Abstract
Semen parameters were evaluated by a variety of tests. A polarizing microscope (PM) which allows, in a single step, for the evaluation of the number, motility, viability of sperm using the phenomenon of birefringence was used. This approach avoids a Papanicolaou staining procedure modified for sperm (PAP) performed in fixed material. The aim of this study was to examine the birefringence of sperm structures, in live cells, for the direct analysis of morphology on fresh samples. Semen samples from 15 men of recently proven fertility and from 66 male patients who attended our center for semen analysis were examined by polarization microscope (PM) analysis in order to find an index representing a pool of sperm with normal morphology and progressive motility. The receiver operating characteristics (ROC) curves were used to determine the performance of the proposed diagnostic method. The difference between the two areas under the ROC curves (PAP=0.76 and PM=0.82) was quantitatively not significant (P=0.308); however, the curve of the PM method was always higher than the curve of PAP, revealing that, qualitatively, PM was more sensitive than PAP. The PM index can represent the percentage of motile sperm with normal morphology, which is the actual pool of sperm that can reach and fertilize the oocyte. We suggest a PM diagnostic cut-off value of 20%, since this value was the lowest found for individuals of proven fertility.
| Abbreviations | ||
| PM: | = | polarizing microscope |
| PAP: | = | Papanicolau staining |
| WHO: | = | World Health Organization |
| CASA: | = | computer assisted semen analyzer |
| ICSI: | = | intracytoplasmic sperm injection |
| ROC: | = | receiver operating characteristics |
INTRODUCTION
A significant percentage of human infertility can be ascribed to the male partner. However, the problem of semen evaluation seems to be far from solution. The minimum requirements for semen analysis and semen parameter standards were established in 1951 by the American Fertility Association [Abarbanel et al. Citation1951]. The first semen parameter utilized, the sperm count, has been found to be insufficient [Seibel and Zilberstein Citation1995] because of the absence of any qualitative information and due to a discrepancy between sperm concentration and fertilizing ability. Moreover, natural, day to day variations occur in spermatozoa concentration [Huszar et al. Citation1988]. Other parameters subsequently used were motility, that are now typically evaluated using computerized systems [Barratt et al. Citation1993; Barrat Citation1995; De Geyter et al. Citation1995], sperm penetration [Yanagimachi et al. Citation1976], acrosomal function [Kennedy et al. Citation1989; De Jonge et al. Citation1993; Bartoov et al. Citation1994; Yang et al. Citation1994; Parinaud et al. Citation1995], and/or biochemical characterization [Huszar et al. Citation1994].
The ‘strict criteria’ of the evaluation of sperm morphology, performed by light microscopy and automatically computerized [Kruger et al. Citation1993], is particularly interesting. An effort to standardize in vivo thresholds of semen parameters, including the World Health Organization [WHO] and strict morphological criteria in a fertile and subfertile population, has been described [Menkveld et al. Citation2001]. Computer-assisted semen analysis (CASA) has also been used to help evaluate sperm morphology and motility [Graves et al. Citation2005]. However, CASA technology has been criticized due to difficulties in achieving optimum set-up procedures and because of operational difficulties [Agarwal et al. Citation2003]. It is clear that the analysis of a single sperm feature or function may not provide enough information for a proper evaluation of a semen sample.
The current WHO [Citation1999] guidelines suggest that a sperm concentration ≥20×106, rapid (a) and slow (b) progressive motility (a+b) ≥50%, and the presence of ≥30% of sperm with normal form [WHO Citation1992], evaluated by PAP criteria, are consistent with normal semen quality. Sperm viability is reflected in the proportion of sperm that are ‘alive’ as determined by either dye exclusion or hypo-osmotic swelling; >50% of living cells is considered to be compatible with normality [WHO Citation1999]. The assessment of sperm viability is particularly important in samples in which many sperm are immotile, in order to distinguish between dead immotile or viable immotile sperm.
In this applications note we describe our experience concerning the use of the polarizing microscope (PM) for semen evaluation. This method presents considerable advantages, such as the possibility to analyze, in a single step, the motility, the viability,the morphology, and obviously the concentration of the sample, avoiding the staining of cells smeared on a slide. As reported by Baccetti [Citation2004], viable human spermatozoa are naturally birefringent, whereas in pathological conditions, dead, necrotic spermatozoa are devoid of birefringence due to the absence of conventional sperm texture.
In this study, we analyzed 15 semen samples from men of proven fertility and 66 consecutive semen samples from patients recruited at our Center (Interdepartmental Centre for Research and Therapy of Male Infertility; University of Siena, Siena, Italy). Semen analysis was performed following WHO [Citation1999] guidelines and by PM to compare these methods of semen evaluation towards developing a diagnostic cut off value for the PM method.
Sample Preparation and Analysis
Semen samples were obtained from 15 men of proven fertility (aged 22 to 40 y) with normal karyotype and without anatomical problems and infections. These fertile men had fathered one or more children during the past two years. At the same time, 66 consecutive semen samples from male patients (aged 26 to 41 y) recruited at the Interdepartmental Centre for Research and Therapy of Male Infertility were evaluated. In this group of patients, some men attended our Center for infertility problems and others just to assess their fertility status. Semen evaluation was performed by PM and by light microscope following WHO [Citation1999] guidelines. All donors and patients gave their informed consent to the research.
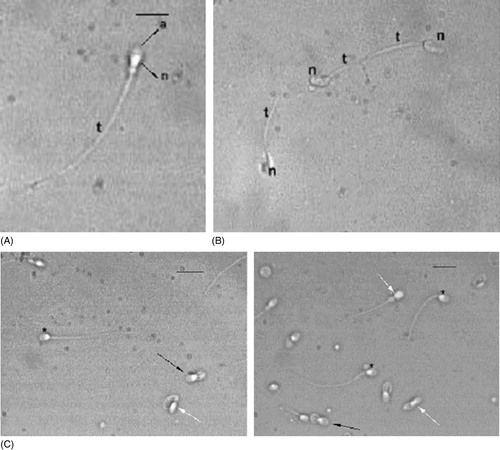
Semen samples were collected by masturbation after 4 d of sexual abstinence and examined after liquefaction for 30 min at 37°C. Volume, pH, sperm concentration, and motility were quantified according to WHO [Citation1999] guidelines. A Burker counting chamber was used for performing the analysis. Sperm morphology was assessed by the Papanicolaou test modified for spermatozoa (PAP). Ejaculated human spermatozoa were washed two times in PBS buffer, smeared on pre-cleaned glass slides, air dried, and fixed in an equal volume of ethanol 95% and ether, for 5–15 min. The fixed slides were stained using the protocol outlined in the WHO [Citation1992] guidelines. An ejaculate was considered with normal sperm morphology if at least 30% of sperm exhibited normal morphology. A sperm was considered abnormal if at least one organelle among acrosome, nucleus, or flagellum was present in anomalous form. Two hundred sperm were evaluated from each sample using a Leitz Aristoplan microscope (Leica, Wetzlar, Germany) with 63× bright field objective and a 10× ocular. The same studied semen samples were analyzed in the Burker counting chamber using a polarizing microscope (63× objective and a 10× ocular, Leica Microsystems DM EP). Spermatozoa were categorized following the different motility grades (rapid and slow progressive motility, nonprogressive motility, immotile sperm), the viability and the morphology were assessed by the natural birefringence of nucleus and flagellum. Normal sperm nucleus and normal tail show positive anisotropy according to Wiener [Citation1912]. Scores were collected and the sperm concentration was evaluated with optimum calculation for the Burker chamber. A normal progressively motile spermatozoon examined using the PM method, shows a non luminous acrosome and a luminous and normal sized compact nucleus and flagellum (). The non birefringent acrosome is identified since it is not bright () and therefore its presence or absence (), its shape, its dimension, and the possible presence of vacuoles are easily assessed. The subacrosomal region, not covered by the acrosome, is uniformly birefringent in normal spermatozoa ( and ), since the chromatin probably shows a normal structure. Therefore, when a nucleus is well shaped but not birefringent, it is considered to be anomalous because the chromatin has lost its typical nucleoprotein texture. A totally birefringent sperm head is devoid of the acrosome, which could be reacted or absent (). The tail of viable, motile spermatozoa must show positive birefringence, reflecting the microtubular organization of the axoneme. The normal mitochondrial helix must also be birefringent, because it is characterized by a particular filamentous texture (). When an organelle loses its typical structure, the birefringence disappears and the spermatozoon is considered dead (). One of the main advantages of the PM method is the possibility to discriminate the immotile but birefringent spermatozoa, since they could be recovered for possible use in intracytoplasmic sperm injection (ICSI), as they are living cells with a normal structure but without motility.
Figure 1 Spermatozoa observed using the PM. (A) Normal cell shows a well shaped acrosome (a) covering the anterior part of the head. It is not birefrigent. The region of the nucleus devoid of acrosome is birefringent (n) as is the entire tail (t). Bar 12.7 μm. (B) Dead abnormal spermatozoa observed. The heads (n) and the tails (t) are not birefringent. (C) Examples of different sperm populations, observed. Normal spermatozoa (*), spermatozoa with normal acrosomes and nuclei but rolled up axonemes (black arrows), spermatozoa with altered nuclei and reduced or absent acrosomes (white arrows) are shown in the bottom panels. Bar 18 μm.
The PM evaluation allows for obtaining a PM index expressing the percentage of sperm with normal morphology and with progressive motility. These two parameters can be evaluated at the same time in the same spermatozoon without staining. Birefringence clearly identifies the various organelles, due to their different response to polarized light. Semen parameters from 15 men of proven fertility are reported in , along with the PM index. The lowest observed PM index was 20% in patients 7 and 10. Semen characteristics of 66 patients are reported in . Among them 13 exhibit normal semen parameters according to WHO guidelines; sperm concentrations ranged between 0.8×106 to 200×106 (52 men showed normal sperm concentration) and sperm progressive motility (a+b) from 6% to 60% (15 men showed normal motility). By the PAP method [WHO Citation1999], 44 of the 66 patients showed a normal sperm morphology (≥30%). The PM index ranged from 4% to 50%.
Table 1 Spermiogram Data of Men of Proven Fertility.
Table 2 Semen Parameters of 66 Consecutive Patients.
Sperm viability was evaluated in 26 of 66 patients examined in order to explore if the PM method, compared with eosin staining, is able to correctly discriminate viable from dead spermatozoa. Viability was assessed by 0.5% eosin Y staining in 0.9% NaCl. A drop of fresh semen was mixed with a drop of eosin solution on a microscope slide covered with a cover slip and examined after 30 s with a Leitz Aristoplan microscope (63X objective, 10X ocular). These slides were assessed immediately. Viable spermatozoa were unstained (white) and dead cells were stained (red). Two hundred sperm were evaluated from each sample. The viability was evaluated in the same samples also by PM, using birifringent properties of sperm cells. The results from the eosin staining and PM, reported in , were compared using the Bland-Altman method (ninety-five percent confidence intervals were computed), which revealed no differences between the two methods. The values obtained by eosin and PM showed a random distribution with a mean of differences between the two methods of 1.52±1.96 (SD 1.92) (lower limit −2.24; upper limit 5.28).
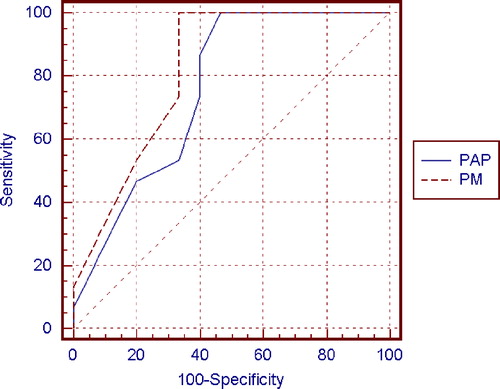
The receiver operating characteristics (ROC) curve procedure () was undertaken to describe the accuracy of the two methods in terms of the relationship between sensitivity and specificity. A modification of the Wilcoxon rank sum statistic was used to determine whether the two methods were significantly different. A curve was produced for the percentages of normal sperm morphology evaluated by the PAP method in 15 fertile controls and in 15 random sampled patients (, in bold). Sample size remained constant in order to obtain diagnostic accuracy [Zweig and Campbell Citation1993]. Another curve was calculated for the percentage of normal sperm morphology evaluated by the PM method in the same 30 individuals. Certified MedCalc 8.0 statistical programs were used for these analyses. The PM cut-off point was determined by interpolation from the point of intersection of the lines of specificity and sensitivity. The overall performance of the ROC test was quantified by computing the area under each curve. The difference between the two areas under the ROC curves (PAP=0.76, confidence interval 0.57–0.89 and PM=0.82, confidence interval 0.63–0.93) was not quantitatively significant (P=0.308); however, the curve of the PM method was always higher than the curve of PAP (), revealing that PM is qualitatively more sensitive than PAP. The cut-off value for the PAP method was 36% (sensitivity 100%; specificity 53.3) and for the PM method the cut-off value was 24% (sensitivity 100%; specificity 67.7).
CONCLUSIONS
The standardization and quality control of basic semen analysis requires an easy and reliable technique. The most widely used parameters for semen evaluation are sperm concentration, motility, and morphology examined by typical staining procedures and, when necessary, viability estimated by eosin or eosin-nigrosin. In this study the natural birefringence of spermatozoa, detectable under polarized light, was used to assess the sperm morphology and viability in living cells, directly in the counting cell chamber (Burker chamber), avoiding PAP staining performed in fixed material. The analysis of the various sperm regions can be performed using the differential brightness instead of the efficacy of staining. Sperm birefringence is a typical property of living cells and it is lost in dead cells. To verify whether this method would also be able to correctly identify dead and viable sperm we carried out the eosin test in 26 semen samples and the data obtained confirmed the reliability of PM technique. With this tool we can gather more information and thus increase the rigor of the analysis. The PM index includes the percentage of motile and well shaped sperm that perhaps shows the real fertilizing potential. Motility and morphology are evaluated contemporaneously in the same cell. Conversely, since PAP analysis requires fixation, motility is not considered.
We enrolled a group of men of proven fertility in order to test the reliability of the proposed method. Our aim was to define the lowest value of the PM index as a measure of fertility. This is 20%. The group of fertile men is numerically small but representative, and this must be considered. We then applied the PM method to 66 consecutive semen samples from men referred to our center for infertility to assess fertility status. In order to determine the performance of the diagnostic methods in terms of sensitivity, specificity, and predictive values, ROC curves were used. The higher accuracy of the PM method compared to PAP (0.81 vs. 0.76) was observed. However this difference was not statistically significant. These data support the view that the PM technique should be considered equivalent to the PAP method and used to perform semen analysis.
Applying the ROC curve to values obtained by the two methods used on semen samples from 15 fertile patients and 15 randomly sampled patients, we established a cut-off value for the PM method of 24% and a value of 36% for the PAP method. In our samples the obtained value of PAP was 36%, higher compared to the 30% suggested by WHO [1992]. Because the PM index was 24% in the group examined, we suggest a diagnostic PM cut-off value of 20% since this was the lowest value found in individuals of proven fertility.
We propose the use of natural sperm birefringence as an alternative method to evaluate sperm morphology and viability. The PM index represents the percentage of motile sperm with normal morphology. Attempts to provide semen quality scores able to summarize meaningful information on the quality of semen specimens were proposed by Agarwal et al. [Citation2003] that have already characterized 2 scores developed by a principal component analysis of semen characteristics.
For potential clinical application, the PM index may be more helpful in assisted reproductive technologies, when sperm concentration may not be as essential as the other parameters. In particular, Baccetti [Citation2004] proposed the application of PM to the ICSI technique, introducing polarizing and analyzing lenses in an inverted microscope model. This idea was further developed and sperm head birefringence characteristics have been reputed to be a new criterion for sperm selection in ICSI [Gianaroli et al. Citation2008] allowing to even verify that sperm with reacted acrosome are more prone to support the development of viable embryos [Gianaroli et al. Citation2010].
We are aware that the examined patient group is small and that the definitive evaluation of this system needs more extensive use in other laboratories in order to compare and standardize the results. However, we are confident in proposing the PM method, which could be very useful in semen samples with immotile but viable spermatozoa, as it can easily identify living cells by highlighting different birefringent properties.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Abarbanel, A. R., Brown, W. E., Greulich, W. W. et al. (1951) Evaluation of the barren marriage. Minimal procedures. Fertil Steril 2:1–14.
- Agarwal, A., Sharma, R. K. and Nelson, D. R. (2003) New semen quality scores developed by principal component analysis of semen characteristics. J Androl 24:343–352.
- Baccetti, B. (2004) Microscopical advances in assisted reproduction. J Submicrosc Cytol Pathol 36:333–339.
- Barratt, C. L., Tomlinson, M. J. and Cooke, I. D. (1993) Prognostic significance of computerized motility analysis for in vivo fertility. Fertil Steril 60:520–525.
- Barratt, C. L. (1995) On the accuracy and clinical value of semen laboratory tests. Hum Reprod 10:250–252.
- Bartoov, B., Reichart, M., Eltes, F., Lederman, H. and Kedem, P. (1994) Relation of human sperm acrosin activity and fertilization in vitro. Andrologia 26:9–15.
- Boyle, C. A., Khoury, M. J., Katz, D. F., Annest, J. L., Kresnow, M. J., De Stefano, F. and Schrader, S. M. (1992) The relation of computer-based measures of sperm morphology and motility to male infertility. Epidemiology 3:239–246.
- Chan, P. J., Corselli, J. U., Jacobson, J. D., Patton, W. C. and King, A. (1996) Correlation between intact sperm acrosome assessed using the Spermac stain and sperm fertilizing capacity. Arch Androl 36:25–27.
- De Geyter, C., De Geyter, M., Selter, S., Schneider, H. P. G. and Nieschlag, E. (1995) Prospective evaluation of computerized analysis of sperm motion for the prediction of sperm function in IVF. Abstract 095, 11th Annual Meeting ESHRE, Hamburg, DE pp. 48–49.
- De Jonge, C. J., Tarchala, S. M., Rawlins, R. G., Binor, Z. and Radwanska, E. (1993) Acrosin activity in human spermatozoa in relation to semen quality and in-vitro fertilization. Hum Reprod 8:253–257.
- Gianaroli, L., Magli, M. C., Collodel, G., Moretti, E., Ferraretti, A. P. and Baccetti, B. (2008) Sperm head's birefringence: A new criterion for sperm selection. Fert Steril 90:104–112.
- Gianaroli, L., Magli, M. C., Ferraretti, A. P., Crippa, A., Lappi, M., Capitani, S. and Baccetti, B. (2010) Birefringence characteristics in sperm heads allow for the selection of reacted spermatozoa for intracytoplasmic sperm injection. Fertil Steril 93:807–813.
- Graves, J. E., Higdon, H. L. 3rd, Boone, W. R. and Blackhurst, D. W. (2005) Developing techniques for determining sperm morphology in today's andrology laboratory. J Assist Reprod Genet 22:219–225.
- Huszar, G., Vigue, L. and Corrales, M. (1988) Sperm creatine phosphokinase activity as a measure of sperm quality in normospermic, variablespermic, and oligospermic men. Biol Reprod 38:1061–1066.
- Huszar, G., Vigue, L. and Oehninger, S. (1994) Creatine kinase immunocytochemistry of human sperm-hemizona complexes: Selective binding of sperm with mature creatine kinase-staining pattern. Fertil Steril 61:136–142.
- Kennedy, W. P., Kaminski, J. M., Van der Ven, H. H., Jeyendran, R. S., Reid, D. S., Blackwell, J., et al. (1989) A simple, clinical assay to evaluate the acrosin activity of human spermatozoa. J Androl 10:221–231.
- Kruger, T. F., Acosta, A. A., Simmons, K. F., Swanson, R. J., Matta, J. F. and Oehninger, S. (1988) Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 49:112–117.
- Kruger, T. F., DuToit, T. C., Franken, D. R., Acosta, A. A., Oehninger, S. C., Menkveld, R. and Lombard, C. J. (1993) A new computerized method of reading sperm morphology (strict criteria) is as efficient as technician reading. Fertil Steril 59:202–209.
- Macleod, I. C. and Irvine, D. S. (1995) The predictive value of computer-assisted semen analysis in the context of a donor insemination programme. Hum Reprod 10:580–586.
- Menkveld, R., Stander, F. S., Kotze, T. J., Kruger, T. F. and van Zyl, J. A. (1990) The evaluation of morphological characteristics of human spermatozoa according to strict criteria. Hum Reprod 5:586–592.
- Menkveld, R., Wong, W. Y., Lombard, C. J., Wetzels, A. M., Thomas, C. M., Merkus, H. M. and Steegers-Theunissen, R. P. (2001) Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: An effort towards standardization of in-vivo thresholds. Hum Reprod 16:1165–1171.
- Parinaud, J., Vieitez, G., Moutaffian, H., Richoilley, G. and Labal, B. (1995) Relevance of acrosome function in the evaluation of semen in vitro fertilizing ability. Fertil Steril 63:598–603.
- Seibel M.M. and Zilberstein M. (1995). The shape of sperm morphology. Hum Reprod 10:247–248.
- Yanagimachi, R., Yanagimachi, H. and Rogers, B. J. (1976) The use of zona-free animal ova as a test-system for the assessment of the fertilizing capacity of human spermatozoa. Biol Reprod 15:471–476.
- Yang, Y. S., Chen, S. U., Ho, H. N., Chen, H. F., Lien, Y. R., Lin, H. R., et al. (1994) Acrosin activity of human sperm did not correlate with IVF. Arch Androl 32:13–19.
- Wiener, O. (1912) Die Theorie des Mischkorpers fur das Feld der stationaren Stomung. Erste abandlung. Die mittel Wertsatze fur Kraft, Polarisation und Energie. 32Bd, I-IV, pp. 509–604.
- World Health Organization (1992) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, Cambridge, UK pp:1–107.
- World Health Organization (1999) WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 4th ed, Cambridge University Press, Cambridge, UK.
- Zweig, M. H. and Campbell, G. (1993) Receiver-Operating Characteristic (ROC) Plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577.