Abstract
Reproductive biology is considered a specialty field, however, an argument can be made that it is instead generally applicable to many fields of biology. The one-cell embryo is presented here as a model system for the study of eukaryotic DNA replication, apoptotic DNA degradation, and signaling mechanisms between the cytoplasm and nucleus. Two unique aspects of this system combine to make it particularly useful for the study of chromatin function. First, the evolutionary pressure that lead to the extreme condensation of mammalian sperm DNA resulted in a cell with virtually inert chromatin, no DNA replication or transcription ongoing in the sperm cell, and all of the cells in a G0 state. This chromatin is suddenly transformed into actively transcribing and replicating DNA upon fertilization. Therefore, the sperm chromatin is poised to become active but does not yet posses sufficient components present in somatic chromatin structure for all these processes. The second unique aspect of this system is that the one cell embryo houses two distinct nuclei, termed pronuclei, through the first round of DNA synthesis. This means the sperm cell can be experimentally manipulated to test the affects of the various treatments on the biological functions of interest. Experimental manipulations of the system have already revealed a certain level of plasticity in the coordination of both the timing of DNA synthesis in the two pronuclei and in the response to cellular signals by each pronucleus involved with the progression through the G1/S checkpoint, including the degradation of DNA in the paternal pronucleus. The fact that two nuclei in the same cytoplasm can undergo different responses infers a level of autonomy in the nuclear control of the cell cycle. Thus, the features of mammalian fertilization can provide unique insights for the normal biology of the cell cycle in somatic cells.
Introduction: fertilization as a unique model for chromatin function
Reproductive biology is usually considered a specialty field but a strong argument can be made that it is, in fact, generally applicable to many other fields in biology. The field has had uniquely important impacts in the area of DNA function. It encompasses a key element of evolution, the mixing of genes from two different organisms to provide greater variety for the progeny. The molecular mechanisms that control this genetic variety are found in meiosis. The unique chromatin structures of the synaptonemal complex, in which the two homologous chromosomes from each pair come together, have provided valuable insight into the structure of mitotic chromosomes. Studies of the mechanisms by which homologous chromosomes trade DNA sequences, i.e., crossing-over, have resulted in important insights into DNA repair, mutation research, and chromosomal translocations in cancer [Liang et al. Citation2010].
One important model reproductive biology has provided to the study of chromatin structure is fertilization. With no detectable transcription, DNA replication, or DNA repair mechanisms, sperm DNA is nearly functionally inert [Yanagimachi Citation1994]. Yet, when this genome is inserted into the oocyte at fertilization, the chromatin is reorganized and all of these processes become activated de novo. This provides two set points for studying chromatin function. The first is the preparatory phase in the condensed sperm nucleus. The chromatin in these cells is not yet actively engaged in any of these processes but must be in a state that is able to participate in any of these pathways upon receiving the correct biochemical signals. The sperm nucleus therefore provides a model to identify the key elements that must be present for biologically viable chromatin. The second is the activation of the events after fertilization. The sperm chromatin thus represents a virgin state of active DNA in the sense that this DNA has not yet been involved in any functional process. This presents an opportunity to examine functions of interest from a different viewpoint than is often available in the somatic cell.
We discuss how our laboratory is using the sperm chromatin model to investigate two important chromatin functions, DNA replication and DNA degradation during apoptosis, and detail key questions in each area of study for which the sperm cell is an ideal model. The main hypothesis of this manuscript is that while many important differences between the one cell embryo and somatic cells exist, there are enough similarities that studying one can shed important insight on the other. One of the best examples of how the study of the reproduction can lead to important discoveries about somatic cell regulation is the discovery of cyclins controlling the cell cycle. Early studies on Xenopus oocytes identified an M-phase promoting factor, or MPF, that controlled the progression of oocytes that were arrested in meitotic prophase to activate the mitotic machinery [Reynhout and Smith Citation1974; Wasserman and Smith Citation1978]. It was later discovered that MPF was composed of CDK and cyclin complexes [Maller et al. Citation1989; Moreno et al. Citation1989a] now known to control the progression of the cell cycle in somatic cell. In 1989, Moreno and colleagues stated that “Considerable advances have been made recently in our understanding of how the cell cycle timing of mitosis is regulated. This has come about because links have been established between two independent areas of research, one based on a genetic approach using the fission yeast Schizosaccharomyces pombe and the second based on a biochemical approach using Xenopus and starfish oocytes [Moreno et al. Citation1989b].” In this review we suggest that similar links between reproductive biology and somatic cell regulation can provide important insights into DNA replication and the degradation of DNA during apoptosis.
Regulation of mammalian DNA replication: the need for licensing
One of the important biological processes that seems to be initiated de novo in sperm DNA is the licensing of origins of replication. Before discussing the role of the sperm model in this process, we will first briefly review licensing. The 6 billion base pairs of the human (and mouse) genome are replicated in the cell with 103 to 105 different start sites, called replication origins, dispersed throughout the genome [Pardoll et al. Citation1980; Vogelstein et al. Citation1980]. In most mouse and human cell lines in culture, the entire genome is replicated completely in a highly coordinated manner during S-phase that lasts an average of 6 h, and each segment is only replicated once. Different parts of the genome can be replicated at independent times [Stubblefield Citation1975], and in different structural foci in the nucleus [Berezney Citation1991; Citation2002]. DNA replication occurs at a fixed site within all cells, from bacteria [Lemon and Grossman Citation1998] to mammals [Berezney and Coffey Citation1975; Jackson and Cook Citation1986; Pardoll et al. Citation1980]. That is, the replication complex remains stationary while the template DNA moves through it during replication (). Because there are so many different replication origins that are replicated at different times, researchers in the field began to question how the cell efficiently prevented any of these origins from firing more than once during S-phase, which would result in ‘rereplicating’ parts of the genome (). Blow and Laskey [Citation1988] proposed that a ‘licensing factor’ binds to DNA replication origins during G1 in the preparation for DNA synthesis, and subsequently provided experimental evidence for its existence [Blow Citation1993; Leno et al. Citation1992]. We now know that licensing is a highly regulated event with the coordinated sequential loading of several different proteins ([Takeda and Dutta Citation2005]; ). Licensing prevents rereplication of any segment of the genome, and failure to license can lead to checkpoint activation that halts DNA synthesis.
Figure 1. DNA is Replicated at a Fixed Site. DNA origins are prepared for replication by licensing (blue circles; Figs. 2 and 3). During replication, DNA is pulled through the replication machinery, which rests at a fixed site on the nuclear matrix. Newly replicated strands of DNA emerge (red) and remain attached to the nuclear matrix at sites that are not licensed for DNA replication (green circles).
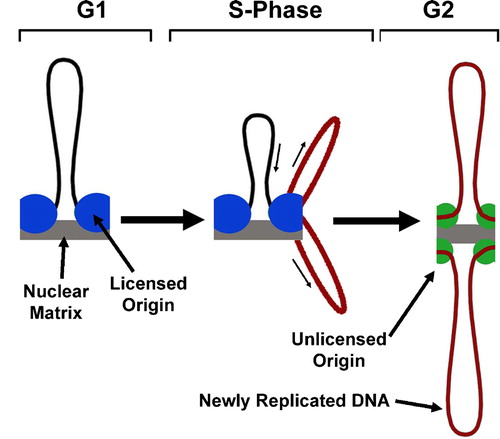
Figure 2. The Need for Licensing in Mammalian DNA Replication. A. DNA is replicated at several hundred thousand independent start sites called origins. B. To ensure that each origin only fires once during S-Phase, each origin is ‘licensed’ by the ORC complex. C. The license is removed when the origin is replicated, and cannot be added to any origins until the next G1 phase.
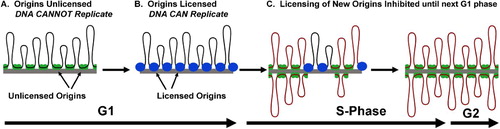
Figure 3. Licensing of Mammalian Replication Origins. A. ORC2L-5L remain on the origin throughout the cell cycle (the fate of ORC6L is currently unknown). B. Licensing begins with the binding of ORC1L, which C recruits CDC6 and CTD1. D. This complex recruits the DNA helicases MCM2-7, and the origin is fully licensed. E. CDK2/CCNA2 and CDK/CCNE1 initiate the entry into S-phase and the removal of ORC1L, CDC6, and CDT1, thereby preventing new licensing. At this point, a number of other proteins are loaded onto the replication fork. Adapted from Takeda and Dutta [Citation2005].
![Figure 3. Licensing of Mammalian Replication Origins. A. ORC2L-5L remain on the origin throughout the cell cycle (the fate of ORC6L is currently unknown). B. Licensing begins with the binding of ORC1L, which C recruits CDC6 and CTD1. D. This complex recruits the DNA helicases MCM2-7, and the origin is fully licensed. E. CDK2/CCNA2 and CDK/CCNE1 initiate the entry into S-phase and the removal of ORC1L, CDC6, and CDT1, thereby preventing new licensing. At this point, a number of other proteins are loaded onto the replication fork. Adapted from Takeda and Dutta [Citation2005].](/cms/asset/8bf2ff44-878e-4cd7-a290-e5f64eb2bace/iaan_a_505679_f0003_b.jpg)
Licensing begins in early G1, soon after mitosis, when the origin recognition complex protein, ORC1L (origin recognition complex, subunit 1–like S. cerevisiae), binds to a complex of five related proteins ORC2L-5L, which in mammals remains bound to the DNA throughout the cell cycle () [DePamphilis Citation2005; Thomae et al. Citation2008]. The ORC complex recruits CDT1 and CDC6, which in turn recruits the MCM2-7 helicase complex. At this stage, the origin is considered licensed, and DNA synthesis can be initiated at this site in S-phase. The CDK2/CCNA2 and CDK2/CCNE1 complexes activate the cell to progress to S phase. At this point, replication begins and all new licensing is inhibited when CDK phosphorylates CDC6 releasing it and CDT1 from the DNA. PCNA and a host of DNA synthesis initiation proteins then bind, and finally DNA polymerase attaches to the origin complex. The MCM2-7 helicase may remain associated with the replication fork during replication. ORC2L-5L remain bound to the origin of replication, and ORC1L cycles off the complex, to return in the next round of DNA synthesis. Recent evidence suggests that some of these same factors are also important for licensing in the mammalian one cell embryo [Borsuk and Czolowska Citation2010].
DNA replication in the mammalian one cell embryo
Mammalian fertilization has one attribute that makes it an excellent model to study DNA replication; the maternal and paternal genomes are segregated into separate nuclei, called pronuclei, until the first mitotic division [Sirlin and Edwards Citation1959]. An important consequence of this segregation is that the two genomes are replicated in separate pronuclei in the same cytoplasm (). After fertilization, the sperm chromatin decondenses to form the male pronucleus, while the metaphase II plate of the oocyte completes division and the maternal chromatin decondenses to form the female pronucleus. Both pronuclei undergo one round of DNA synthesis before the DNA condenses into mitotic chromosomes which join at a single metaphase plate where the paternal and maternal genomes are united for the first time. Several reports have suggested DNA replication in mice begins in the male pronucleus up to 2 h before it does in the female when embryos are generated by normal mating [Bouniol-Baly et al. Citation1997; Ferreira and Carmo-Fonseca Citation1997; Howlett and Bolton Citation1985; Luthardt and Donahue Citation1973], while in IVF this asynchrony was less apparent [Aoki and Schultz Citation1999; Howlett and Bolton Citation1985]. Specifically, Aoki and Schultz [Citation1999] reported that while male and female pronuclear DNA synthesis began synchronously, the male pronucleus completed DNA synthesis 1 h before the female. Our recently published data using ICSI, have not detected any differences in the initiation of DNA replication within 1 h windows [Yamauchi et al. Citation2009].
Figure 4. Experimental Manipulation of Mouse Fertilization to Study DNA Replication and Degradation. A. In fertilization by ICSI or IVF, the female (F) and male (M) pronuclei are fully decondensed by about 4 h, and initiate DNA synthesis simultaneously at about 5.5 h. B. When sperm nuclear halos are injected into ooctyes 3 h after parthenogenetic activation of the oocyte, the male pronucleus initiates DNA synthesis after the female pronucleus. C. When sperm nuclei are treated to induce fragmentation of the paternal genome prior to injection, the male pronuclei develop normally until the initiation of S-phase at which point the DNA is degraded.
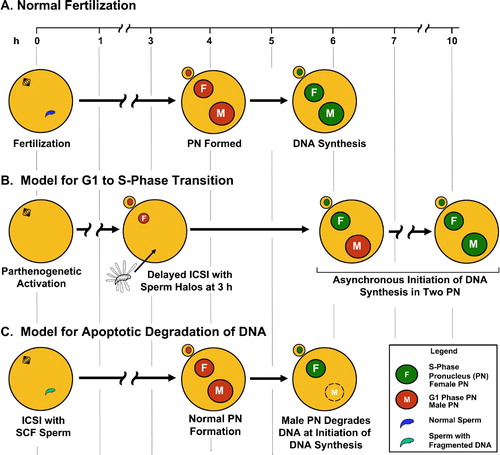
The simultaneous initiation of DNA synthesis in both pronuclei when IVF or ICSI are used suggests that either both nuclei have near identical molecular clocks for the timing of replication or that they both respond to a cytoplasmic signal to initiate DNA synthesis. Mammalian cell free DNA replication systems have revealed that the cytoplasm does exert some control over DNA replication in the nucleus [DePamphilis et al. Citation2006; Takeda and Dutta Citation2005], supporting the latter model. However, the exact molecular mechanisms for this control are not well understood. One possibility is the CDK-cyclin complexes CDK2/CCNA2 and CDK2/CCNE1. At least one study has shown that these complexes shuttle between the cytoplasm and the nucleus [Jackman et al. Citation2002]. Either possibility, complete nuclear autonomy or cytoplasmic control, would have implications for DNA synthesis studies in somatic cells, but the one cell embryo, which contains two nuclei in the same cytoplasm, is the most likely to provide a framework in which to distinguish them.
Experimental manipulation of the initiation of DNA synthesis initiation in mouse pronuclei
While evidence suggests the two pronuclei initiate replication together, there is some flexibility in the timing of DNA synthesis in the one cell embryo. For example, it is possible to experimentally manipulate fertilization such that the male pronucleus initiates DNA replication up to two hours after the female pronucleus. Two laboratories have demonstrated this by injecting mouse oocytes with spermatozoa after parthenogenic activation. In some cases delayed injection of spermatozoa into oocytes resulted in an embryo that does develop to the birth of a live pup [Kishigami et al. Citation2004; Maleszewski et al. Citation1999]. We recently used the same technique to test whether DNA synthesis was initiated at the normal time when the sperm nucleus was injected after oocyte activation [Yamauchi et al. Citation2009]. We found that in some of the oocytes injected with mouse sperm nuclei, the male pronucleus initiated DNA synthesis at least 2 h after the female pronucleus e.g., , except that sperm nuclei were injected at 2 h post activation. See injection of halos below). Asynchronous initiation of two nuclei in the same cytoplasm was also shown by another group by fusing immature oocytes with parthenogenetically activated oocytes [Borsuk and Czolowska Citation2010].
These data suggest that even if the pronuclei normally initiate DNA synthesis together, as discussed above, there are some circumstances in which the two pronuclei can be in different phases, G1 and S, in the same cytoplasm. Understanding the mechanisms that control the plasticity of the cytoplasm, as well as the limits of that plasticity, will have important consequences for models of the cytoplasmic and nuclear communication during the G1/S transition.
The sperm matrix is necessary for DNA replication: evidence that the sperm nucleus provides an origin replication demarcation
Mammalian sperm nuclei are remarkably resistant to mechanical stress, even when most of the DNA binding proteins are extracted with high salt and reducing reagents [Ward et al. Citation1989]. This has allowed for the experimental manipulation of ‘sperm nuclear halos’, which are sperm nuclei that contain only a salt resistant nuclear skeleton termed the nuclear matrix, and naked 50 kb loops of DNA attached at their bases to the skeleton [Choudhary et al. Citation1995; Ward et al. Citation1989]. Mouse sperm halos injected into oocytes can form normal paternal chromosomes [Mohar et al. Citation2002], indicating that one complete round of DNA synthesis is possible when the oocyte uses a sperm halo as the template for paternal DNA replication (). More recently, we have demonstrated that when sperm nuclear halos are treated with restriction endonucleases to remove most of the loop DNA, the remaining DNA associated with the sperm nuclear matrix still acts as a template for DNA synthesis [Shaman et al. Citation2007]. Isolated sperm DNA injected into mouse oocytes does not replicate, nor does DNA reconstituted with isolated sperm nuclear matrices [Shaman et al. Citation2007]. These data demonstrate that it is the in situ chromatin organization of DNA associated with the sperm nuclear matrix that is required for DNA replication in the male pronucleus.
This finding has many implications for the regulation of mammalian DNA replication. First, it supports many older reports that DNA replication occurs on the nuclear matrix in somatic cells [Berezney and Coffey Citation1975; Dijkwel and Hamlin Citation1995; Jackson and Cook Citation1986; Pardoll et al. Citation1980]. Second, the binding of the 6 ORC proteins is resistant to 50 mM KCl, but not to the 2 M NaCl, used to make sperm halos [Rowles et al. Citation1999]. Thus, sperm halos are very unlikely to contain the earliest known origin binding proteins. However, the origins must have been identified by the replication machinery in the oocyte because the DNA was replicated completely. Mammalian replication origins are not determined by DNA sequence, therefore an epigenetic marker for DNA replication origins must be present in sperm nuclear halos to which ORC proteins bind after fertilization.
This suggests the possibility that the sperm nucleus and the events that occur in the paternal chromatin shortly after fertilization may provide the means through which we may answer one of the most perplexing questions in the field regarding mammalian origin of replication. Replication in bacteria and yeast initiates at defined DNA sequences, but mammalian origins are not dependent on DNA sequence [Costa and Blow Citation2007; Gilbert Citation2001; Robinson and Bell Citation2005]. ORC proteins, which remain on the origins throughout the cell cycle in mammals, are not likely to be the direct origin demarcation entities because in Xenopus, which also has sequence independent origins, all six ORC proteins cycle on and off the origins [DePamphilis Citation2005; Sun et al. Citation2002]. A major question in this field is “how do cells determine where and when to assemble pre-RCs, and how do they ensure sufficient sites are distributed throughout the genome to allow its complete duplication within a reasonable period of time? [DePamphilis et al. Citation2006].” In other words, what defines a mammalian origin of replication? Our data suggest that sperm halos contain this information in some form, possibly the attachment sites of DNA to the sperm nuclear matrix.
Nuclear autonomy in DNA degradation: implications for apoptosis
The plasticity of the relationship between the cytoplasm and the two pronuclei extends beyond simply the timing of the initiation of DNA replication. It is possible to experimentally manipulate mouse spermatozoa so that the nuclear responses of the cellular signals to cross the G1/S border are different (). Mouse sperm nuclei that are treated with Mn2+ and Ca2+ degrade their DNA to 50 kb fragments in a reversible manner reminiscent of TOP2B cleavage [Shaman et al. Citation2006]. The fragments can be religated by treatment with EDTA. Under certain conditions, an unidentified nuclease then irreversibly digests the sperm DNA. This TOP2 – nuclease interaction is similar to the normal apoptotic degradation of DNA in somatic cells, in which DNA is first fragmented by TOP2, then digested by nucleases [Durrieu et al. Citation2000; Gromova et al. Citation1995; Hars et al. Citation2006; Lagarkova et al. Citation1995; Li et al. Citation1999]. When mouse spermatozoa are treated with Mn2+ and Ca2+ and then injected into oocytes, the male pronucleus appears to develop normally for the first 5 h [Yamauchi et al. Citation2007a]. At the start of DNA synthesis, however, all of the DNA in the male pronucleus is degraded, while the DNA in the female pronucleus is replicated normally (). The DNA degradation of the male pronucleus can be inhibited by aphidicolin, an inhibitor of DNA replication [Yamauchi et al. Citation2007b], confirming the relationship between DNA degradation and the initiation of DNA replication.
These results have two implications for studies in the biology of the one cell embryo, in the relationship between the cytoplasm and nucleus in transitioning the G1/S border, and in apoptotic degradation of DNA. First, the data suggest that two nuclei in the same cytoplasm can respond differently to the cellular signals to cross the G1/S border. This indicates a certain level of autonomy in the two zygotic pronuclei which may have implications for the nuclear – cytoplasmic signaling in somatic cells. Second, in this case, at least, it appears that apoptosis-like DNA degradation can occur without the normal cytoplasmic signals because the female pronucleus was unaffected. Neither implication could have been easily identified in most other cells types that have only one nucleus. It must be emphasized that with regard to this last point we have not yet demonstrated that the DNA degradation observed in the one cell embryo is mediated by topoisomerase 2, so that it is not yet clear how it is mechanistically related to apoptosis in somatic cells. However, if a relationship can be established, it might suggest that DNA degradation and DNA replication share some common molecular pathways.
Finally, one other aspect of apoptotic DNA degradation in somatic cells may be more fully revealed by studying sperm chromatin. As mentioned above, sperm chromatin can be induced to degrade in the male pronucleus after fertilization by incubation with divalent cations, however, this degradation can also be induced in mature spermatozoa outside of the oocyte [Boaz et al. Citation2008]. Both the TOP2B reversible degradation of DNA to 50 kb fragments, which we have termed sperm chromatin fragmentation or SCF, and the subsequent nuclease digestion of the DNA to very small DNA sizes occur when spermatozoa isolated from the vas deferens are treated with divalent cations. Spermatozoa isolated from epididymus only exhibit the TOP2B SCF degradation, suggesting that the nuclease is supplied by the vas deferens. We have recently provided evidence that the nuclease that is associated with TOP2B DNA fragmentation is activated by EGTA chelated to calcium, a novel cofactor for nucleases given that most require divalent cations for activation and are actually inhibited by EGTA [Boaz et al. Citation2008; Dominguez and Ward Citation2009]. The existence of a nuclease that contributes to apoptotic-like DNA degradation in the male reproductive tract is somewhat controversial because it does not seem immediately consistent with the major biological goal of producing sperm cells with pristine copies of the paternal genome. We have suggested that this may represent a kind of checkpoint for the integrity of sperm DNA [Ward and Ward Citation2004], but this has not yet been validated. However, if this mechanism can be more fully elucidated, it may contribute to the identification of the mechanism by which nucleases and TOP2 normally interact during apoptosis.
Conclusions
All of the experiments discussed in this manuscript have important implications for the field of reproductive biology, itself, but we have focused on how they also impact other fields of cell biology. Mammalian sperm chromatin represents the least functional DNA known, and the oocyte provides the mechanism for embellishing that inert chromatin with the components necessary for function. The combination of the sperm and the activating oocyte provides a unique model for chromatin function through the window of this unique activation of inert DNA.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Abbreviations
| h: | = | hour(s) |
| MPF: | = | M-phase promoting factor |
| ORC: | = | origin recognition complex protein |
| SCF: | = | sperm chromatin fragmentation. |
Acknowledgments
This work was supported by NIH Grant No. R01HD060722.
References
- Aoki, E. and Schultz, R.M. (1999) DNA replication in the 1-cell mouse embryo: stimulatory effect of histone acetylation. Zygote 7:165–172.
- Berezney, R. (1991) Visualizing DNA replication sites in the cell nucleus. Semin Cell Biol 2:103–115.
- Berezney, R. (2002) Regulating the mammalian genome: the role of nuclear architecture. Adv Enzyme Regul 42:39–52.
- Berezney, R. and Coffey, D.S. (1975) Nuclear protein matrix: association with newly synthesized DNA. Science 189:291–293.
- Blow, J.J. (1993) Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J Cell Biol 122:993–1002.
- Blow, J.J. and Laskey, R.A. (1988) A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332:546–548.
- Boaz, S.M., Dominguez, K.M., Shaman, J.A. and Ward, W.S. (2008) Mouse spermatozoa contain a nuclease that is activated by pretreatment with EGTA and subsequent calcium incubation. J Cell Biochem 103:1636–1645.
- Borsuk, E. and Czolowska, R. (2010) Factors engaged in reactivation of DNA replication in the nuclei of growing mouse oocytes introduced into the cytoplasm of parthenogenetic one-cell embryos. Int J Dev Biol 54:21–31.
- Bouniol-Baly, C., Nguyen, E., Besombes, D. and Debey, P. (1997) Dynamic organization of DNA replication in one-cell mouse embryos: relationship to transcriptional activation. Exp Cell Res 236:201–211.
- Choudhary, S.K., Wykes, S.M., Kramer, J.A., Mohamed, A.N., Koppitch, F., Nelson, J.E., (1995) A haploid expressed gene cluster exists as a single chromatin domain in human sperm. J Biol Chem 270:8755–8762.
- Costa, S. and Blow, J.J. (2007) The elusive determinants of replication origins. EMBO Rep 8:332–334.
- DePamphilis, M.L. (2005) Cell cycle dependent regulation of the origin recognition complex. Cell Cycle 4:70–79.
- DePamphilis, M.L., Blow, J.J., Ghosh, S., Saha, T., Noguchi, K. and Vassilev, A. (2006) Regulating the licensing of DNA replication origins in metazoa. Curr Opin Cell Biol 18:231–239.
- Dijkwel, P.A. and Hamlin, J.L. (1995) Origins of replication and the nuclear matrix: the DHFR domain as a paradigm. Int Rev Cytol 162A:455–484.
- Dominguez, K. and Ward, W.S. (2009) A novel nuclease activity that is activated by Ca(2+) chelated to EGTA. Syst Biol Reprod Med 55:193–199.
- Durrieu, F., Samejima, K., Fortune, J.M., Kandels-Lewis, S., Osheroff, N. and Earnshaw, W.C. (2000) DNA topoisomerase IIalpha interacts with CAD nuclease and is involved in chromatin condensation during apoptotic execution. Curr Biol 10:923–926.
- Ferreira, J. and Carmo-Fonseca, M. (1997) Genome replication in early mouse embryos follows a defined temporal and spatial order. J Cell Sci 110(Pt 7):889–897.
- Gilbert, D.M. (2001) Making sense of eukaryotic DNA replication origins. Science 294:96–100.
- Gromova, I.I., Nielsen, O.F. and Razin, S.V. (1995) Long-range fragmentation of the eukaryotic genome by exogenous and endogenous nucleases proceeds in a specific fashion via preferential DNA cleavage at matrix attachment sites. J Biol Chem 270:18685–18690.
- Hars, E.S., Lyu, Y.L., Lin, C.P. and Liu, L.F. (2006) Role of apoptotic nuclease caspase-activated DNase in etoposide-induced treatment-related acute myelogenous leukemia. Cancer Res 66:8975–8979.
- Howlett, S.K. and Bolton, V.N. (1985) Sequence and regulation of morphological and molecular events during the first cell cycle of mouse embryogenesis. J Embryol Exp Morphol 87:175–206.
- Jackman, M., Kubota, Y., den Elzen, N., Hagting, A. and Pines, J. (2002) Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol Biol Cell 13:1030–1045.
- Jackson, D.A. and Cook, P. R. (1986) Replication occurs at a nucleoskeleton. Embo J 5:1403–1410.
- Kishigami, S., Wakayama, S., Nguyen, V.T. and Wakayama, T. (2004) Similar time restriction for intracytoplasmic sperm injection and round spermatid injection into activated oocytes for efficient offspring production. Biol Reprod 70:1863–1869.
- Lagarkova, M.A., Iarovaia, O.V. and Razin, S.V. (1995) Large-scale fragmentation of mammalian DNA in the course of apoptosis proceeds via excision of chromosomal DNA loops and their oligomers. J Biol Chem 270:20239–20241.
- Lemon, K.P. and Grossman, A.D. (1998) Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science 282:1516–1519.
- Leno, G.H., Downes, C.S. and Laskey, R.A. (1992) The nuclear membrane prevents replication of human G2 nuclei but not G1 nuclei in Xenopus egg extract. Cell 69:151–158.
- Li, T.K., Chen, A.Y., Yu, C., Mao, Y., Wang, H. and Liu, L.F. (1999) Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev 13:1553–1560.
- Liang, Y., Gao, H., Lin, S.Y., Peng, G., Huang, X., Zhang, P., (2010) BRIT1/MCPH1 is essential for mitotic and meiotic recombination DNA repair and maintaining genomic stability in mice. PLoS Genet 6:e1000826.
- Luthardt, F.W. and Donahue, R.P. (1973) Pronuclear DNA synthesis in mouse eggs. An autoradiographic study. Exp Cell Res 82:143–151.
- Maleszewski, M., Borsuk, E., Koziak, K., Maluchnik, D. and Tarkowski, A.K. (1999) Delayed sperm incorporation into parthenogenetic mouse eggs: sperm nucleus transformation and development of resulting embryos. Mol Reprod Dev 54:303–310.
- Maller, J., Gautier, J., Langan, T.A., Lohka, M.J., Shenoy, S., Shalloway, D., (1989) Maturation-promoting factor and the regulation of the cell cycle. J Cell Sci 12(Suppl):53–63.
- Mohar, I., Szczygiel, M.A., Yanagimachi, R. and Ward, W.S. (2002) Sperm nuclear halos can transform into normal chromosomes after injection into oocytes. Mol Reprod Dev 62:416–420.
- Moreno, S., Hayles, J. and Nurse, P. (1989a) Regulation of p34cdc2 protein kinase during mitosis. Cell 58:361–372.
- Moreno, S., Hayles, J. and Nurse, P. (1989b) Regulation of the cell cycle timing of mitosis. J Cell Sci 12(Suppl):1–8.
- Pardoll, D.M., Vogelstein, B. and Coffey, D.S. (1980) A fixed site of DNA replication in eucaryotic cells. Cell 19:527–536.
- Reynhout, J.K. and Smith, L.D. (1974) Studies on the appearance and nature of a maturation-inducing factor in the cytoplasm of amphibian oocytes exposed to progesterone. Dev Biol 38:394–400.
- Robinson, N.P. and Bell, S.D. (2005) Origins of DNA replication in the three domains of life. Febs J 272:3757–3766.
- Rowles, A., Tada, S. and Blow, J.J. (1999) Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J Cell Sci 112(Pt 12):2011–2018.
- Shaman, J.A., Prisztoka, R. and Ward, W.S. (2006) Topoisomerase IIB and an extracellular nuclease interact to digest sperm DNA in an apoptotic-like manner. Biol Reprod 75:741–748.
- Shaman, J.A., Yamauchi, Y. and Ward, W.S. (2007) The Sperm Nuclear Matrix is Required for Paternal DNA Replication. J Cell Biochem 102:680–688.
- Sirlin, J.L. and Edwards, R.G. (1959) Timing of DNA synthesis in ovarian oocyte nuclei and pronuclei of the mouse. Exp Cell Res 18:190–194.
- Stubblefield, E. (1975) Analysis of the replication pattern of Chinese hamster chromosomes using 5-bromodeoxyuridine suppression of 33258 Hoechst fluorescence. Chromosoma 53:209–221.
- Sun, W.H., Coleman, T.R. and DePamphilis, M.L. (2002) Cell cycle-dependent regulation of the association between origin recognition proteins and somatic cell chromatin. Embo J 21:1437–1446.
- Takeda, D.Y. and Dutta, A. (2005) DNA replication and progression through S phase. Oncogene 24:2827–2843.
- Thomae, A.W., Pich, D., Brocher, J., Spindler, M.P., Berens, C., Hock, R., (2008) Interaction between HMGA1a and the origin recognition complex creates site-specific replication origins. Proc Natl Acad Sci USA 105:1692–1697.
- Vogelstein, B., Pardoll, D.M. and Coffey, D.S. (1980) Supercoiled loops and eucaryotic DNA replicaton. Cell 22:79–85.
- Ward, M.A. and Ward, W.S. (2004) A model for the function of sperm DNA degradation. Reprod Fertil Dev 16:547–554.
- Ward, W.S., Partin, A.W. and Coffey, D.S. (1989) DNA loop domains in mammalian spermatozoa. Chromosoma 98:153–159.
- Wasserman, W.J. and Smith, L.D. (1978) The cyclic behavior of a cytoplasmic factor controlling nuclear membrane breakdown. J Cell Biol 78:R15–R22.
- Yamauchi, Y., Shaman, J.A., Boaz, S.M. and Ward, W.S. (2007b) Paternal pronuclear DNA degradation is functionally linked to DNA replication in mouse oocytes. Biol Reprod 77:407–415.
- Yamauchi, Y., Ward, M.A. and Ward, W.S. (2009) Asynchronous DNA 605 replication and origin licensing in the mouse one cell embryo. J Cell Biochem 107:214–223.
- Yamauchi, Y., Shaman, J.A. and Ward, W.S. (2007a) Topoisomerase II mediated breaks in spermatozoa cause the specific degradation of paternal DNA in fertilized oocytes. Biol Reprod 76:666–672.
- Yanagimachi, R. (1994) Stability of the mammalian sperm nucleus. Zygote 2:383–384.