Abstract
Intrauterine growth restriction (IUGR) is a disease responsible for neonatal morbidity and mortality and perinatal death affecting 8% of all pregnancies. In sheep, IUGR that mimics the human IUGR disease closely can be brought on by environmental hyperthermia. Endothelial nitric oxidase synthase (eNOS) and nitric oxide (NO) are important in the regulation of blood flow in the fetal-placental circulation and are modulated by several factors including hypoxia. eNOS activity is also regulated by the phosphorylation of ERK1/2 and AKT proteins in various tissues. In a hyperthermic (HT) ovine model of IUGR with systemic hypertension and increased blood flow resistance, our objective was to determine the relationship between p-ERK, p-AKT, eNOS, and NO concentrations in the placenta, uterine, and umbilical vessels at mid-gestation and near-term.
Eight pregnant ewes were exposed to hyperthermic conditions for either 55 or 80 days to induce IUGR. Sheep necropsies were performed at mid-gestation and near-term for collection of placentomes, umbilical vessels, and the uterine artery. Tissues were assessed for eNOS mRNA and protein, and p-ERK and p-AKT protein. Blood was collected for NO determination at the time of necropsy. Placental insufficiency and IUGR (PI-IUGR) pregnancies demonstrated: 1) reduced placental weight at mid-gestation and reduced placental and fetal weight near-term, 2) no changes in eNOS protein concentration in the uterine artery and umbilical vessels, but an increase in NO in umbilical vein blood at both time points, 3) no significant changes in signal transduction makers (ERK/AKT) in placental tissue at mid-gestation but a significant increase near-term in cotyledon tissues, and 4) an increase in p-AKT in the uterine vessels at term. The near-term findings of increased placental p-ERK and p-AKT proteins and umbilical vein NO concentration suggest one mechanism responsible for the increase in placental eNOS previously described in this PI-IUGR model characterized by fetal systemic hypertension and abnormal umbilical artery Doppler velocimetry.
| Abbreviations | ||
| IUGR: | = | intrauterine growth restriction |
| eNOS: | = | endothelial nitric oxidase synthase |
| NO: | = | nitric oxide |
| HT: | = | hyperthermic |
| PI-IUGR: | = | placental insufficiency and IUGR |
| ERK1/2: | = | extracellular signal-regulated kinase ½ |
| AKT: | = | protein kinase B |
INTRODUCTION
Intrauterine growth restriction (IUGR) is an obstetric complication known to increase the risk for fetal and infant morbidity and mortality [Bernstein et al. Citation2000; Sankaran and Kyle Citation2009]. This disease is characterized by a decrease in fetal and placental weight, increased blood flow resistance, and hypoxia. IUGR is also associated with the development of long-term adverse health problems for the newborn and adult [Bernstein et al. Citation2000; Joss-Moore and Lane Citation2009]. While there are a number of causes of IUGR, abnormal placentation with placental insufficiency is the most common, affecting 3-5% of all pregnancies [Kingdom et al. Citation2000]. During pregnancy there is significant elevation of blood flow through the uterus and placenta in order for the placenta to grow and meet the demands of the growing fetus [Reynolds and Redmer Citation2001]. IUGR is characterized by a decrease in placental size and increased blood flow resistance. Disruptions in vasoactive modulators such as nitric oxide (NO) are known to be present during IUGR [Galan et al. Citation2001; Thaete et al. Citation2004]. NO is a potent vasodilator synthesized by the enzyme endothelial nitric oxide synthase (eNOS) and is known to regulate placental blood flow [Pallares and Gonzalez-Bulnes Citation2008; Umar and van der Laarse Citation2009; Myatt et al. Citation1997; Citation1991]. In an ovine model of placental insufficiency and IUGR (PI-IUGR) induced with hyperthermic (HT) exposure, placental and umbilical artery eNOS protein is decreased at mid-gestation in the placenta, but increased near-term [Arroyo et al. Citation2006; Galan et al. Citation2001]. These eNOS data provided a mechanism describing the reduced blood flow, hypertension, and increased resistance to blood flow that is characteristic of the HT ovine IUGR fetus [Galan et al. Citation2002; Citation1999a; Citation1999b; Citation1998]. However, the effect on NO concentration and the signaling mechanisms remain unknown.
The extracellular signal-regulated kinase 1/2 (ERK1/2) and the protein kinase B (AKT) pathways are found in many cell types playing various roles. While the ERK protein is usually associated with cell proliferation, the AKT proteins are associated with cell survival in many types of tissues. Reports had previously shown that eNOS activity is regulated by the phosphorylation (p) of ERK and AKT proteins in various tissues. These signaling proteins have not been determined in the ovine model of PI-IUGR but they had been shown to be decreased in other models of IUGR [Ain et al. Citation2005].
Our ovine model of PI-IUGR has numerous features characteristic of IUGR in humans, including asymmetrical fetal growth with an increase in umbilical Doppler velocimetry indices and systemic blood pressure, as well as a reduction of placental size [Galan et al. Citation2001; Citation1999b]. IUGR has been induced by a variety of techniques including uterine artery ligation, umbilical artery ligation, nutritional restriction, hypoxia, high altitude exposure, hyperthermic exposure, carunculectomy, caruncle embolization, and administration of vasoactive agents [Block et al. Citation1984; Galan et al. Citation2001; Giles et al.Citation1989; Miller et al. Citation2009; Molnar and Hertelendy Citation1992; Moore et al. Citation2004; Schober et al. Citation2009; Skarsgard et al. Citation2001; Turner and Trudinger Citation2009; Yallampalli and Garfield Citation1993]. IUGR in humans is most commonly defined by an estimated fetal weight below 10th percentile for gestational age. Unlike the human definition, a clear weight cut-off is not standard in animal models, but rather a comparison of birth weight between control and exposed groups is typically used. More specifically, the HT model of IUGR shows a graded IUGR response depending on the duration of HT exposure during pregnancy. Beginning at 40 days of gestation, sheep exposed for 80 days show a greater degree of IUGR than those exposed for 55 days [Galan et al. Citation1999b]. Exposure beyond this level of hyperthermia results in more severe growth restriction, but a prohibitively expensive fetal death rate. The sheep exposed to high ambient temperature throughout pregnancy develop the most severe IUGR that closely mimics multiple features seen in the human IUGR fetus [Bell et al. Citation1989; Citation1987; Galan et al. Citation1999a; Molnar et al. Citation1992; Thureen et al. Citation1992]. In the present study our goal was to determine underlying mechanisms for altered eNOS protein expression by assessing different end points in placental, umbilical, and uterine tissues. We chose to study this model to further define the molecular mechanisms related to eNOS and signaling proteins not previously defined in IUGR. In addition, using this model enabled us to define these mechanisms earlier in pregnancy in a controlled fashion that is not achievable in humans. We chose to study these tissues at two time points, mid-gestation (95 days of gestation; dGA) and near-term (130 dGA), two important time points in ovine pregnancy relative to fetal and placental growth. To accomplish this goal we set forth the following objectives comparing HT treated animals and controls: 1) to asses eNOS mRNA and protein concentration at mid-gestation in the uterine and umbilical vessel, 2) to determine the NO concentration in the blood from the umbilical and uterine circulations, and 3) to determine the activation (phosphorylation) of ERK (p-ERK) and AKT (p-AKT) in the placenta, uterine, and umbilical vessels.
RESULTS
Gestational ages and fetal and placental weights are shown in . Gestational ages of animals were not significantly different between control and PI-IUGR pregnancies at each time point. At mid-gestation, PI-IUGR pregnancies showed a significant decrease in placental weight (2.4-fold; 440±50 vs. 186±18; p<0.004), but not fetal weight. However, near-term there was a significant decrease in both placental (2.0-fold; 348.7±21.02 vs. 168.7±43.2; p≤0.004) and fetal (1.8-fold; 2914±201g vs. 1718±433g; p≤0.008) weights in the PI-IUGR pregnancies. Hemodynamics and blood gas analysis is shown in . There was a significant increase in S/D ratios (1.0-fold 3.0±0.34 vs. 3.8±0.18) and mean systemic blood pressure (1.3-fold 41±1.53 mmHg vs. 44.3±1.71 mmHg) associated with IUGR pregnancies near-term. There was a significant decrease in fetal O2 saturation and partial pressure of oxygen associated with IUGR pregnancies at this gestational age. There were no pregnancy losses in our studies.
TABLE 1 Necropsy Age, Placental and Fetal Weights in IUGR and Control Animals.
TABLE 2 Near-Term Fetal Hemodynamic and Blood Gas Results.*
eNOS mRNA and Protein Concentrations
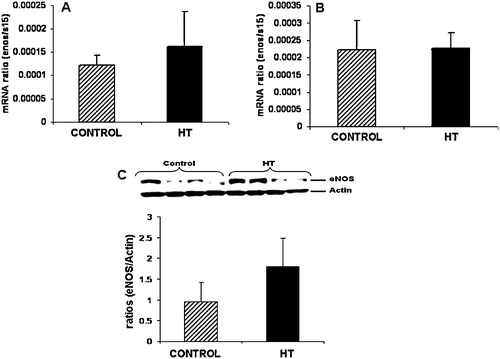
Umbilical vein eNOS mRNA concentration was similar between groups at mid-gestation () or near-term (). Western blot results for eNOS in the umbilical vein at mid-gestation is shown in Figure 1C demonstrating no differences in eNOS protein in the umbilical vein during IUGR.
FIGURE 1 Umbilical vein eNOS protein and mRNA. No significant differences were observed for eNOS mRNA in the umbilical vein at mid-gestation (A) and near-term (B) in treated animals vs. controls. No significant difference in eNOS protein expression was observed for the umbilical vein at mid-gestation during heat induced IUGR in the sheep (C).
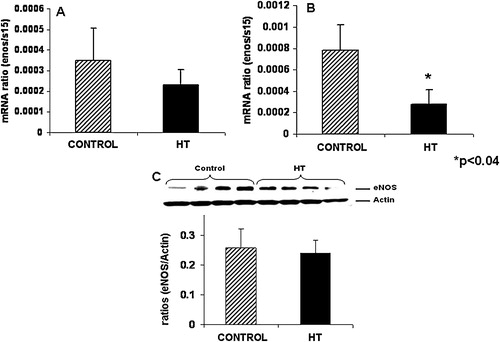
Umbilical artery eNOS mRNA or protein concentration did not show any significant difference at mid-gestation in treated animals as compared to controls ( and ). A 2.8-fold decrease in eNOS mRNA concentration was observed in the umbilical artery of treated animals near-term ().
FIGURE 2 Umbilical artery eNOS protein did not change at mid-gestation during IUGR in the sheep. Umbilical artery eNOS mRNA concentrations were similar between control and IUGR groups at mid-gestation (A) while a significant decrease was observed for eNOS mRNA in the IUGR umbilical artery near-term (B). Umbilical artery eNOS protein is similar between treated and control animals (C).
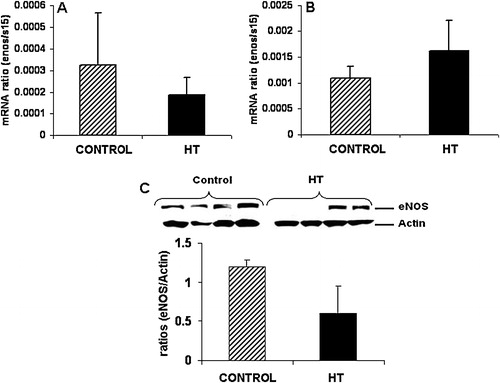
Uterine artery eNOS mRNA did not show any difference with treatment at mid-gestation or near-term ( and ). Western blot analysis for eNOS in the uterine artery did not show any differences for this protein in IUGR animals as compared to controls ().
FIGURE 3 Uterine artery eNOS protein is decreased in IUGR animals. No significant differences were observed for eNOS mRNA concentration in the uterine artery of treated animals at mid-gestation (A) and near-term (B). A 2.0-fold non-significant decrease was observed for eNOS protein in the uterine artery of treated animals vs. controls (C).
NO Determination
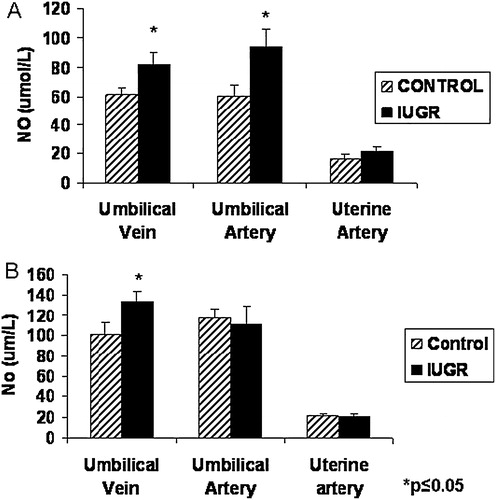
The concentration of nitric oxide was significantly increased in the blood from the umbilical vessels at mid-gestation in treated animals as compared to controls (). Uterine artery blood analysis did not show any significant differences for NO at this gestational period. Near-term, NO in the umbilical vein blood was increased in PI-IUGR pregnancies. Neither the uterine or umbilical artery showed significant changes in NO with treatment at near term ().
FIGURE 4 Plasma NO concentration in the uterine artery and umbilical vessels of HT treated animals. At mid-gestation, NO was significantly increased in both umbilical vessels (A) while at term, only plasma from blood obtained from the umbilical vein showed a significant increase for NO near-term (B).
Placental ERK and AKT Assessment
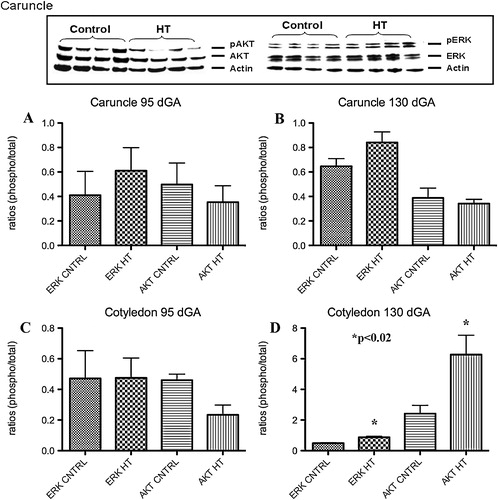
A characteristic western for AKT and ERK is shown in . No significant differences for p-ERK and p-AKT were observed in the placental caruncle (maternal compartment) as compared to controls ( and ) at either gestational period studied. Cotyledon tissues (fetal side) showed no significant differences for p-ERK or p-AKT at mid-gestation (). A 1.8-fold increase (p<0.01) in p-ERK and a 2.4-fold increase (p<0.02) for p-AKT were observed in this tissue near-term ().
FIGURE 5 Placental p-ERK and p-AKT during IUGR. A characteristic western for ERK and AKT is shown in top panel of this figure. No significant differences were observed for these proteins in the caruncle at any gestational point studies (A and B). A significant increase in p-ERK and p-AKT was observed in the cotyledon only near term during HT treatment in the sheep (C and D).
Umbilical and Uterine Vessels ERK and AKT Assessment
A characteristic western for AKT and ERK is shown in the top of . At mid-gestation, PI-IUGR pregnancies showed a significant 2.3-fold increase (p<0.002) in p-ERK in the umbilical veins with no differences in p-AKT (). In contrast, near-term, the umbilical vein showed no significant differences for p-ERK and p-AKT with treatment as compared to controls ().
FIGURE 6 p-ERK and p-AKT in the uterine artery and umbilical vessels. Compared to controls, umbilical vein p-ERK was significantly increased in treated animals at mid-gestation, but not near-term (A and B). At mid-gestation, p-ERK was increased in the umbilical artery of treated animals, while p-AKT was decreased (C and D). Uterine artery p-AKT was increased only near-term in IUGR animals (E and F).
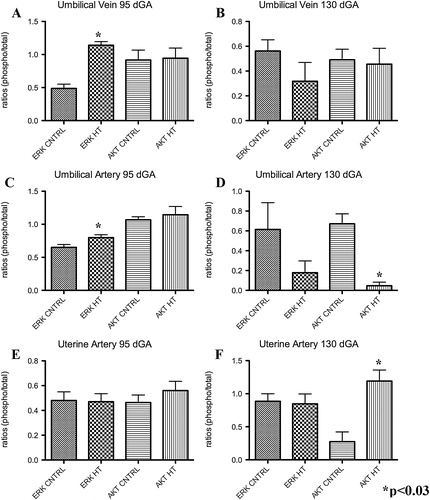
No significant differences were observed in p-ERK and p-AKT within the umbilical artery () at mid-gestation. In contrast, the umbilical artery exhibited a significant 14.2 fold decrease in p-AKT in near-term PI-IUGR pregnancies (). Similarly, no differences were detected between groups in the uterine artery tissues at mid-gestation for p-ERK and p-AKT (). In comparison there was a 4.3 fold increase (p<0.03) for p-AKT yet no difference in p-ERK at near-term in the sheep PI-IUGR pregnancies ().
DISCUSSION
We studied two important time points in ovine pregnancy, mid-gestation, when placental growth is at its peak, and near-term when fetal growth is at its maximum. In our study, we found that PI-IUGR placental weights were significantly decreased at both gestational periods as previously reported [Arroyo et al. Citation2006; Galan et al. Citation2001]. Interestingly, fetal weight was only significantly decreased near-term and not at mid-gestation in the PI-IUGR pregnancies, a finding likely reflecting that at mid-gestation the fetus is just entering the exponential portion of the fetal growth curve thus making it more difficult to detect differences in growth.
summarizes the eNOS, NO, p-AKT, and p-ERK results from this study and the eNOS data from the previous studies in cotyledon and caruncles tissues [Arroyo et al. Citation2006; Galan et al. Citation2001]. NO and eNOS were examined because they are important regulators of vasodilation. In the present study, no significant differences were observed in eNOS mRNA and protein concentration in the umbilical vein, umbilical artery, or uterine artery of HT animals at mid-gestation. Near-term, only umbilical artery eNOS mRNA was significantly decreased which is consistent with the findings of a decrease in eNOS protein level previously obtained in this vessel at this gestational period [Arroyo et al. Citation2006]. In this model of IUGR, these results suggest eNOS is transcriptionally regulated in these vessels. NO was increased in the serum from the umbilical vein at both mid-gestation and near-term gestational points. In contrast, serum NO only increased in the umbilical artery at mid-gestation while there was no change observed in serum NO from the uterine artery at any gestational period. The general lack of eNOS changes in the umbilical artery and uterine artery is understandable as these are the conduit and not the resistance vessels. The finding of an increase NO in the blood from the umbilical vein is likely due to the transfer of NO produced upstream in the cotyledon where eNOS protein is increased [Arroyo et al. Citation2006].
TABLE 3 Summary of Results Obtained in Study.
For the placental signaling studies, the placentomes were separated into caruncles and cotyledons reflecting the maternal and fetal sides of the placenta, respectively. Placental eNOS experiments were not repeated as this data has been published previously. In studies that assess important signaling proteins such as AKT and ERK, it is common to find only one or the other that is affected and, as such, both are often simultaneously studied [Boyd et al. Citation2003; Dimmeler et al. Citation1998; Jo et al. Citation1997; Sumpio et al. Citation2005; Tseng et al. Citation1995]. At mid-gestation, the cotyledon (fetal side) did not show any differences in p-AKT or p-ERK as compared to controls. In contrast, our near-term studies showed increases in both of these proteins. There was no change in p-ERK or p-AKT in the maternal side of the placenta (caruncle). This is similar to the lack of change in other proteins in the caruncle related to vasoregulation such as eNOS and VEGF [Arroyo et al. Citation2006; Galan et al. Citation2001; Regnault et al. Citation2003]. This is consistent with the idea that hyperthermic exposure and IUGR in this model has its primary effect on the fetal side of the placenta.
The near-term HT PI-IUGR ovine pregnancy model demonstrates reduced absolute uterine and umbilical (ml/min) blood flows, increased umbilical artery Doppler velocimetry indices [Galan et al. Citation1998], and fetal systemic hypertension [Galan et al. Citation2005]. Accordingly, we chose to study the uterine artery and umbilical vessels for activation of ERK and AKT anticipating that there may be alterations in their protein concentration. At mid-gestation, umbilical veins of PI-IUGR pregnancies showed a significant increase in p-ERK, but not p-AKT, and no significant differences in the uterine or umbilical artery expression of p-ERK or p-AKT. In the near-term HT pregnancies, no differences were observed for either protein in the umbilical veins, which may again be explained by the primary conduit functions of these vessels.
It is natural to draw associations and to speculate what these findings represent relative to what is known in the HT-induced model of IUGR. While the HT model of IUGR closely simulates many of the features seen in the human IUGR fetus, the model is limited by a long gestation, epitheliochorial placentation, and expense. Additional characteristics of this model can be found in a review by Regnault et al. [Citation2003]. Several characteristics of this IUGR model suggest marked disruption of normal vascular development and dysregulation. This is reflected by a variety of molecular and physiologic parameters such as increased umbilical artery Doppler velocimetry (e.g. increased impedance to placental blood flow) and fetal hypertension [Galan et al. Citation2005; Citation1998]. These physiologic findings are reconciled when viewing the abnormal vascular casts of the villous tree in this model [Regnault et al. Citation2002b]. VEGF, eNOS, and Angiopoitein 2 abnormalities in the cotyledon provide molecular support for abnormal vascular development and function [Arroyo et al. Citation2006; Galan et al. Citation2001; Hagen et al. Citation2005; Regnault et al. Citation2003]. The results of the current study when taken collectively with those of others provide indirect evidence that shear stress may play a role in vasoregulation in this model of IUGR. Shear stress or the frictional force produced by blood flow is known to modulate physiological and pathological processes in the cells. Some of the processes regulated by shear stress are vascular tone, vessel remodeling, hemostasis, and others [Boyd et al. Citation2003]. Shear stress induces eNOS, mRNA, and protein expression in endothelial cells [Ranjan et al. Citation1995; Uematsu et al. Citation1995]. Induction of genes by shear stress in endothelial cells is mediated, in part, by ERK and AKT. Western blot analysis of the near-term cotyledon tissues showed a significant increase for both p-ERK and p-AKT in PI-IUGR animals, and shear stress has been shown to be a regulator of eNOS activity, p-ERK, and p-AKT proteins. This pattern of increased p-AKT and p-ERK, as markers of shear stress, is consistent with the increase in eNOS protein content in the cotyledon of HT IUGR pregnancies [Arroyo et al. Citation2006; Regnault et al. Citation1999].
Our previous placental and umbilical and uterine vessel eNOS studies [Regnault et al. Citation2002a; Citation2002b] lead to the investigation of the activation of ERK and AKT in an ovine model of IUGR. The ERK pathway is involved in cellular proliferation and differentiation in the cells and this protein is phosphorylated during shear stress in endothelial cells [Azuma et al. Citation2001; Citation2000; Sumpio et al. Citation2005]. AKT protein is similarly phosphorylated by shear stress in endothelial cells [Dimmeler et al. Citation1998; Kudo et al. Citation2000; Wedgwood et al. Citation2003; Wyatt et al. Citation2004). Dimmeler et al. [Citation1998] demonstrated that eNOS activation is mediated by the phosphorylation of AKT in cultured endothelial cells, thus demonstrating a link between eNOS and AKT activation [Dimmeler et al. Citation1998]. Although an association between eNOS, NO, and p-AKT and p-ERK in IUGR has not been previously shown they collectively serve as surrogate markers of shear stress in this model of PI-IUGR with umbilical artery Doppler abnormalities and fetal systemic hypertension. We also recognize that there could be several mechanisms that regulate eNOS and the ERK and AKT signaling. In vitro pharmacologic studies are underway to better understand potential mechanisms for the altered shear stress and vasoreactivity suspected in this model of IUGR.
MATERIALS AND METHODS
Animal Care
This study was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. Mixed-breed (Columbia-Rambouillet) ewes with time-dated singleton pregnancies (total of 16 animals) were used for this study. Ewes were exposed to environmental conditions as previously described [Regnault et al. Citation1999; Thureen et al. Citation1992] and consisting of maintaining the temperature at 40oC for 12 h during the day and decreased to 35oC at night and the humidity kept between 35% and 40%. Animals were separated into two groups based on length of HT exposure and gestational age at necropsy. In the first group, four ewes were housed in the environmental chamber for 55 days beginning at 40 dGA (term=147 days) and four ewes were housed at ambient temperature (20±2°C) to serve as controls. These animals underwent necropsy at 95 days of gestation (dGA; mid-gestation). In the second group, four ewes were exposed to HT conditions for 80 days (near-term) and were removed to control conditions at approximately 120 days gestation. An additional four ewes were kept at ambient temperature to use as controls. All animals in this group were euthanized at 130 dGA. Ewes were pair-fed and offered water ad libitum. At the time of laparotomy and hysterotomy prior to maternal and fetal euthanasia, blood was drawn and collected by a 20g needle and 10cc syringe directly from the uterine and umbilical vessels. Following euthanasia, fetal and placentome weights were recorded. The placentomes were separated into cotyledon (fetal) and caruncle (maternal) components, and frozen in liquid nitrogen for western blot analysis. Uterine artery and umbilical vessels were excised and dissected carefully from each other and frozen in liquid nitrogen for protein analysis.
RNA Extraction and cDNA Synthesis
RNA was extracted from the collected tissues using the TRI REAGENT method. A total of 100 mg of tissues were homogenized in 1 ml of TRI REAGENT (Sigma, Saint Louis, MO, USA). Samples were centrifuged, chloroform extracted, precipitated, and washed in cold 75% ethanol. Samples were purified using a Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA, USA). cDNA was produced through reverse transcription using the First-Strand cDNA Synthesis protocol of SuperScript III (Invitrogen, Carlsbad, CA, USA). Total RNA (5 μg) was mixed with 50 μM of oligo (dT) primers, 10 mM dNTP mix and DEPC-treated water. Samples were incubated at 65°C for 5 min and then placed on ice for at least 1 min. Ten μl of cDNA synthesis mix (10× RT buffer, 25 mM MgCl2, 0.1 M DTT, RNase out and SuperScript III RT) was added to each sample and then incubated at 50°C for 60 min. Reactions were terminated by incubation at 70°C for 15 min. RNase H (1 μl) was added to each sample and then incubated at 37°C for 20 min. Samples were stored at −20°C until needed.
Real Time PCR
Quantitative Real Time Syber Green PCR was used to quantify eNOS mRNA. Each cDNA (10ng) was subject to real time PCR using our Ovine eNOS forward (5′-TGC ATG ACA TTG AGA GCA AAG GGC-3′) and ovine eNOS reverse (5′-ATG TCC TCG TGA TAG CGT TGC TGA-3′), and compared to a standard curve generated by known quantities of eNOS cDNA to determine starting quantity. To normalize our eNOS data, sample cDNA were subjected to real-time PCR using primers (forward 5′-TCA ACC AGG TGG AGA TCA ACG-3′ and reverse 5′-TGC TTT ACG GGC TTG TAG GTG-3′) for ribosomal protein S15, and a standard curve of known quantities of S15 cDNA. The amplification efficiencies were 98% and 99% for eNOS and S15, respectively. All results were expressed as the ratio of the eNOS value by the S15 control.
Western Blot Analysis
Vessel, cotyledon, and caruncle tissues were homogenized in protein lysis buffer and proteins were separated on a 10% SDS-PAGE and subject to eNOS western blot analysis as previously described by Arroyo et al. [Citation2006]. Western blot was also performed using antibodies against rabbit p-ERK, ERK, p-AKT, or AKT (dilution of 1:500; Cell signalling, Dancers, MA, USA). Caruncles and cotyledons were not assessed for eNOS protein as it has been previously published [Aramburu et al. Citation2006; Galan et al. Citation2001]. A secondary anti-rabbit IgG-HRP antibody (dilution 1:5,000; Cell signalling, Dancers, MA, USA) was incubated for 1 h at room temperature. The membranes were rinsed and incubated with chemiluminescent substrate (Pierce, Rockford, IL, USA) for 5 min. The emission of light was detected using X-ray film. Each membrane was stripped of antibodies and reprobed utilizing antibody against mouse beta-actin (dilution 1:4,000; MP Biomedicals, Aurora, OH, USA) to confirm loading consistencies in each lane. Presence of these proteins was confirmed by densitometry and quantified. Results were compared to the untreated controls.
NO Determination
Uterine artery and umbilical vessel blood samples were collected prior to euthanization. Briefly, as suggested by the manufacturer (Assay Designs, Ann Arbor, MI, USA) blood samples were ultrafiltrated through a 10,000 MWCO filter. After filtration, a solution of NADH was added to the samples followed by the addition of the Nitrate Reductase solution. Samples were incubated at 37°C for 30 min. After incubation, the Griess reagents were added and samples were incubated for 10 min at room temperature. The optical densities of the samples were determined at 540–570 nm.
Statistical Analysis
Comparisons of the following end-points were made between control and PI-IUGR pregnancies: fetal and placental weights, eNOS mRNA and protein concentration, NO production and p-ERK and p-AKT western blot analysis. Treatment effects were determined using Mann-Whitney test with p<0.05 considered significant.
ACKNOWLEDGMENTS
We thank Bradley Ziebell for the technical assistance he rendered. This study was supported by NIH grant R01 HL071990.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Ain, R., Canham, L. N. and Soares, M. J. (2005) Dexamethasone-induced intrauterine growth restriction impacts the placental prolactin family, insulin-like growth factor-II and the Akt signaling pathway. J Endocrinol 185:253–63.
- Aramburu, J., Drews-Elger, K., Estrada-Gelonch, A., Minguillon, J., Morancho, B., Santiago, V. and Lopez-Rodriguez, C. (2006) Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol 72:1597–1604.
- Arroyo, J. A., Anthony, R. V., Parker, T. A. and Galan, H. L. (2006) Differential expression of placental and vascular endothelial nitric oxide synthase in an ovine model of fetal growth restriction. Am J Obstet Gynecol 195:771–777.
- Azuma, N., Akasaka, N., Kito, H., Ikeda, M., Gahtan, V., Sasajima, T. and Sumpio, B. E. (2001) Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol 280:H189–H197.
- Azuma, N., Duzgun, S. A., Ikeda, M., Kito, H., Akasaka, N., Sasajima, T. and Sumpio, B. E. (2000) Endothelial cell response to different mechanical forces. J Vasc Surg 32:789–794.
- Bell, A. W., McBride, B. W., Slepetis, R., Early, R. J. and Currie, W. B. (1989) Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J Anim Sci 67:3289–3299.
- Bell, A. W., Wilkening, R. B. and Meschia, G. (1987) Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol 9:17–29.
- Bernstein, I. M., Horbar, J. D., Badger, G. J., Ohlsson, A. and Golan, A. (2000) Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 182:198–206.
- Block, B. S., Llanos, A. J. and Creasy, R. K. (1984) Responses of the growth-retarded fetus to acute hypoxemia. Am J Obstet Gynecol 148:878–885.
- Boyd, N. L., Park, H., Yi, H., Boo, Y. C., Sorescu, G. P., Sykes, M. and Jo, H. (2003) Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol 285:H1113–H1122.
- Dimmeler, S., Assmus, B., Hermann, C., Haendeler, J. and Zeiher, A. M. (1998) Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res 83:334–341.
- Galan, H. L., Anthony, R. V., Rigano, S., Parker, T. A., de Vrijer, B., Ferrazzi, E., Wilkening, R. B. and Regnault, T. R. (2005) Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 192:272–279.
- Galan, H. L., Ferrazzi, E. and Hobbins, J. C. (2002) Intrauterine growth restriction (IUGR): biometric and Doppler assessment. Prenat Diagn 22:331–337.
- Galan, H. L., Hussey, M. J., Barbera, A., Ferrazzi, E., Chung, M., Hobbins, J. C. and Battaglia, F. C. (1999a) Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol 180:1278–1282.
- Galan, H. L., Hussey, M. J., Chung, M., Chyu, J. K., Hobbins, J. C. and Battaglia, F. C. (1998) Doppler velocimetry of growth-restricted fetuses in an ovine model of placental insufficiency. Am J Obstet Gynecol 178:451–456.
- Galan, H. L., Jozwik, M., Rigano, S., Regnault, T. R., Hobbins, J. C., Battaglia, F. C. and Ferrazzi, E. (1999b) Umbilical vein blood flow determination in the ovine fetus: comparison of Doppler ultrasonographic and steady-state diffusion techniques. Am J Obstet Gynecol 181:1149–1153.
- Galan, H. L., Regnault, T. R., Le Cras, T. D., Tyson, R. W., Anthony, R. V., Wilkening, R. B. and Abman, S. H. (2001) Cotyledon and binucleate cell nitric oxide synthase expression in an ovine model of fetal growth restriction. J Appl Physiol 90:2420–2426.
- Giles, W. B., Trudinger, B. J., Stevens, D., Alexander, G. and Bradley, L. (1989) Umbilical artery flow velocity waveform analysis in normal ovine pregnancy and after carunculectomy. J.Dev.Physiol 11:135–138.
- Hagen, A. S., Orbus, R. J., Wilkening, R. B., Regnault, T. R. and Anthony, R. V. (2005) Placental expression of angiopoietin-1, angiopoietin-2 and tie-2 during placental development in an ovine model of placental insufficiency-fetal growth restriction. Pediatr Res 58:1228–1232.
- Jo, H., Sipos, K., Go, Y. M., Law, R., Rong, J. and McDonald, J. M. (1997) Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J.Biol.Chem. 272:1395–1401.
- Joss-Moore, L. A. and Lane, R. H. (2009) The developmental origins of adult disease. Curr Opin Pediatr 21:230–234.
- Kingdom, J., Huppertz, B., Seaward, G. and Kaufmann, P. (2000) Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol 92:35–43.
- Kudo, T., Izutsu, T. and Sato, T. (2000) Telomerase activity and apoptosis as indicators of ageing in placenta with and without intrauterine growth retardation. Placenta 21:493–500.
- Miller, S. L., Loose, J. M., Jenkin, G. and Wallace, E. M. (2009) The effects of sildenafil citrate (Viagra) on uterine blood flow and well being in the intrauterine growth-restricted fetus. Am J Obstet Gynecol 200:102 e1–7.
- Molnar, M. and Hertelendy, F. (1992) N omega-nitro-L-arginine, an inhibitor of nitric oxide synthesis, increases blood pressure in rats and reverses the pregnancy-induced refractoriness to vasopressor agents. Am J Obstet Gynecol 166:1560–1567.
- Moore, L. G., Shriver, M., Bemis, L., Hickler, B., Wilson, M., Brutsaert, T., Parra, E. and Vargas, E. (2004) Maternal adaptation to high-altitude pregnancy: an experiment of nature-a review. Placenta 25 (Suppl A): S60–71.
- Myatt, L., Brewer, A. and Brockman, D. E. (1991) The action of nitric oxide in the perfused human fetal-placental circulation. Am J Obstet Gynecol 164:687–692.
- Myatt, L., Eis, A. L., Brockman, D. E., Kossenjans, W., Greer, I. and Lyall, F. (1997) Inducible (type II) nitric oxide synthase in human placental villous tissue of normotensive, pre-eclamptic and intrauterine growth-restricted pregnancies. Placenta 18:261–268.
- Pallares, P. and Gonzalez-Bulnes, A. (2008) Intrauterine growth retardation in endothelial nitric oxide synthase-deficient mice is established from early stages of pregnancy. Biol Reprod 78:1002–1006.
- Ranjan, V., Xiao, Z. and Diamond, S. L. (1995) Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol 269:H550–H555.
- Regnault, T. R., de Vrijer, B. and Battaglia, F. C. (2002a) Transport and metabolism of amino acids in placenta. Endocrine 19:23–41.
- Regnault, T. R., de Vrijer, V. B., Galan, H. L., Davidsen, M. L., Trembler, K. A., Battaglia, F. C., Wilkening, R. B. and Anthony, R. V. (2003) The relationship between transplacental O2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J.Physiol 550:641–656.
- Regnault, T. R., Galan, H. L., Parker, T. A. and Anthony, R. V. (2002b) Placental development in normal and compromised pregnancies—a review. Placenta 23 (Suppl A):S119–129.
- Regnault, T. R., Orbus, R. J., Battaglia, F. C., Wilkening, R. B. and Anthony, R. V. (1999) Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency. J Endocrinol 162:433–442.
- Reynolds, L. P. and Redmer, D. A. (2001) Angiogenesis in the placenta. Biol Reprod 64:1033–1040.
- Sankaran, S. and Kyle, P. M. (2009) Aetiology and Pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol 6:765–777.
- Schober, M. E., McKnight, R. A., Yu, X., Callaway, C. W., Ke, X. and Lane, R. H. (2009) Intrauterine growth restriction due to uteroplacental insufficiency decreased white matter and altered NMDAR subunit composition in juvenile rat hippocampi. Am J Physiol Regul Integr Comp Physiol 296: R681–R692.
- Skarsgard, E. D., Amii, L. A., Dimmitt, R. A., Sakamoto, G., Brindle, M. E. and Moss, R. L. (2001) Fetal therapy with rhIGF-1 in a rabbit model of intrauterine growth retardation. J Surg Res 99:142–146.
- Sumpio, B. E., Yun, S., Cordova, A. C., Haga, M., Zhang, J., Koh, Y. and Madri, J. A. (2005) MAPKs (ERK1/2, p38) and AKT can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (CD31) in vascular endothelial cells. J Biol Chem 280:11185–11191.
- Thaete, L. G., Dewey, E. R. and Neerhof, M. G. (2004) Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J Soc Gynecol Investig 11:16–21.
- Thureen, P. J., Trembler, K. A., Meschia, G., Makowski, E. L. and Wilkening, R.B. (1992) Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol 263:R578–R585.
- Tseng, H., Peterson, T. E. and Berk, B. C. (1995) Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res 77:869–878.
- Turner, A. J. and Trudinger, B. J. (2009) A modification of the uterine artery restriction technique in the guinea pig fetus produces asymmetrical ultrasound growth. Placenta 30:236–240.
- Uematsu, M., Ohara, Y., Navas, J. P., Nishida, K., Murphy, T. J., Alexander, R. W., Nerem, R. M. and Harrison, D. G. (1995) Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol 269:C1371–C1378.
- Umar, S. and van der Laarse, A. (2009) Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem [Epub ahead of print].
- Wedgwood, S., Mitchell, C. J., Fineman, J. R. and Black, S. M. (2003) Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am J Physiol Lung Cell Mol Physiol 284:L650–L662.
- Wyatt, A. W., Steinert, J. R. and Mann, G. E. (2004) Modulation of the L-arginine/nitric oxide signalling pathway in vascular endothelial cells. Biochem Soc Symp 71:143–156.
- Yallampalli, C. and Garfield, R. E. (1993) Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol 169:1316–1320.