Abstract
Polycyclic aromatic hydrocarbons (PAHs) are released into the environment as a result of incomplete fossil fuel combustion from industrial furnaces, wood-burning stoves, and automobile exhaust fumes; however, the primary source of human exposure to these compounds is cigarette smoke. Embryonic and fetal loss after treatment with high doses of PAHs have been well documented in animal studies; however, few studies have addressed the reproductive consequences of long-term, low-level exposure to these chemicals. We previously reported that low doses of PAHs administered to ICR mice over a period of 9 weeks prior to conception resulted in early embryonic resorptions, whereby treated dams lost approximately 50% of their litter. During the course of these studies, we observed greater numbers of infiltrating uterine natural killer (uNK) cells into the placenta of PAH-exposed conceptuses. While exposure to high levels of PAHs has been shown to be immunosuppressive, increasing evidence suggests that chronic, low-dose exposure to PAHs may stimulate immune cells. Thus, we hypothesized that low-dose, chronic PAH exposure in our mouse model is mediating embryonic resorption by hyperstimulating maternal immune cells. In this review of the literature, we outline the rationale of our argument and present preliminary data, focussing upon PAH-mediated alterations in uNK cell dynamics and how these changes may be linked to early embryonic resorptions.
| Abbreviations | ||
| AhR: | = | aryl hydrocarbon receptor |
| BaP: | = | benzo(a)pyrene |
| DMBA: | = | 7,12-dimethylbenz(a)anthracene |
| NKT: | = | natural killer T cell |
| PAH: | = | polycylic aromatic hydrocarbon |
| TH1: | = | cell-mediated immunity |
| TH2: | = | humoral immunity |
| TH17 cell: | = | pro-inflammatory Tcell producing IL-17 |
| Treg: | = | regulatory Tcell |
| uDC: | = | uterine dendritic cell |
| uNK: | = | uterine natural killer cell |
INTRODUCTION
Cigarette smoking has been associated with a number of reproductive hazards including increased risk of spontaneous abortion during natural or assisted conception [Kline et al. Citation1977; Neal et al. Citation2005; Ness et al. Citation1999], and delayed conception or infertility [Alderete et al. Citation1995; Howe et al. Citation1985; Joesoef et al. Citation1993]. In addition, such negative effects have been documented for both active and passive (i.e. second-hand) smoking, using both human epidemiological and animal model studies (reviewed in [Cnattingius Citation2004; Higgins Citation2002; Sharara et al. Citation1998; Witschi et al. Citation1997]). While early pregnancy loss has previously been linked to excessive cell death [Baek Citation2004; Hardy et al. Citation2001] and some mechanisms surrounding female subfertility and exposure to tobacco smoke as it relates to ovarian function have been described [Shiverick and Salafia Citation1999; Zenzes et al. Citation1995], the physiological and molecular mechanisms involved in toxicant-mediated embryo demise are unclear.
Cigarettes are known to contain over 4,000 different chemicals, including a group of compounds known as polycyclic aromatic hydrocarbons (PAHs), which are considerably detrimental to human health [Hoffmann and Hoffmann Citation1997]. While PAHs are also known environmental pollutants generated by fossil fuel combustion, car exhaust, and forest fires [Grimmer et al. Citation1983], or through the consumption of smoked and grilled foods [Howard and Fazio Citation1980], the major source of human exposure is through the use of tobacco products [Becher and Bjorseth Citation1983]. For the purposes of this review, focus will be placed upon the putative role of PAHs in mediating immune-mediated abortion.
SMOKING AND SPONTANEOUS ABORTION
Smoking before and during pregnancy has dramatically declined in the past few decades within most of North America and Europe [Cnattingius Citation2004]; however, certain groups of women with high levels of tobacco product consumption during their reproductive years still exist. Such groups include women from varying ethnic backgrounds, of low socioeconomic status, and those having lower levels of education [Fingerhut et al. Citation1990; Heaman and Chalmers Citation2005; Kahn et al. Citation2002]. Moreover, a recent study from the United States reports that while the rate of smoking during pregnancy declined from 37% in 1989 to 12.2% in 2000, the highest rates of gestational smoking were observed in older teenagers and women in their early 20s [Ventura et al. Citation2003].
Exposure to tobacco smoke has consistently been associated with female sub-fertility in human populations [Cnattingius Citation2004; Higgins Citation2002]. In addition, laboratory animals exposed to cigarette smoke have also exhibited reduced fecundity, as evidenced by reduced litter size and increased resorptions [Izzotti et al. Citation2003; Khan et al. Citation2008], poor embryo quality [Huang et al. Citation2008], low fetal weight [Esposito et al. Citation2008]), and fetal demise [Farkas et al. Citation2006]. Early evidence connecting smoking during pregnancy with the risk of spontaneous miscarriage was controversial, with some groups reporting a positive association [Himmelberger et al. Citation1978; Kline et al. Citation1977] and others reporting no association [Harlap and Shiono Citation1980]. However, later epidemiological [Chatenoud et al. Citation1998; George et al. Citation2006] and meta-analyses [Augood et al. Citation1998] provided additional data supporting the concept that maternal smoking increased the risk of spontaneous abortion. Moreover, exposure to second-hand smoke during pregnancy [Venners et al. Citation2004] or childhood [Meeker et al. Citation2007] has also been implicated in recurrent pregnancy loss. Finally, more recent data obtained from fertility clinics indicate a positive association between maternal exposure to tobacco smoke and the rate of spontaneous abortions [Lintsen et al. Citation2005; Waylen et al. Citation2009].
Approximately one-third of post-implantation pregnancies are lost [Wilcox et al. Citation1988; Zinaman et al. Citation1996]; however, the mechanisms of spontaneous abortion are not well understood. Impaired uterine receptivity has been shown to play a role in pregnancy loss [Donaghay and Lessey Citation2007] and maternal exposure to cigarette smoke is thought to be involved in perturbing the uterine environment (for reviews, see [Shiverick and Salafia Citation1999; Soares and Melo Citation2008]). While a high incidence of cytogenetic abnormalities is found in early aborted specimens in the overall population [Warren and Silver Citation2008], the products of abortion in women who smoke cigarettes are often karyotypically normal [Kline et al. Citation1995]. A pivotal, clinical study recently reported that maternal use of tobacco products was associated with reduced uterine receptivity in females implanted with embryos obtained through oocyte donation and subsequent in vitro fertilization (IVF) [Soares et al. Citation2007]. This was corroborated by a separate group, that observed that exposure to both mainstream and sidestream smoke reduced implantation and pregnancy rates in IVF patients’ morphological normal embryos [Neal et al. Citation2005].
To ensure optimal embryo implantation, the uterine lining must be well developed and the uterine musculature must remain quiescent. In vitro studies reveal that endometrial cells held in cigarette-smoke-conditioned medium exhibit decreased proliferative capacity [Khorram et al. Citation2008] and reduced secretion of trophoblast chemoattractants [Thirkill et al. Citation2006]. In addition, smoking-related sub-fertility may be linked to poor endometrial vascularity, altered endothelial cell expression patterns [Soghomonians et al. Citation2004], and reduced blood flow [Raine-Fenning et al. Citation2004]. These diminished uterine conditions, coupled with the fact that cigarette smoke enhances uterine muscle contractility [Egawa et al. Citation2003; Nakamoto et al. Citation2006] may well be responsible for the recurrent pregnancy loss in smoking mothers.
POLYCYCLIC AROMATIC HYDROCARBONS
Polycyclic aromatic hydrocarbons have long been known to exert toxic effects on a variety of organs, including those of the reproductive system (reviewed in [Pocar et al. Citation2005]). The most serious outcomes of tested PAHs include impaired pre-implantation embryo development, early embryo mortality, stillbirths, intrauterine growth retardation, and reduced neonatal survival [Cnattingius Citation2004; Fischer Citation2000; Sram Citation1999]. Benzo(a)pyrene (BaP) and dimethylbenz(a)anthracence (DMBA) are two well-known PAHs which have carcinogenic properties and whose toxic effects include the formation of DNA and protein adducts, in addition to triggering the expression of xenobiotic-metabolizing enzymes through binding to the aryl hydrocarbon receptor (AhR). AhR is an intracellular receptor that plays a major role as a xenobiotic sensor. It is a member of the basic helix-loop-helix family of transcription factors and has a broad range of tissue expression [Frericks et al. Citation2007].
Animal studies using various types and sources of PAHs have also shown that exposure to these toxins can lead to unfavorable reproductive outcomes. It was recently shown, in rats, that mid-gestation inhalation of aerosolized BaP resulted in decreased fetal survival in a dose-dependent manner [Archibong et al. Citation2002]; similar results have also been reported in pregnant rats [Bui et al. Citation1986] and mice [Mackenzie and Angevine Citation1981] exposed to BaP. Resorption of the entire litter was observed in pregnant mice orally exposed to carbon black oil, which is a petroleum refinery by-product, containing several classes of hydrocarbons, including PAHs [Hansen et al. Citation2000]. Additionally, early studies in mice demonstrated the transplacental capacity of PAHs [Bulay and Wattenberg Citation1971] and human studies have shown significant accumulation of PAHs in the placenta [Gladen et al. Citation2000], umbilical cord endothelium [Hansen et al. Citation1992], and neonatal white blood cells [Whyatt et al. Citation2001] from babies born to mothers who smoke.
ENVIRONMENTAL PAHS AND PREGNANCY
Both epidemiological and clinical studies of human populations have reported numerous deleterious reproductive outcomes due to compounds such as BaP, dioxins, polychlorinated biphenyls (PCBs), and particulate matter that is found in air, soil, and water (reviewed in [Mlynarcikova et al. Citation2005; Sharara et al. Citation1998; Sram Citation1999]). Exposure to environmental pollution during pregnancy is associated with many adverse outcomes, including intrauterine fetal growth restriction, preterm delivery, and increased perinatal mortality [Cnattingius Citation2004; Sharara et al. Citation1998; Sram Citation1999]. A key study was conducted in the Czech Republic which demonstrated the association between environmental pollution and neonatal mortality [Bobak and Leon Citation1992]. This was followed by several studies in the USA and China where regions considered to have high levels of pollution were linked to low birth weight, sudden infant death syndrome, and neonatal mortality [Wang et al. Citation1997; Woodruff et al. Citation1997].
In addition to chronic exposure to pollutants, disasters such as the terrorist attacks and subsequent collapse of the buildings of the World Trade Centre (WTC) in New York on September 11th, 2001, can result in acute exposure to compounds such as PAHs, PCBs, and heavy metals [Lioy et al. Citation2002; Offenberg et al. Citation2003]. Studies of mothers living in close proximity to the WTC at the time of the attacks revealed an increased incidence of DNA adduct formation in maternal and fetal white blood cells [Perera et al. Citation2005a] that was associated with BaP exposure [Perera et al. Citation2005b]. Lastly, a similar study revealed that neonates born to mothers exposed to the various pollutants in the aftermath of the WTC attacks, exhibited reduced birth weight, length, and head circumference [Lederman et al. Citation2004]. In light of such profound effects on female reproduction and neonatal outcome, continued investigations and elucidation of ideal models to study the impact of environmental pollution on fetal and maternal health is warranted.
CIGARETTE SMOKE, PAHS, AHR, AND THE IMMUNE SYSTEM
Exposure to either mainstream or side-stream tobacco smoke has been well-known to have immunomodulatory effects. Moreover, cigarette smokers have been shown to exhibit states of immunosuppression and immunostimulation even within the same individual [Klareskog et al. Citation2007]. While smokers are notoriously susceptible to infections and exhibit diminished cell-mediated immunity [Sopori Citation2002] they also present with higher levels of inflammatory diseases [Kitamura and Kasai Citation2007], autoantibody production, leukoctyosis, and increased risk of rheumatoid arthritis, systemic lupus erythematosus and Graves’ disease [Costenbader and Karlson Citation2006; Klareskog et al. Citation2007; Vestergaard et al. Citation2002]. Maternal smoking during pregnancy is associated with higher cord blood IgE (which does not cross the placenta) [Magnusson Citation1986], stronger neonatal lymphoproliferation [Noakes et al. Citation2003], and increased rates of asthma and allergy in children [Gilliland et al. Citation2001; Magnusson Citation1986]. In mice, cigarette smoke stimulated oligoclonal expansion of T lymphocytes, leading to a persistent, adaptive T cell immune response [Motz et al. Citation2008] and in utero exposure resulted in elevated levels of circulating white blood cells in the offspring [Ng and Zelikoff Citation2008].
Numerous reports indicate that while high levels of PAHs are immunosuppressive by triggering apoptosis in various types of immune cells [Booker and White Citation2005; Dean et al. Citation1986], low-level exposure to PAHs can stimulate the immune system [Burchiel and Luster Citation2001; Molina and Shoenfeld Citation2005], resulting in pathologies such as asthma and autoimmune disorders. It has become increasingly apparent that both the magnitude and duration (i.e. acute or chronic) of PAH exposure are important factors in natural and experimental systems, leading to vastly different immunological outcomes. For example, coke oven workers are chronically exposed to PAHs and a recent study demonstrated that these people exhibit reduced T-cell proliferative capacity but enhanced natural killer (NK) cell activity [Karakaya et al. Citation2004]. In laboratory animals, low doses of PAHs in pregnant dams elicited elevated hypersensitivity, antibody, and mitogenic responses in offspring, whereas high doses had either no effect or reduced immunological response [Das et al. Citation1990]. Another group showed that low-dose BaP exposure in neonatal rats favoured cell-mediated immunity (TH1) whereas high doses of BaP shifted the balance towards humoral immunity (TH2) [Matiasovic et al. Citation2008]. To complicate matters, the same chemical can result in either immune suppression or exacerbation/induction of autoimmune disorders depending on the biological age and genetic background of the subject (reviewed in [Holladay and Smialowicz Citation2000; Yusuf et al. Citation2007]). Lastly, PAHs themselves can cause allergic contact hypersensitivity, upregulating TH1 immunity, and reducing TH2 immunity in the affected areas, and, therefore possibly contribute to diseases such as dermatitis, chronic obstructive pulmonary disease, and food and nasal allergies [Kadkhoda et al. Citation2004; Rushton Citation2007; Yusuf et al. Citation2007]
It is hypothesized that some of the immunological outcomes observed after PAH exposure may be mediated through the AhR, as a number of immune cell types are capable of AhR activation [Germolec et al. Citation1996]. Moreover, AhR-deficient mice exhibit immune pathologies including reduced lymphocyte numbers in the spleen and lymph nodes, and enhanced inflammatory responses, particularly in the gut, lung, and urinary tract [Fernandez-Salguero et al. Citation1995, Citation1997; Schmidt et al. Citation1996]. Interestingly, trans-genic mice expressing a constitutively active form of AhR in T- and B-lymphocytes exhibited diminished populations of these cell [Andersson et al. Citation2003; Nohara et al. Citation2005] while expression in keratinocytes resulted in inflammatory skin lesions [Tauchi et al. Citation2005]. Additionally, it was shown that regulatory T cell (Treg) and pro-inflammatory T cell (producing IL-17, hence TH17) differentiation—the dysregulation of which is thought to impair self-tolerance [Stevens and Bradfield Citation2008]—is controlled by AhR [Quintana et al. Citation2008]. This is an important factor to consider given that Treg cells appear crucial for successful pregnancy [von Rango Citation2008; Zenclussen Citation2005]. Furthermore, a role for AhR in environmental-toxin-induced autoimmunity was recently reported where experimental autoimmune encephalomyelitis was exacerbated in AhR wildtype, but not AhR-deficient mice and this was shown to be mediated by TH17 cells. Finally, the development of AhR-regulated, TH17-cell-mediated autoimmunity was linked to environmental toxins [Veldhoen et al. Citation2008] and optimal culture conditions for TH17 cell differentiation requires natural AhR agonists in the culture medium [Veldhoen et al. Citation2009]. Thus, it appears that PAH ligation to AhR can exert diverse immunological outcomes depending on tissue and cell type.
Signalling events downstream of AhR-ligand binding in immune cells can lead to a number of different physiological outcomes. In the context of this review, the possible mechanisms by which AhR can alter the proliferative and cytolytic capacities of immune cells are of particular interest. Nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) is a key transcription factor involved in regulating the immune response. Interactions between AhR and NF-κB, resulting in either enhancement or suppression of NF-κB activity, have been described in murine hepatoma [Tian et al. Citation1999] and human breast cells [Kim et al. Citation2000]. While the two transcription factors are known to physically associate, whether AhR has a positive or negative effect on NF-κB activity appears to be contextually specific and thus, must be determined for different cell types within particular environments. Also important in immune cell signaling, extracellular signal-related kinases are activated by AhR ligands in macrophages, via AhR-dependent and -independent mechanisms [Cheon et al. Citation2007; Lecureur et al. Citation2005]. Interestingly, both NF-κB and ERK activation have been shown to be responsible for upregulating human NK cell proliferation and cytotoxicity [Liang et al. Citation2005] and the triggering of these two pathways may be responsible for the embryonic resorption phenotype observed in BaP-exposed dams. A larger population of uNK cells having dysregulated cytotoxic capabilities would pose a likely threat to fetal trophoblast cells attempting to invade the maternal tissues and establish the placental bed.
THE PLACENTA
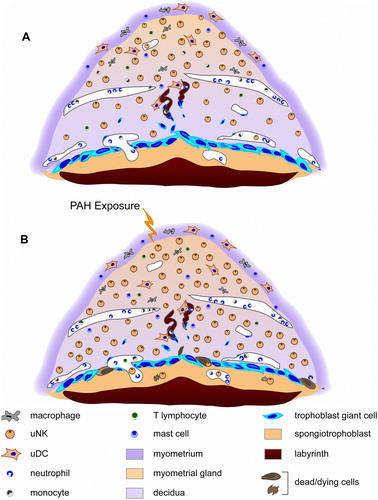
The placenta is a life-sustaining organ mediating the physiological exchange of oxygen, nutrients, and waste between mother and fetus. Recent comparative studies between mouse and human placenta [Carter Citation2006; Georgiades et al. Citation2002] have revealed striking similarities in cellular mechanisms and tissue framework, providing justification for using the mouse placenta as an animal model of human placentation. During murine implantation, the embryo attaches to the uterine wall and induces the decidual reaction, whereby the endometrial cells proliferate and differentiate into morphologically and functionally distinct decidual cells, thus forming the placental decidua. The murine embryo at implantation (day 4.5 post coitum, d4.5) has a simple trophectoderm surrounding the blastocyst, which will eventually give rise to all trophoblast cells in the placenta (reviewed in [Cross Citation2005]). This cell type defines the boundaries between the mother and fetus. is a schematic representation of the early mouse placenta at d9.5, the time at which the major compartments of the placenta are established. Trophoblast giant cells comprise the border at the maternal-fetal interface and have a role in invasion, vascular remodeling, and hormone production [(Cross Citation2005]. The spongiotrophoblast region provides structure and support to the labyrinth and consists of variable-sized, hormone-producing cells that appear to have limited proliferative capabilities [Cross et al. Citation2002]. Large, maternal arterial canals pass through the spongiotrophoblast and branch out into the labyrinth, which consists of a dense meshwork of fetal capillaries and trophoblast-lined maternal blood spaces, between which lies two layers of syncytiotro-phoblast cells. The labyrinth is the site of maternal-fetal exchange and facilitates the transfer of maternally-derived nutrients and oxygen to pass to the fetus.
Figure 1 Schematic representation of d9.5 mouse placenta with associated maternal immune cell types. (A) Normal d9.5 mouse placenta. Chronic, low-level exposure to PAHs prior to conception (B) is hypothesized to stimulate some of these immune cell populations, thus effecting embryonic resorptions in outbred ICR mice.
IMMUNOLOGY OF PREGNANCY
During a successful pregnancy, the mother must become transiently tolerant to semi-allogeneic, fetal antigens. In fact, in special cases such as ovum donation or surrogate pregnancy, completely allogenic fetal tissues must be accepted by the maternal immune system. During the process of hemochorial placenta-tion (e.g., in humans and rodents), fetal trophoblast cells are found in close proximity to maternal immune cells, lining the maternal spiral arteries and blood spaces, putting these cells in direct contact with maternal immune cells. Thus, the immune system of the mother must be tightly regulated in order to establish and maintain a healthy pregnancy.
Pregnancy is considered to be an inflammatory state [Sargent et al. Citation2006] as evidenced by activated endothelium and leukocytes and also by alterations in the maternal plasma protein profile [Redman and Sargent Citation2003]. Additionally, the hormonal milieu during pregnancy exerts a bias against systemic cell-mediated immunity (TH1) and a skewing towards the TH2 response. This TH2 shift was coined the Wegmann hypothesis [Wegmann et al. Citation1993] and such a phenomenon has been reported by several groups [Lin et al. Citation1993; Piccinni and Romagnani Citation1996; Sacks et al. Citation2001]. Moreover, a shift towards TH1 during pregnancy has been associated with spontaneous abortion and intrauterine growth restriction [Hill et al. Citation1995; Marzi et al. Citation1996], further supporting the idea that an optimal balance between TH1 and TH2 cytokines are crucial for successful pregnancy. Not to be outdone by the adaptive immune system, the innate compartment is steadily gaining interest as an important element of gestational health. Recent reports have shown that innate immune factors such as complement, monocytes and granulocytes become systemically active during pregnancy [Luppi et al. Citation2002; Osorio et al. Citation2008; Richani et al. Citation2005]. There is some evidence that such activation—along with the presence of γδ T cells and uNK cells [Hunt et al. Citation1998]—may be an attempt to protect mother and fetus against infection; however, additional studies are required to fully elucidate the function of innate immunity during gestation.
As reiterated, upon implantation, the cells lining the uterus transform into a highly specialized tissue known as the decidua. The maternal decidua and the myometrial gland of the placenta contain a number of different immune cells (A). A predominant characteristic of the decidua is the influx of a distinctive population of maternal lymphocytes known as uterine natural killer (uNK) cells. However, there are a number of other immune cell types within the decidua, including macrophages, Treg cells, uterine dendritic cells (uDC), CD8+ T lymphocytes (both αβ and γδ T cells, with a greater proportion of γδ cells), and a subset of lymphocytes, natural killer T (NKT) cells [Dang et al. Citation2000; Hunt Citation2006; Seavey and Mosmann Citation2008]. Neutrophils and monocytes circulate throughout the maternal placental vasculature and have been shown to infiltrate areas of infection, damage, or resorption [Girardi Citation2008; Girardi et al. Citation2003; Rogerson et al. Citation2003]. Lastly, mast cells have been reported to exist within the murine myometrium during normal pregnancy [Wordinger et al. Citation1986] and in greater numbers in the decidua during abortion [Widayati et al. Citation2004]. In humans, increased numbers of mast cells in the uterus are associated with failed pregnancies [Arck et al. Citation2001; Marx et al. Citation1999].
UTERINE NATURAL KILLER CELLS
During the first half of pregnancy, uNKs comprise the majority of immune cells in both human and murine decidua (for review see [Croy et al. Citation2002; Moffett and Loke Citation2006]). In the mouse, before blastocyst implantation at approximately d4.5, uNK cells are small and agranular [Parr et al. Citation1991; Peel Citation1989]; however, after implantation promotes uterine decidualization the uNK cells begin to proliferate rapidly [Peel Citation1989]. Moreover, these cells begin to gain unique characteristics, acquiring cytoplasmic granules containing perforin, serine proteases, phosphatises, and other molecules [Croy and Kiso Citation1993; Croy et al. Citation1997]. In addition, uNK cytoplasm starts to expand and the cells begin to secrete IFN-γ [Croy et al. Citation2003a], TNF-α [Wu et al. Citation2006], VEGF, and placenta growth factor [Hanna et al. Citation2006]. Peak numbers of uNK cells are attained by d10.5 during normal murine pregnancy and these numbers start to decline at approximately d12.5 [Delgado et al. Citation1996; Kusakabe et al. Citation1999]. During mid-to-late gestation in the mouse, there are fewer uNK cells in the decidua basalis and are predominantly localized to the mesometrial gland [Peel Citation1989].
Uterine natural killer cells have been proposed to perform a number of different functions during pregnancy. It is firmly established that these cells play a crucial role in maternal spiral artery modification during both human [Hanna et al. Citation2006; Parham Citation2004] and murine placentation [Croy et al. Citation2003b]. Mice genetically ablated for NK and uNK lineages do not exhibit normal spiral artery transformation, have smaller placenta, and low-birth weight offspring [Ashkar et al. Citation2000; Guimond et al. Citation1998]. In humans, poor physiological transformation of the maternal placental vasculature is associated with preeclampsia and uNK cells have been implicated in the pathogenesis of this disease [Zhang and Tian Citation2007]. In addition to spiral artery modification, uNK cells are also believed to be involved in protection against infection [Pejcic-Karapetrovic et al. Citation2007], endometrial angiogenesis [Quenby et al. Citation2009], decidual differentiation [Guimond et al. Citation1998], and trophoblast chemoattraction, invasion [Hanna et al. Citation2006], and recognition [Sharkey et al. Citation2008]. Unfortunately, while the physiological roles of uNK cells are somewhat clear, the precise pathways of activation and the mechanisms by which cytolysis is inhibited, are unclear.
A healthy pregnancy requires the presence of cytokine-producing, cytolytically-quiescent uNK cells. In fact, uNK cells have been shown to closely associate with trophoblast [King et al. Citation1996] and it is believed that human trophoblast are resistant to uNK-mediated lysis [King and Loke Citation1990]. Nonetheless, a number of different studies have linked this cell type to deleterious gestational outcomes in mice, including spontaneous abortion. Early work suggested that cell-mediated immune mechanisms were responsible for fetal loss as such cell types were evident at abortion sites in murine uteri after xenogeneic transplantation [Croy et al. Citation1982]. Moreover, examination of the resorption sites of an allogeneic, abortion-prone mouse model (CBA/J female × DBA/2J male) revealed excessive infiltration of uNK cells at implantation sites just before and after spontaneous abortion [Gendron and Baines Citation1988]. Depletion of NK cells (by injections of anti-asialo GM1 antibody) in this same mouse model resulted in a reduction of the number of resorption sites, while enhancement of NK activity (via poly I:C injections) increased the resorption rate [de Fougerolles and Baines Citation1987]. These combined data served to underscore the role of uNK cells in murine abortion and develop the theory that these cells are the causative effectors in CBA x DBA resorption [Chaouat Citation2003]. A recently published xenogeneic model showed that vole embryos transferred to the uterus of a CD1 mouse were excluded by d11 of gestation, with excessive infiltration of uNK cells at the implantation site and destruction of the trophoblastic barriers [Widayati et al. Citation2004]. Furthermore, while transfer of vole embryos to the uteri of SCID mice yielded longer survival rates, these xenogeneic transfers were still ultimately rejected while allogeneic embryos in SCID uteri survived, suggesting some NK involvement in fetal rejection [Widayati et al. Citation2004]. Lastly, inflammation-induced fetal loss in IL-10-deficient mice was reported to be mediated by uNK cells [Murphy et al. Citation2005], once again cementing the role of this cell type in murine spontaneous abortion.
In vitro and in vivo studies of normal and spontaneously aborted human placenta have corroborated much of the work in mouse models. High peripheral NK activity [Matsubayashi et al. Citation2005] and elevated IFN-γ secretion from peripheral blood mononuclear cells [Lee et al. Citation2005] have been reported to be associated with recurrent pregnancy loss. In addition, decidual lymphocytes collected from early miscarriages exhibited a significantly greater capacity to kill cultured trophoblast cell lines in comparison to decidual lymphocytes from age-matched, elective abortions [Olivares et al. Citation2002]. In humans, CD56brightCDl6− NK cells represent the vast majority of immune cells in the early pregnancy decidua [Moffett-King Citation2002; Nishikawa et al. Citation1991]. A recent study showed that accumulation of CD56bright, granulysin-positive uNK cells in the decidua basalis was linked to pregnancy loss in humans [Nakashima et al. Citation2008]. Interestingly, the numbers of CD56bright, perforin-positive or granzyme B-positive uNK cells did not differ between spontaneously aborted and normal control samples, suggesting that constituents within uNK granules may be a better prognosticator for recurrent pregnancy loss than the magnitude of uNK cell numbers at the implantation site or the level of NK activity in the peripheral blood. This is an important consideration since there is some contention in the literature as to depth of uNK cell involvement in recurrent pregnancy loss in humans and whether sero-therapeutics such as immunization and antibody injections are efficacious in preventing recurrent spontaneous abortion (for review see [Christiansen et al. Citation2005]). While it is not disputed that components of the immune system, such as leukocytes, cytokines and complement play key roles in pregnancy loss, the exact nature and mechanisms of these factors remain enigmatic.
CHRONIC EXPOSURE TO PAHS: A MOUSE MODEL
We previously reported a murine model [Detmar et al. Citation2006] designed to mimic the phenomenon observed in human populations where women will cease smoking upon attempting, or acquiring knowledge of, conception, typically due to fetal health concerns [Cnattingius et al. Citation1992; Fingerhut et al. Citation1990]. Using two different mouse strains chronically exposed to PAHs prior to conception, we were able to recapitulate a number of conditions observed in women smokers [Cnattingius Citation2004; Gruslin et al. Citation2001; Higgins Citation2002; Shiverick and Salafia Citation1999]. Exposure to PAHs using inbred, C57Bl/6 mice included alterations in placental vasculature, IUGR and altered cell death patterns observed in C57Bl/6 mice [Detmar et al. Citation2008], whereas outbred ICR mice exhibited increased rates of spontaneous abortion [Detmar et al. Citation2006]. In addition to providing information regarding tobacco use during pregnancy, we pose that this would be a valuable experimental model in which to assess the effects of gestational exposure to environmental pollutants, many of which trigger the AhR pathway (reviewed in [Pocar et al. Citation2005]) and lead to deleterious gestational outcomes [Cnattingius Citation2004; Sharara et al. Citation1998; Sram Citation1999] similar to that seen in smoking mothers.
Polycyclic aromatic hydrocarbons such as BaP and DMBA are known potent immune suppressors in both humans and mice (reviewed in [Burchiel and Luster Citation2001]). High doses (50–150 mg/kg) given orally or subcutaneously, can suppress both humoral and cell-mediated immunity [Booker and White Citation2005; Dean et al. Citation1986; Ward et al. Citation1984], in some cases, even after the PAH has been cleared from the animal [Ward et al. Citation1986]. On the contrary, low doses of PAHs have been shown to enhance the immune response, leading to increased IgE and cytokine production, and activating macrophages [Burchiel and Luster Citation2001]. In our particular model, the mice were treated with a final, cumulative dose of 12 mg/kg of total PAHs (i.e. 6 mg/kg BaP + 6 mg/kg DMBA) over nine weeks, which could be considered a low dose (compare with a cumulative dose of 50–150 mg/kg in [Burchiel and Luster Citation2001] and daily doses of 40–80 mg/kg for one month in [Booker and White Citation2005]). Therefore, given that maternal immune cells have been implicated in spontaneous abortion and that low-dose PAH exposure can activate the immune system, it is our hypothesis that PAHs lead to maternal immune cell stimulation, which subsequently attack and destroy placental cells, thus mediating embryonic loss (A and B).
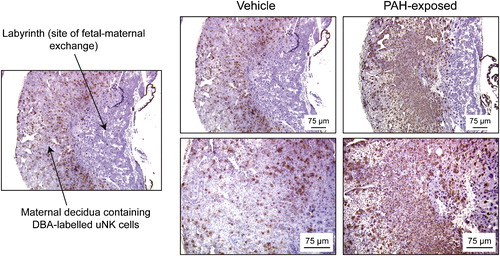
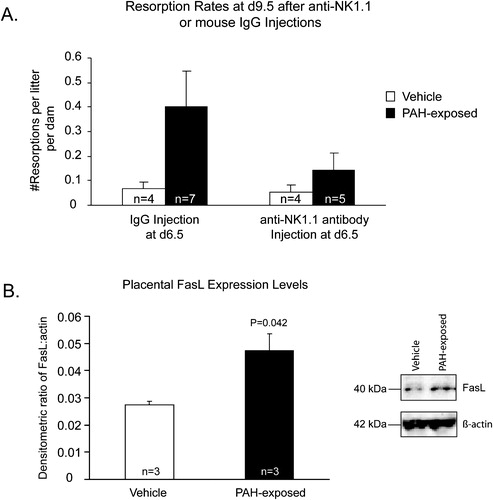
In light of the potential immunotoxic or immune-enhancing effects on the resident uNK population, we applied lectin histochemistry to assess the status of uNK infiltration in early-gestation placenta. From these experiments, we observed increased numbers of uNK cells in d9.5 PAH-treated decidua, compared with vehicle-treated decidua (). Thus, there did not appear to be a suppression of this particular cell type in the placenta at this time point, but an enhancement. Moreover, depletion of NK1.1+ cells by anti-NK1.1 antibody injections yielded reduced resorption rates in PAH-exposed dams (A). While this reduction has not yet reached statistical significance (P = 0.062), we anticipate that it will occur by increasing the sample size (currently underway). Nonetheless, these are encouraging data and serve to provide a basis for continued investigations into putative PAH-induced, uNK-mediated embryonic resorption. Since PAHs can affect numerous immune cell types, some of which are found in both human and murine placenta (e.g. macrophages, dendritic cells, neutrophils, T lymphocytes), analyses of the numbers and activity levels of these cell types still need to be determined. Assessment of classical cell death marker activation using immunohistochemistry, caspase-3 assays, and terminal deoxynucleotidyl transferase dUTP-mediated nick-end labelling (TUNEL) against the embryonic and placental tissues is warranted to determine whether the cell death cascade has been triggered in conceptuses from PAH-exposed dams.
FIGURE 2 Polycyclic aromatic hydrocarbon treatment in ICR dams alters levels of uNK cells in early placental decidua. Histomicrographs depict d9.5 placental sections from dams exposed to vehicle and PAHs, with indicated PAH-exposed placentae exhibiting upregulated populations of maternal uNK cells. Histochemistry employing biotinylated Dolichos biflorus agglutinin lectin was used as a marker of murine uNK cells, which are stained brown. Sections were counter-stained with hematoxylin. Photomicrographs in the lower panels on the right are higher magnifications of the upper panels.
Exploring the possible molecular mechanisms involved in PAH-induced, immune-mediated resorptions have been initiated and focus upon molecules associated with cell death pathways. The Fas (CD95, Apo-1)/FasL (Fas ligand; CD95L, Apo-1L) pathway is a major apoptotic pathway responsible for transducing external death cues, resulting in the demise of the cell. FasL binds to Fas receptor, triggering Fas oligomerization and forming a trimeric complex. Both maternal decidual [Qiu et al. Citation2005] and fetal trophoblast [Aschkenazi et al. Citation2002; Hunt et al. Citation1997] cells express FasL, which is thought to play an important role in maternal immune tolerance to the fetus. While detailed mechanisms have not yet been determined, it is believed that cells bearing this ligand will bind to Fas receptor on the surface of maternal immune cells, thus eliminating potentially cytotoxic threats (reviewed in [Bogovic Crncic et al. Citation2005; Jerzak and Bischof Citation2002]). An initial study exploring cell-death-associated proteins revealed that FasL expression is upregulated in d15.5, PAH-exposed placenta (B). In this model, embryonic resorptions occur between d7.5–d10.5 and viable embryos (approximately 50–60% of the litter) would ultimately survive the remainder of gestation, parturition, and achieve adulthood. Thus, increased expression of FasL in d15.5 placentae may reflect successful protection of fetal trophoblast from a PAH-induced, hyper-stimulated maternal immune system. However, further investigations into the molecular mechanisms involved in PAH-induced embryonic resorptions are required to fully establish whether maternal immune cell activation or loss of tolerance is responsible for the observed phenotype. Nonetheless, when the aforementioned data are encompassed as a whole, they provide encouraging support towards the hypothesis that chronic, low-dose exposure to PAHs may hyperstimulate maternal uNK cells and trigger embryonic resorption early in gestation.
SUMMARY
Maternal cigarette smoking has been associated with a number of deleterious gestational outcomes, including spontaneous abortion. In our animal model of chronic, low-dose PAH exposure prior to conception, ICR females exhibited significantly reduced litter sizes attributed to early embryonic resorption. Due to increased numbers of infiltrating uNK cells in PAH-treated placentae and the increased resorption rate, we hypothesized that this normally cytolytically quiescent cell type might become activated in the presence of PAHs, thus eliminating trophoblast and killing the embryo. Preliminary data suggest that uNK cells may be involved in PAH-mediated embryonic demise; however, continued investigation into this possibility is required and is currently ongoing. Nevertheless, the concept that low-dose, chronic PAH exposure may be responsible for immune-mediated pregnancy loss is an intriguing one. Since PAHs can be found not only in tobacco smoke, but also in environmental pollution, the results of such studies have the potential to impact a far greater number of people and may lead to tighter regulations regarding tobacco consumption and pollution control.
FIGURE 3 Polycyclic aromatic hydrocarbon treatment in ICR dams affects maternal uNK dynamics and upregulates placental FasL expression. Graphs depicting (A) resorption rates at d9.5 in vehicle- and PAH-exposed dams after treatment with monoclonal anti-NK1.1 or murine IgG (control) antibodies. Mice were injected with antibody at d4.5 and d7.5 and PAH-exposed, anti-NK1.1-treated dams are exhibiting a trend towards decreased resorption rates compared with PAH-exposed dams treated with IgG. (B) FasL expression, as assessed by Western blotting, is significantly (Student's t-test) upregulated in d15.5, PAH-exposed placental lysates. Bars represent average values ± SE, with white bars indicating vehicle-exposed and black bars indicating PAH-exposed mice/samples.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Alderete, E., Eskenazi, B. and Sholtm, R. (1995) Effect of cigarette smoking and coffee drinking on time to conception. Epidemiology 6:403–408.
- Andersson, P., Ridderstad, A., McGuire, J., Pettersson, S., Poellinger, L. and Hanberg, A. (2003) A constitutively active aryl hydrocarbon receptor causes loss of peritoneal B1 cells. Biochem Biophys Res Commun 302:336–341.
- Archibong, A. E., Inyang, F., Ramesh, A., Greenwood, M., Nayyar, T., Kopsombut, P., Hood, D. B. and Nyanda, A. M. (2002) Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed to inhalation to benzo(a)pyrene. Reproductive Toxicology 16:801–808.
- Arck, P. C., Rose, M., Hertwig, K., Hagen, E., Hildebrandt, M. and Klapp, B. F. (2001) Stress and immune mediators in miscarriage. Hum Reprod 16:1505–1511.
- Aschkenazi, S., Straszewski, S., Verwer, K. M., Foellmer, H., Rutherford, T. and Mor, G. (2002) Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol Reprod 66:1853–1861.
- Ashkar, A. A., Di Santo, J. P. and Croy, B. A. (2000) Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 192:259–270.
- Augood, C., Duckitt, K. and Templeton, A. A. (1998) Smoking and female infertility: A systematic review and meta-analysis. Hum Reprod 13:1532–1539.
- Baek, K. H. (2004) Aberrant gene expression associated with recurrent pregnancy loss. Mol Hum Reprod 10:291–297.
- Becher, G. and Bjorseth, A. (1983) Determination of exposure to poly-cyclic aromatic hydrocarbons by analysis of human urine. Cancer Lett 17:301–311.
- Bobak, M. and Leon, D. A. (1992) Air pollution and infant mortality in the Czech Republic, 1986–88. Lancet 340:1010–1014.
- Bogovic Crncic, T., Laskarin, G., Juretic, K., Strbo, N., Dupor, J., Srsen, S., Randic, L., Le Bouteiller, P., Tabiasco, J. and Rukavina, D. (2005) Perforin and Fas/FasL cytolytic pathways at the maternal-fetal interface. Am J Reprod Immunol 54:241–248.
- Booker, C. D. and White, K. L., Jr. (2005) Benzo(a)pyrene-induced anemia and splenomegaly in NZB/WF1 mice. Food Chem Toxicol 43:1423–1431.
- Bui, Q. Q., Tran, M. B. and West, W. L. (1986) A comparative study of the reproductive effects of methadone and benzo(a)pyrene in the pregnant and pseudopregnant rat. Toxicology 42:195–204.
- Bulay, O. M. and Wattenberg, L. W. (1971) Carcinogenic effects of polycyclic hydrocarbon carcinogen administration to mice during pregnancy on the progeny. J Natl Cancer Inst 46:397–402.
- Burchiel, S. W. and Luster, M. I. (2001) Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin Immunol 98:2–10.
- Carter, A. M. (2006) Animal models of human placentation—a review. Placenta 28(Suppl A):S41–S47.
- Chaouat, G. (2003) Innately moving away from the Th1/Th2 paradigm in pregnancy. Clin Exp Immunol 131:393–395.
- Chatenoud, L., Parazzini, F., di Cintio, E., Zanconato, G., Benzi, G., Bortolus, R. and La Vecchia, C. (1998) Paternal and maternal smoking habits before conception and during the first trimester: Relation to spontaneous abortion. Ann Epidemiol 8:520–526.
- Cheon, H., Woo, Y. S., Lee, J. Y., Kim, H. S., Kim, H. J., Cho, S., Won, N. H. and Sohn, J. (2007) Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced TNF-alpha production in differentiated THP-1 human macrophages. Exp Mol Med 39:524–534.
- Christiansen, O. B., Nybo Andersen, A. M., Bosch, E., Daya, S., Delves, P. J., Hviid, T. V., Kutteh, W. H., Laird, S. M., Li, T. C. and van der Ven, K. (2005) Evidence-based investigations and treatments of recurrent pregnancy loss. Fertil Steril 83:821–839.
- Cnattingius, S. (2004) The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res 6(Suppl 2):S125–140.
- Cnattingius, S., Lindmark, G. and Meirik, O. (1992) Who continues to smoke while pregnant? Journal of Epidemiology and Community Health 46:218–221.
- Costenbader, K. H. and Karlson, E. W. (2006) Cigarette smoking and autoimmune disease: What can we learn from epidemiology? Lupus 15:737–745.
- Cross, J. C. (2005) How to make a placenta: Mechanisms of trophoblast cell differentiation in mice–a review. Placenta 26(Suppl A):S3–9.
- Cross, J. C., Hemberger, M., Lu, Y., Nozaki, T., Whiteley, K., Masutani, M. and Adamson, S. L. (2002) Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol 187:207–212.
- Croy, B. A., Chantakru, S., Esadeg, S., Ashkar, A. A. and Wei, Q. (2002) Decidual natural killer cells: Key regulators of placental development (a review). Journal of Reproductive Immunology 57:151–168.
- Croy, B. A., He, H., Esadeg, S., Wei, Q., McCartney, D., Zhang, J., Borzychowski, A., Ashkar, A. A., Black, G. P., Evans, S. S., et al. (2003a) Uterine natural killer cells: Insights into their cellular and molecular biology from mouse modelling. Reproduction 126:149–160.
- Croy, B. A., Esadeg, S., Chantakru, S., van den Heuvel, M., Paffaro, V. A., He, H., Black, G. P., Ashkar, A. A., Kiso, Y. and Zhang, J. (2003b) Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. Journal of Reproductive Immunology 59:175–191.
- Croy, B. A. and Kiso, Y (1993) Granulated metrial gland cells: A natural killer cell subset of the pregnant murine uterus. Microsc Res Tech 25:189–200.
- Croy, B. A., McBey, B. A., Villeneuve, L. A., Kusakabe, K., Kiso, Y and van den Heuvel, M. (1997) Characterization of the cells that migrate from metrial glands of the pregnant mouse uterus during explant culture. J Reprod Immunol 32:241–263.
- Croy, B. A., Rossant, J. and Clark, D. A. (1982) Histological and immunological studies of post implantation death of Mus caroli embryos in the Mus musculus uterus. J Reprod Immunol 4:277–293.
- Dang, Y., Beckers, J., Wang, C. R. and Heyborne, K. D. (2000) Natural killer 1.1 ( + ) alpha beta T cells in the periimplantation uterus. Immunology 101:484–491.
- Das, S. N., Paul, B. N., Saxena, A. K. and Ray, P. K. (1990) Effect of in utero exposure to hexachlorocyclohexane on the developing immune system of mice. Immunopharmacol Immunotoxicol 12:293–310.
- de Fougerolles, A. R. and Baines, M. G. (1987) Modulation of the natural killer cell activity in pregnant mice alters the spontaneous abortion rate. J Reprod Immunol 11:147–153.
- Dean, J. H., Ward, E. C., Murray, M. J., Lauer, L. D., House, R. V., Stillman, W., Hamilton, T. A. and Adams, D. O. (1986) Immunosuppression following 7,12-dimethylbenz[a]anthracene exposure in B6C3F1 mice—II. Altered cell-mediated immunity and tumor resistance. Int J Immunopharmacol 8:189–198.
- Delgado, S. R., McBey, B. A., Yamashiro, S., Fujita, J., Kiso, Y. and Croy, B. A. (1996) Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukoc Biol 59:262–269.
- Detmar, J., Rabaglino, T., Taniuchi, Y., Oh, J., Acton, B. M., Benito, A., Nunez, G. and Jurisicova, A. (2006) Embryonic loss due to exposure to polycyclic aromatic hydrocarbons is mediated by Bax. Apoptosis 11:1413–1425.
- Detmar, J., Rennie, M. Y., Whiteley, K. J., Qu, D., Taniuchi, Y., Shang, X., Casper, R. F., Adamson, S. L., Sled, J. G. and Jurisicova, A. (2008) Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. American journal of physiology. Endocrinology and metabolism 295:E519–530.
- Donaghay, M. and Lessey, B. A. (2007) Uterine receptivity: Alterations associated with benign gynecological disease. Semin Reprod Med 25:461–475.
- Egawa, M., Yasuda, K., Nakajima, T., Okada, H., Yoshimura, T., Yuri, T., Yasuhara, M., Nakamoto, T., Nagata, F. and Kanzaki, H. (2003) Smoking enhances oxytocin-induced rhythmic myometrial contraction. Biol Reprod 68:2274–2280.
- Esposito, E. R., Horn, K. H., Greene, R. M. and Pisano, M. M. (2008) An animal model of cigarette smoke-induced in utero growth retardation. Toxicology 246:193–202.
- Farkas, S., Hussein, J., Ariano, R. E., Sitar, D. S. and Hasan, S. U. (2006) Prenatal cigarette smoke exposure: Pregnancy outcome and gesta-tional changes in plasma nicotine concentration, hematocrit, and carboxyhemoglobin in a newly standardized rat model. Toxicol Appl Pharmacol 214:118–125.
- Fernandez-Salguero, P., Pineau, T., Hilbert, D. M., McPhail, T., Lee, S. S., Kimura, S., Nebert, D. W., Rudikoff, S., Ward, J. M. and Gonzalez, F. J. (1995) Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726.
- Fernandez-Salguero, P. M., Ward, J. M., Sundberg, J. P. and Gonzalez, F. J. (1997) Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 34:605–614.
- Fingerhut, L. A., Kleinman, J. C. and Kendrick, J. S. (1990) Smoking before, during, and after pregnancy. American Journal of Public Health 80:541–544.
- Fischer, B. (2000) Receptor-mediated effects of chlorinated hydrocarbons. Andrologia 32:279–283.
- Frericks, M., Meissner, M. and Esser, C. (2007) Microarray analysis of the AHR system: Tissue-specific flexibility in signal and target genes. Toxicol Appl Pharmacol 220:320–332.
- Gendron, R. L. and Baines, M. G. (1988) Infiltrating decidual natural killer cells are associated with spontaneous abortion in mice. Cell Immunol 113:261–267.
- George, L., Granath, F., Johansson, A. L., Anneren, G. and Cnattingius, S. (2006) Environmental tobacco smoke and risk of spontaneous abortion. Epidemiology 17:500–505.
- Georgiades, P., Ferguson-Smith, A. C. and Burton, G. J. (2002) Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23:3–19.
- Germolec, D. R., Henry, E. C., Maronpot, R., Foley, J. F., Adams, N. H., Gasiewicz, T. A. and Luster, M. I. (1996) Induction of CYP1A1 and ALDH-3 in ymphoid tissues from Fisher 344 rats exposed to 2,3,7,8-tetrachlorodibenzodioxin (TCDD). Toxicol Appl Pharmacol 137:57–66.
- Gilliland, F. D., Li, Y F. and Peters, J. M. (2001) Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 163:429–436.
- Girardi, G. (2008) Guilty as charged: All available evidence implicates complement's role in fetal demise. Am J Reprod Immunol 59:183–192.
- Girardi, G., Berman, J., Redecha, P., Spruce, L., Thurman, J. M., Kraus, D., Hollmann, T. J., Casali, P., Caroll, M. C., Wetsel, R. A., et al. (2003) Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 112:1644–1654.
- Gladen, B. C., Zadorozhnaja, T. D., Chislovska, N., Hryhorczuk, D. O., Kennicutt, M. C., 2nd and Little, R. E. (2000) Polycyclic aromatic hydrocarbons in placenta. Hum Exp Toxicol 19:597–603.
- Grimmer, G., Stober, W., Jacob, J., Mohr, U., Schoene, K., Brune, H. and Misfeld, J. (1983) Inventory and biological impact of polycyclic carcinogens in the environment. Exp Pathol 24:3–13.
- Gruslin, A., Qiu, Q. and Tsang, B. K. (2001)X-linked inhibitor of apoptosis protein expression and the regulation of apoptosis during human placental development. Biol Reprod 64:1264–1272.
- Guimond, M. J., Wang, B. and Croy, B. A. (1998) Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med 187:217–223.
- Hanna, J., Goldman-Wohl, D., Hamani, Y., Avraham, I., Greenfield, C., Natanson-Yaron, S., Prus, D., Cohen-Daniel, L., Arnon, T. I., Manaster, I., et al. (2006) Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12:1065–1074.
- Hansen, C., Sorensen, L. D., Asmussen, I. and Autrup, H. (1992) Trans-placental exposure to tobacco smoke in human-adduct formation in placenta and umbilical cord blood vessels. Teratog Carcinog Mutagen 12:51–60.
- Hansen, J. M., Reynolds, P. R., Booth, G. M., Schaalje, G. and Seegmiller, R. E. (2000) Developmental toxicity of carbon black oil in mice. Teratology 62:227–232.
- Hardy, K., Spanos, S., Becker, D., Iannelli, P., Winston, R. M. and Stark, J. (2001) From cell death to embryo arrest: Mathematical models of human preimplantation embryo development. Proc Natl Acad Sci USA 98:1655–1660.
- Harlap, S. and Shiono, P. H. (1980) Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet 2:173–176.
- Heaman, M. I. and Chalmers, K. (2005) Prevalence and correlates of smoking during pregnancy: A comparison of aboriginal and non-aboriginal women in manitoba. Birth 32:299–305.
- Higgins, S. (2002) Smoking in pregnancy. Current Opinion in Obstetrics and Gynecology 14:145–151.
- Hill, J. A., Polgar, K. and Anderson, D. J. (1995)T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. Jama 273:1933–1936.
- Himmelberger, D. U., Brown, B. W., Jr. and Cohen, E. N. (1978) Cigarette smoking during pregnancy and the occurrence of spontaneous abortion and congenital abnormality. Am J Epidemiol 108:470–479.
- Hoffmann, D. and Hoffmann, I. (1997) The changing cigarette, 1950–1995. J Toxicol Environ Health 50:307–364.
- Holladay, S. D. and Smialowicz, R. J. (2000) Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect 108(Suppl 3): 463–473.
- Howard, J. W. and Fazio, T. (1980) Analytical methodology and reported findings of polycyclic aromatic hydrocarbons in foods. J Assoc Off Anal Chem 63:1077–1104.
- Howe, G., Westhoff, C., Vessey, M. and Yeates, D. (1985) Effects of age, cigarette smoking and other factors on fertility: Findings in a large prospective study. British Medical Journal 290:1697–1700.
- Huang, J., Okuka, M., McLean, M., Keefe, D. L. and Liu, L. (2008) Effects of cigarette smoke on fertilization and embryo development in vivo. http://www.fertstert.org/article/S0015–0282(08)03563–2/pdf 28 February 2009.
- Hunt, J. S. (2006) Stranger in a strange land. Immunol Rev 213:36–47.
- Hunt, J. S., Chu, W. and Miller, L. (1998) Nonspecific immunity in the uterus and placenta. Trophoblast Research 12:125–134.
- Hunt, J. S., Vassmer, D., Ferguson, T. A. and Miller, L. (1997) Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol 158:4122–4128.
- Izzotti, A., Balansky, R. M., Cartiglia, C., Camoirano, A., Longobardi, M. and De Flora, S. (2003) Genomic and transcriptional alterations in mouse fetus liver after transplacental exposure to cigarette smoke. Faseb J 17:1127–1129.
- Jerzak, M. and Bischof, P. (2002) Apoptosis in the first trimester human placenta: The role in maintaining immune privilege at the maternal-foetal interface and in the trophoblast remodelling. Eur J Obstet Gynecol Reprod Biol 100:138–142.
- Joesoef, M. R., Beral, V., Aral, S. O., Rolfs, R. T. and Cramer, D. W. (1993) Fertility and use of cigarettes, alcohol, marijuana and cocaine. Annals of Epidemiology 3:592–594.
- Kadkhoda, K., Pourpak, Z., Akbar Pourfathallah, A. and Kazemnejad, A. (2004) The ex vivo study of synergistic effects of polycyclic aromatic hydrocarbon, benzo(a)pyrene with ovalbumin on systemic immune responses by oral route. Toxicology 199:261–265.
- Kahn, R. S., Certain, L. and Whitaker, R. C. (2002) A reexamination of smoking before, during, and after pregnancy. Am J Public Health 92:1801–1808.
- Karakaya, A., Ates, I. and Yucesoy, B. (2004) Effects of occupational polycyclic aromatic hydrocarbon exposure on T-lymphocyte functions and natural killer cell activity in asphalt and coke oven workers. Hum ExpToxicol 23:317–322.
- Khan, H. M., Khan, M. Y. and Minhas, L. A. (2008) Effect of passive tobacco smoking on fertility of female mice. J Coll Physicians Surg Pak 18:708–712.
- Khorram, O., Han, G. and Magee, T. (2008) Cigarette smoke inhibits endometrial epithelial cell proliferation through a nitric oxide-mediated pathway. http://www.fertstert.org/article/S0015–0282(08)04101 -0/pdf 6 March 2009.
- Kim, D. W., Gazourian, L., Quadri, S. A., Romieu-Mourez, R., Sherr, D. H. and Sonenshein, G. E. (2000) The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 19:5498–5506.
- King, A., Jokhi, P. P., Burrows, T. D., Gardner, L., Sharkey, A. M. and Loke, Y. W. (1996) Functions of human decidual NK cells. Am J Reprod Immunol 35:258–260.
- King, A. and Loke, Y W. (1990) Human trophoblast and JEG chorio-carcinoma cells are sensitive to lysis by IL-2-stimulated decidual NK cells. Cell Immunol 129:435–448.
- Kitamura, M. and Kasai, A. (2007) Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett 252:184–194.
- Klareskog, L., Padyukov, L. and Alfredsson, L. (2007) Smoking as a trigger for inflammatory rheumatic diseases. Curr Opin Rheumatol 19:49–54.
- Kline, J., Levin, B., Kinney, A., Stein, Z., Susser, M. and Warburton, D. (1995) Cigarette smoking and spontaneous abortion of known karyotype. Precise data but uncertain inferences. Am J Epidemiol 141:417–427.
- Kline, J., Stein, Z. A., Suser, M. and Warburton, D. (1977) Smoking: A risk factor for spontaneous abortion. New England Journal of Medicine 297:793–796.
- Kusakabe, K., Okada, T., Sasaki, F. and Kiso, Y (1999) Cell death of uterine natural killer cells in murine placenta during placentation and preterm periods. J Vet Med Sci 61:1093–1100.
- Lecureur, V., Ferrec, E. L., N'Diaye, M., Vee, M. L., Gardyn, C., Gilot, D. and Fardel, O. (2005) ERK-dependent induction of TNFalpha expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett 579:1904–1910.
- Lederman, S. A., Rauh, V., Weiss, L., Stein, J. L., Hoepner, L. A., Becker, M. and Perera, F. P. (2004) The effects of the World Trade Center event on birth outcomes among term deliveries at three lower Manhattan hospitals. Environ Health Perspect 112:1772–1778.
- Lee, J., Choi, B. C., Cho, C., Hill, J. A., Baek, K. H. and Kim, J. W. (2005) Trophoblast apoptosis is increased in women with evidence of TH1 immunity. Fertil Steril 83:1047–1049.
- Liang, S., Zhang, J., Wei, H., Sun, R. and Tian, Z. (2005) Differential roles of constitutively activated ERK1/2 and NF-kappa B in cytotoxicity and proliferation by human NK cell lines. Int Immunopharmacol 5:839–848.
- Lin, H., Mosmann, T. R., Guilbert, L., Tuntipopipat, S. and Wegmann, T. G. (1993) Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol 151:4562–4573.
- Lintsen, A. M., Pasker-de Jong, P. C., de Boer, E. J., Burger, C. W., Jansen, C. A., Braat, D. D. and van Leeuwen, F. E. (2005) Effects of subfertility cause, smoking and body weight on the success rate of IVF. Hum Reprod 20:1867–1875.
- Lioy, P. J., Weisel, C. P., Millette, J. R., S. Eisenreich, S., Vallero, D., Offenberg, J., Buckley, B., Turpin, B., Zhong, M., Cohen, M. D., . (2002) Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect 110:703–714.
- Luppi, P., Haluszczak, C., Betters, D., Richard, C. A., Trucco, M. and DeLoia, J. A. (2002) Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol 72:874–884.
- Mackenzie, K. M. and Angevine, D. M. (1981) Infertility in mice exposed in utero to benzo(a)-pyrene. Biology of Reproduction 24:183–191.
- Magnusson, C. G. (1986) Maternal smoking influences cord serum IgE and IgD levels and increases the risk for subsequent infant allergy. J Allergy Clin Immunol 78:898–904.
- Marx, L., Arck, P., Kieslich, C., Mitterlechner, S., Kapp, M. and Dietl, J. (1999) Decidual mast cells might be involved in the onset of human first-trimester abortion. Am J Reprod Immunol 41:34–40.
- Marzi, M., Vigano, A., Trabattoni, D., Villa, M. L., Salvaggio, A., Clerici, E. and Clerici, M. (1996) Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol 106:127–133.
- Matiasovic, J., Leva, L., Maskova, J., Kummer, V and Faldyna, M. (2008) Effects of postnatal exposure to benzo[a]pyrene on the immunity of immature rats. Veterinarni Medicina 53:93–100.
- Matsubayashi, H., Shida, M., Kondo, A., Suzuki, T., Sugi, T., Izumi, S., Hosaka, T. and Makino, T. (2005) Preconception peripheral natural killer cell activity as a predictor of pregnancy outcome in patients with unexplained infertility. Am J Reprod Immunol 53:126–131.
- Meeker, J. D., Missmer, S. A., Vitonis, A. F., Cramer, D. W. and Hauser, R. (2007) Risk of spontaneous abortion in women with childhood exposure to parental cigarette smoke. Am J Epidemiol 166:571–575.
- Mlynarcikova, A., Fickova, M. and Scsukova, S. (2005) Ovarian intra-follicular processes as a target for cigarette smoke components and selected environmental reproductive disruptors. Endocr Regul 39:21–32.
- Moffett-King, A. (2002) Natural killer cells and pregnancy. Nat Rev Immunol 2:656–663.
- Moffett, A. and Loke, C. (2006) Immunology of placentation in eutherian mammals. Nat Rev Immunol 6:584–594.
- Molina, V and Shoenfeld, Y (2005) Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity 38:235–245.
- Motz, G. T., Eppert, B. L., Sun, G., Wesselkamper, S. C., Linke, M. J., Deka, R. and Borchers, M. T. (2008) Persistence of lung CD8 Tcell oligoclonal expansions upon smoking cessation in a mouse model of cigarette smoke-induced emphysema. J Immunol 181:8036–8043.
- Murphy, S. P., Fast, L. D., Hanna, N. N. and Sharma, S. (2005) Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol 175:4084–4090.
- Nakamoto, T., Yasuda, K., Yasuhara, M., Nakajima, T., Mizokami, T., Okada, H. and Kanzaki, H. (2006) Cigarette smoke extract enhances oxytocin-induced rhythmic contractions of rat and human preterm myometrium. Reproduction 132:343–353.
- Nakashima, A., Shiozaki, A., Myojo, S., Ito, M., Tatematsu, M., Sakai, M., Takamori, Y., Ogawa, K., Nagata, K. and Saito, S. (2008) Granulysin produced by uterine natural killer cells induces apoptosis of extravillous trophoblasts in spontaneous abortion. Am J Pathol 173:653–664.
- Neal, M. S., Hughes, E. G., Holloway, A. C. and Foster, W. G. (2005) Side-stream smoking is equally as damaging as mainstream smoking on IVF outcomes. Hum Reprod 20:2531–2535.
- Ness, R. B., Grisso, J. A., Hirschinger, N., Markovic, N., Shaw, L. M., Day, N. L. and Kline, J. (1999) Cocaine and tobacco use and the risk of spontaneous abortion. New England Journal of Medicine 340:333–339.
- Ng, S. P. and Zelikoff, J. T. (2008) The effects of prenatal exposure of mice to cigarette smoke on offspring immune parameters. J Toxicol Environ Health A 71:445–453.
- Nishikawa, K., Saito, S., Morii, T., Hamada, K., Ako, H., Narita, N., Ichijo, M., Kurahayashi, M. and Sugamura, K. (1991) Accumulation of CD16-CD56+ natural killer cells with high affinity interleukin 2 receptors in human early pregnancy decidua. Int Immunol 3:743–750.
- Noakes, P. S., Holt, P. G. and Prescott, S. L. (2003) Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy 58:1053–1058.
- Nohara, K., Pan, X., Tsukumo, S., Hida, A., Ito, T., Nagai, H., Inouye, K., Motohashi, H., Yamamoto, M., Fujii-Kuriyama, Y. and Tohyama, C. (2005) Constitutively active aryl hydrocarbon receptor expressed specifically in T-lineage cells causes thymus involution and suppresses the immunization-induced increase in splenocytes. J Immunol 174:2770–2777.
- Offenberg, J. H., Eisenreich, S. J., Chen, L. C., Cohen, M. D., Chee, G., Prophete, C., Weisel, C. and Lioy, P. J. (2003) Persistent organic pollutants in the dusts that settled across lower Manhattan after September 11, 2001. Environ Sci Technol 37:502–508.
- Olivares, E. G., Munoz, R., Tejerizo, G., Montes, M. J., Gomez-Molina, F. and Abadia-Molina, A. C. (2002) Decidual lymphocytes of human spontaneous abortions induce apoptosis but not necrosis in JEG-3 extravillous trophoblast cells. Biol Reprod 67:1211–1217.
- Osorio, Y., Bonilla, D. L., Peniche, A. G., Melby, P. C. and Travi, B. L. (2008) Pregnancy enhances the innate immune response in experi mental cutaneous leishmaniasis through hormone-modulated nitric oxide production. J Leukoc Biol 83:1413–1422.
- Parham, P. (2004) NK cells and trophoblasts: Partners in pregnancy. J Exp Med 200:951–955.
- Parr, E. L., Parr, M. B., Zheng, L. M. and Young, J. D. (1991) Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol Reprod 44:834–841.
- Peel, S. (1989) Granulated metrial gland cells. Adv Anat Embryol Cell Biol 115:1–112.
- Pejcic-Karapetrovic, B., Gurnani, K., Russell, M. S., Finlay, B. B., Sad, S. and Krishnan, L. (2007) Pregnancy impairs the innate immune resistance to Salmonella typhimurium leading to rapid fatal infection. J Immunol 179:6088–6096.
- Perera, F. P., Tang, D., Rauh, V., Lester, K., Tsai, W. Y., Tu, Y H., Weiss, L., Hoepner, L., King, J., Del Priore, G. and Lederman, S. A. (2005a) Relationships among polycyclic aromatic hydrocarbon-DNA adducts, proximity to the World Trade Center, and effects on fetal growth. Environ Health Perspect 113:1062–1067.
- Perera, F., Tang, D., Whyatt, R., Lederman, S. A. and Jedrychowski, W. (2005b) DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol Biomarkers Prev 14:709–714.
- Piccinni, M. P. and Romagnani, S. (1996) Regulation of fetal allograft survival by a hormone-controlled Th1- and Th2-type cytokines. Immunol Res 15:141–150.
- Pocar, P., Fischer, B., Klonisch, T. and Hombach-Klonisch, S. (2005) Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction 129:379–389.
- Qiu, Q., Yang, M., Tsang, B. K. and Gruslin, A. (2005) Fas ligand expression by maternal decidual cells is negatively correlated with the abundance of leukocytes present at the maternal-fetal interface. J Reprod Immunol 65:121–132.
- Quenby, S., Nik, H., Innes, B., Lash, G., Turner, M., Drury, J. and Bulmer, J. (2009) Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod 24:45–54.
- Quintana, F. J., Basso, A. S., Iglesias, A. H., Korn, T., Farez, M. F., Bettelli, E., Caccamo, M., Oukka, M. and Weiner, H. L. (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71.
- Raine-Fenning, N. J., Campbell, B. K., Kendall, N. R., Clewes, J. S. and Johnson, I. R. (2004) Quantifying the changes in endometrial vascularity throughout the normal menstrual cycle with three-dimensional power Doppler angiography. Hum Reprod 19:330–338.
- Redman, C. W. and Sargent, I. L. (2003) Pre-eclampsia, the placenta and the maternal systemic inflammatory response—A review. Placenta 24(Suppl A):S21–27.
- Richani, K., Soto, E., Romero, R., Espinoza, J., Chaiworapongsa, T., Nien, J. K., Edwin, S., Kim, Y. M., Hong, J. S. and Mazor, M. (2005) Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med 17:239–245.
- Rogerson, S. J., Pollina, E., Getachew, A., Tadesse, E., Lema, V. M. and Molyneux, M. E. (2003) Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 68:115–119.
- Rushton, L. (2007) Occupational causes of chronic obstructive pulmonary disease. Rev Environ Health 22:195–212.
- Sacks, G. P., Clover, L. M., Bainbridge, D. R., Redman, C. W. and Sargent, I. L. (2001) Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta 22:550–559.
- Sargent, I. L., Borzychowski, A. M. and Redman, C. W. (2006) NK cells and human pregnancy–an inflammatory view. Trends Immunol 27:399–404.
- Schmidt, J. V., Su, G. H., Reddy, J. K., Simon, M. C. and Bradfield, C. A. (1996) Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A 93:6731–6736.
- Seavey, M. M. and Mosmann, T. R. (2008) Immunoregulation of fetal and anti-paternal immune responses. Immunol Res 40:97–113.
- Sharara, F. I., Seifer, D. B. and Flaws, J. A. (1998) Environmental toxicants and female reproduction. Fertil Steril 70:613–622.
- Sharkey, A. M., Gardner, L., Hiby, S., Farrell, L., Apps, R., Masters, L., Goodridge, J., Lathbury, L., Stewart, C. A., Verma, S. and Moffett, A. (2008) Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol 181:39–46.
- Shiverick, K. T. and Salafia, C. (1999) Cigarette smoking and pregnancy I: Ovarian, uterine and placental effects. Placenta 20:265–272.
- Soares, S. R. and Melo, M. A. (2008) Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol 20:281–291.
- Soares, S. R., Simon, C., Remohi, J. and Pellicer, A. (2007) Cigarette smoking affects uterine receptiveness. Hum Reprod 22:543–547.
- Soghomonians, A., Thirkill, T. L., Mariano, N. F., Barakat, A. I. and Douglas, G. C. (2004) Effect of aqueous tobacco smoke extract and shear stress on PECAM-1 expression and cell motility in human uterine endothelial cells. Toxicol Sci 81:408–418.
- Sopori, M. (2002) Effects of cigarette smoke on the immune system. Nat Rev Immunol 2:372–377.
- Sram, R. (1999) Impact of air pollution on reproductive health. Environ Health Perspect 107:A542–543.
- Stevens, E. A. and Bradfield, C. A. (2008) Immunology: Tcells hang in the balance. Nature 453:46–47.
- Tauchi, M., Hida, A., Negishi, T., Katsuoka, F., Noda, S., Mimura, J., Hosoya, T., Yanaka, A., Aburatani, H., Fujii-Kuriyama, Y., et al. (2005) Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol 25:9360–9368.
- Thirkill, T. L., Vedagiri, H. and Douglas, G. C. (2006) Macaque trophoblast migration toward RANTES is inhibited by cigarette smoke-conditioned medium. Toxicol Sci 91:557–567.
- Tian, Y., Ke, S., Denison, M. S., Rabson, A. B. and Gallo, M. A. (1999) Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem 274:510–515.
- Veldhoen, M., Hirota, K., Christensen, J., O'Garra, A. and Stockinger, B. (2009) Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 Tcells. J Exp Med 206:43–49.
- Veldhoen, M., Hirota, K., Westendorf, A. M., Buer, J., Dumoutier, L., Renauld, J. C. and Stockinger, B. (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106–109.
- Venners, S. A., Wang, X., Chen, C., Wang, L., Chen, D., Guang, W., Huang, A., Ryan, L., O'Connor, J., Lasley, B., et al. (2004) Paternal smoking and pregnancy loss: A prospective study using a biomarker of pregnancy. Am J Epidemiol 159:993–1001.
- Ventura, S. J., Hamilton, B. E., Mathews, T. J. and Chandra, A. (2003) Trends and variations in smoking during pregnancy and low birth weight: Evidence from the birth certificate, 1990–2000. Pediatrics 111:1176–1180.
- Vestergaard, P., Rejnmark, L., Weeke, J., Hoeck, H. C., Nielsen, H. K., Rungby, J., Laurberg, P. and Mosekilde, L. (2002) Smoking as a risk factor for Graves’ disease, toxic nodular goiter, and autoimmune hypothyroidism. Thyroid 12:69–75.
- von Rango, U. (2008) Fetal tolerance in human pregnancy—A crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett 115:21–32.
- Wang, X., Ding, H., Ryan, L. and Xu, X. (1997) Association between air pollution and low birth weight: A community-based study. Environ Health Perspect 105:514–520.
- Ward, E. C., Murray, M. J., Lauer, L. D., House, R. V. and Dean, J. H. (1986) Persistent suppression of humoral and cell-mediated immunity in mice following exposure to the polycyclic aromatic hydrocarbon 7,12-dimethylbenz[a]anthracene. Int J Immunopharmacol 8:13–22.
- Ward, E. C., Murray, M. J., Lauer, L. D., House, R. V., Irons, R. and Dean, J. H. (1984) Immunosuppression following 7,12-dimethylbenz[a]anthracene exposure in B6C3F1 mice. I. Effects on humoral immunity and host resistance. Toxicol Appl Pharmacol 75:299–308.
- Warren, J. E. and Silver, R. M. (2008) Genetics of pregnancy loss. Clin Obstet Gynecol 51:84–95.
- Waylen, A. L., Metwally, M., Jones, G. L., Wilkinson, A. J. and Ledger, W. L. (2009) Effects of cigarette smoking upon clinical outcomes of assisted reproduction: A meta-analysis. Hum Reprod Update 15:31–44.
- Wegmann, T. G., Lin, H., Guilbert, L. and Mosmann, T. R. (1993) Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol Today 14:353–356.
- Whyatt, R. M., Jedrychowski, W., Hemminki, K., Santella, R. M., Tsai, W. Y., Yang, K. and Perera, F. P. (2001) Biomarkers of polycyclic aromatic hydrocarbon-DNA damage and cigarette smoke exposures in paired maternal and newborn blood samples as a measure of differential susceptibility. Cancer Epidemiol Biomarkers Prev 10:581–588.
- Widayati, D. T., Ohmori, Y. and Fukuta, K. (2004) Distribution patterns of immunocompetent cells in the pregnant mouse uteri carrying allogeneic mouse and xenogeneic vole embryos. J Anat 205:45–55.
- Wilcox, A. J., Weinberg, C. R., O'Connor, J. F., Baird, , Schlatterer, J. P., Canfield, R. E., Armstrong, E. G. and Nisula, B. C. (1988) Incidence of early loss of pregnancy. N Engl J Med 319:189–194.
- Witschi, H., Joad, J. P. and Pinkerton, K. E. (1997) The toxicology of environmental tobacco smoke. Annual Reviews in Pharmacology and Toxicology 37:29–52.
- Woodruff, T. J., Grillo, J. and Schoendorf, K. C. (1997) The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ Health Perspect 105:608–612.
- Wordinger, R. J., Jackson, F. L. and Morrill, A. (1986) Implantation, deciduoma formation and live births in mast cell-deficient mice (W/Wv). J Reprod Fertil 77:471–476.
- Wu, X., Wei, H., Zhang, J. and Tian, Z. (2006) Increased uterine NK- derived IFN-gamma and TNF-alpha in C57BL/6J mice during early gestation. Cell Mol Immunol 3:131–137.
- Yusuf, N., Timares, L., Seibert, M. D., Xu, H. and Elmets, C. A. (2007) Acquired and innate immunity to polyaromatic hydrocarbons. Toxicol Appl Pharmacol 224:308–312.
- Zenclussen, A. C. (2005) CD4( + )CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol 65:101–110.
- Zenzes, M. T., Wang, P. and Casper, R. F. (1995) Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod 10:3213–3217.
- Zhang, J. and Tian, Z. (2007) UNK cells: Their role in tissue re-modelling and preeclampsia. Semin Immunopathol 29:123–133.
- Zinaman, M. J., Clegg, E. D., Brown, C. C., O'Connor, J. and Selevan, S. G. (1996) Estimates of human fertility and pregnancy loss. Fertil Steril 65:503–509.