μM. Each test chemical was premixed with ethanol to aid dilution; the final ethanol volume was 0.05% of the total culture volume. Positive control medium (plus hCG, minus test chemical) and negative control medium (minus hCG and test chemical) both contained 0.05% ethanol. Tissues were incubated at 34°C in 2.0 ml siliconized tubes rotated at ∼10 rpm. At three time points, 0.5, 1.5, and 2.5 h, the media was removed and replenished with fresh media containing the appropriate triazole and dose concentration. All treatments were replicated in triplicate for each adult rat.
H295R Cells
H295R human adrenocortical carcinoma cell lines were obtained from the American Type Culture Collection (ATCC CRL-2128; ATCC, Manassas, VA) and grown in 75 cm2 flasks with 12.5 ml of supplemented medium at 37°C with a 5% CO2 atmosphere. H295R cultures were performed by Dr. Xiaowei Zhang in the Department of Zoology at Michigan State University, East Lansing, MI. Supplemented medium was a 1:1 mixture of Dulbecco's modified Eagle's medium with Ham's F-12 Nutrient mixture (Sigma, St. Louis, MO) with 15 mM HEPES buffer. The medium was supplemented with 1.2 g/L Na2CO3, ITS + Premix (1 ml Premix/100 ml medium), and 12.5 ml/500 ml NuSerum (BD Biosciences, San Jose, CA). Final component concentrations in the medium were as follows: 15 mM HEPES, 6.25 μg/ml insulin, 6.25 μg/ml transferrin, 6.25 ng/ml selenium, 1.25 mg/ml bovine serum albumin, 5.35 μg/ml linoleic acid, and 2.5% NuSerum. The medium was changed two to three times per week and cells were detached from flasks for subculturing by use of trypsin/ EDTA (Sterile 1X Trypsin-EDTA; Life Technologies Inc., Grand Island, NY). Cells were exposed to triazoles in 6-well Tissue Culture Plates (Nalgene Nunc Inc., Rochester, NY). To minimize potential effects of hormones in the serum during exposures, Nu-Serum was replaced with 2.5% charcoal dextran treated FBS (HyClone Laboratories, Inc. Logan, UT) immediately before cells were exposed to triazoles at 1, 3, 10, 30, or 100 μM concentration in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO) for 48 h. At the end of this culture period media was collected and shipped frozen to EPA for hormone measurements.
H295R Cell Viability
The protocol was adapted from Hilscherova et al. [Citation2004]. Cells were visually inspected under a microscope to evaluate viability and cell numbers. Cell viability was determined using the live/dead cell viability kit (Molecular Probes, Eugene, OR).
Hormone Measurements
Rat intratesticular levels of testosterone, androstenedione, 17alpha-hydroprogesterone, and progesterone were measured from the culture media using coat-a-count 125I radioimmunoassay kits (Diagnostic Products Corporation, Los Angeles, CA). Serum LH was measured using the rat disassociation enhanced lanthanide flourometric immunoassay (DELPHIA) [Haavisto et al. Citation1993]. Serum prolactin (PRL) was measured by radioimmunoassay according to manufacturer's instructions, using materials supplied by the National Hormone and Pituitary Agency for LH and PRL: iodination preparation I-6, reference preparation RP-3, and antisera S-9. Iodination material was radiolabelled with 125I (DuPont/New England Nuclear) by a modification of the chloramine-T method [Greenwood et al. Citation1963]. Con6 control standards (Diagnostic Products Corporation, Los Angeles, CA) were used to verify assay quality. Lactate dehydrogenase (LDH) levels in the media, an indicator of cytotoxicity, were measured using an LDH detection kit (Roche Diagnostics, Indianapolis, IN). Estradiol, progesterone, and testosterone levels in the H295R culture media were assayed in duplicate using appropriate Coat-A-Count radioimmunoassay (RIA) kits (Diagnostic Products Co., Los Angeles, CA) according to manufacturer's instructions. Established serum standards, a low, medium, and high control, were used to quantitate variation among hormone assays. The intra-assay coefficient of variation (CoV) range was 1–13%, and the inter-assay CoV was 14–19% among the low, medium, and high controls for testosterone assays. The androstenedione intra-assay CoV range was 0–9% and inter-assay CoV was 7–17%. The 17alpha-hydroxyprogesterone intra-assay CoV range was 0–12% and inter-assay CoV was 2–11%. The progesterone intra-assay CoV range was 2–12% and inter-assay CoV was 19–21%.
Statistical Analysis
The average of the three technical replicates for each adult rat testis was used for statistical analysis. The litter was the unit of analysis for the neonatal culture. Rat in vitro hormone data and LDH data, both log10 transformed, were analyzed using the SAS GLM procedure (SAS Institute Inc., Cary, NC). Since each adult or litter was exposed to all eight treatments within the testis culture assay, animal or litter was used as a blocking factor within analyses of variance. Pairwise t-tests were used to test for any differences between treatment groups and the control. Rat in vivo hormone data (log 10 transformed), body and tissue weight data were analyzed using a two-tail t-test. Statistical significance between control and treatment was set at p < 0.05. H295R hormone data was analyzed using ANOVA, measures with p < 0.05 were considered significant. Students t-test was used for further comparisons between control and treatment groups.
ACKNOWLEDGMENTS
AKG was supported by U.S. EPA and N.C. State University Cooperative Training Agreement No. CT826512010.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The United States Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. It has undergone Agency review and has been approved for publication.
REFERENCES
- Andersen, H. R., Vinggaard, A. M., Rasmussen, T. H., Gjermandsen, I. M. and Bonefeld-Jørgensen, E. C. (2002) Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol 179:1–12.
- Ayub, M. and Levell, M. J. (1987) Inhibition of testicular 17alpha-hydroxylase and 17,20 lyase but not 3beta-hydroxysteroid dehydrogenase or 17beta-hydroxysteroid oxidoreductase by ketoconazole and other imidazole drugs. J Steroid Biochem 28:521–531.
- Cook, J. C., Klinefelter, G. R., Hardisty, J. H., Sharpe, R. M. and Foster, P. M. D. (1999) Rodent leydig cell tumorigenesis: A review of the physiology, pathology, mechanisms, and relevance to humans. Crit Rev Toxicol 29:169–261.
- Crofton, K. M., Boncek, V. M. and Reiter, L. W. (1988) Hyperactivity induced by triadimefon, a triazole fungicide. Fundam Appl Toxicol 10:459–465.
- Engelhardt, D., Weber, M. M., Miksch, T., Abedinpour, F. and Jaspers, C. (1991) The influence of ketoconazole on human adrenal steroidogenesis: Incubation studies with tissue slices. Clin Endocrinol (Oxf) 35:163–168.
- Ghannoum, M. A. and Rice, L. B. (1999) Antifungal agents: Modes of action, mechanisms of resistance, and correlations of the mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517.
- Goetz, A. K. (2007) Toxicogenomic study of triazole antifungal modes of action. Dissertation. Ph.D. Dissertation, North Carolona State University, Raleigh, NC. URD, etd-05302007–125723. (http://www.lib.ncsu.edu/theses/available/etd-05302007–125723/).
- Goetz, A. K., Ren, H., Schmid, J. E., Blystone, C. R., Thillainadarajah, I., Best, D. S., Nichols, H., Strader, L. F., Narotsky, M. G., Wolf, D. C., et al. (2007) Disruption of testosterone homeostasis as a mode of action for the reproductive toxicity of triazole fungicides in the male rat. Toxicol Sci 95:227–239.
- Goetz, A. K. and Dix, D. J. (2009) Toxicogenomic effects common to triazole antifungals and conserved between rats and humans. Toxicol Appl Pharmacol 238:80–89.
- Gray, L. E., Kelce, W. R., Wiese, T., Tyl, R., Gaido, K., Cook, J., Klinefelter, G., Desaulniers, D., Wilson, E., Zacharewski, T., et al. (1997) Endocrine screening methods workshop: Detection of estrogenic and androgenic hormonal and antihormonal activity for chemicals that act via receptor or steroidogenic enzyme mechanisms. Reprod Toxicol 11:719–750.
- Greenwood, F. C., Hunter, W. M. and Glover, J. S. (1963) The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochem J 89:114–123.
- Hanger, D. P., Jevons, S. and Shaw, J. T. B. (1988) Fluconazole and testosterone: In vivo and in vitro studies. Antimicrob Agents Chemother 32:646–648.
- Haavisto, A. M., Pettersson, K., Bergendahl, M., Perheentupa, A., Roser, J. F. and Huhtaniemi, I. (1993) A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 132:1687–1691.
- Hecker, M. and Giesy, J. P. (2008) Novel trends in endocrine disruptor testing: The H295R Steroidogenesis Assay for identification of inducers and inhibitors of hormone production. Anal Bioannal Chem 390:287–291.
- Hilscherova, K., Jones, P. D., Gracia, T., Newsted, J. L., Zhang, X., Sanderson, J. T., Yu, R. M. K., Wu, R. S. S. and Giesy, J. P. (2004) Assessment of the effects of chemicals on the expression of ten steroidogenic genes in the H295R cell line using real-time PCR. Toxicol Sci 81:78–89.
- Inchem IPCS, FAO/WHO (1985) Joint Meeting on Pesticide Residues: 733. Triadimefon, Pesticide residues in food evaluations Part II Toxicology.
- Inchem IPCS, FAO/WHO (1990) Joint Meeting on Pesticide Residues: 810. Hexaconazole, Pesticide Residues in Food Evaluations in Toxicology.
- Inchem IPCS, FAO/WHO (1995) Joint Meeting on Pesticide Residues: 896. Flusilazole, Pesticide Residues in Food Evaluations Part II Toxicological & Environmental.
- Lloyd, S. C. (1991) Effects of hexaconazole (ICIA523) on the steroidogenic function of isolated rat and human Leydig cells. ICI Central Toxicology Laboratory, Alderley Park, Macclesfield, Cheshire, UK.
- Martin, M. T., Brennan, R., Hu, W., Ayanoglu, E., Lau, C., Ren, H., Wood, C. R., Corton, J. C., Kavlock, R. J. and Dix, D. J. (2007) Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci 97:595–613.
- Okubo, T., Yokoyama, Y., Kano, K., Soya, Y and Kano, I. (2004) Estimation of the estrogenic and antiestrogenic activities of selected pesticides by MCF-7 cell proliferation assay. Arch Environ Contam Toxicol 46:445–453.
- Reeves, R., Thiruchelvam, M. and Cory-Slechta, D. A. (2004a) Development of behavioral sensitization to the cocaine-like fungicide triadimefon is prevented by AMPA, NMDA, DA D1 but not DA D2 receptor antagonists. Toxicol Sci 79:123–136.
- Reeves, R., Thiruchelvam, M. and Cory-Slechta, D. A. (2004b) Expression of behavioral sensitization to the cocaine-like fungicide triadimefon is blocked by pretreatment with AMPA, NMDA and DA D1 receptor antagonists. Brain Research 1008:155–167.
- Roberts, T. R. and Hutson, D. (1999) Metabolic pathways of agrochemicals. Part 2: Insecticides and fungicides. Cambridge, GB: The Royal Society of Chemistry, pp. 1011–1104.
- Taxvig, C., Hass, U., Axelstad, M., Dalgaard, M., Boberg, J., Andersen, H. R. and Vinggaard, A. M. (2007) Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol Sci 100:464–473.
- Taxvig, C., Vinggaard, A. M., Hass, U., Axelstad, M., Metzdorff, S. and Nellemann, C. (2008) Endocrine-disrupting properties in vivo of widely used azole fungicides. Int J Androl 31:170–177.
- Trösken, E. R., Schloz, K., Lutz, R. W., Volkel, W., Zarn, J. A. and Lutz, W. K. (2004) Comparative assessments of the inhibition of recombinant human CYP19 (aromatase) by Azoles used in agriculture and as drugs for humans. Endocrine Research 30:387–394.
- Tully, D. B., Bao, W., Goetz, A. K., Blystone, C. R., Ren, H., Schmid, J. E., Strader, L. F., Wood, C. R., Best, D. R., Narotsky, M. G., et al. (2006) Gene expression profiling in liver and testis of rats to characterize the toxicity of triazole fungicides. Toxicol Appl Pharmacol 215:260–273.
- U.S. EPA (2009) The U.S. Environmental Protection Agency's Strategic Plan for Evaluating the Toxicity of Chemicals. EPA 100/K-09/001, March 2009. Office of the Science Advisor, Science Policy Council. Washington, DC 20460: U.S. Environmental Protection Agency.
- Vinggaard, A. M., Hnida, C., Breinholt, V and Larson, J. C. (2000) Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol in vitro 14:277–234.
- Waeber, C., Reymond, O., Reymond, M. and Lemarchand-Beraud, T. (1983) Effects of hyper- and hypoprolactinemia on gonadtrophin secretion, rat testicular luteinizing hormone/human chorionic gonadotropin receptors and testosterone production by isolated leydig cells. Biol Reprod 28:167–177.
- Walker, Q. D. and Mailman, R. B. (1996) Triadimefon and triadimenol: Effects on monoamine uptake and release. Toxicol Appl Pharmacol 139:227–233.
- Wouters, W., De Coster, R., van Dun, J., Krekels, M. D. W. G., Dillen, A., Raeymaekers, A., Freyne, E., Van Gelder, J., Sanz, G., Venet, M. and Janssen, M. (1990) Comparative effects of the aromatase inhibitor R76713 and of its enantiomers R83839 and R83842 on steroid biosynthesis in vitro and in vivo. J Steroid Biochem Mol Biol 37:1049–1054.
- Zarn, J. A., Bruschweiler, B. J. and Schlatter, J. R. (2003) Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14alpha-demthylase and aromatase. Environ Health Perspect 111:255–261.