Abstract
Inhibins and activins are important regulators of the female reproductive system. Recently, a novel inhibin betaC subunit has been identified. However, only limited data on the expression of this novel inhibin-betaC subunit in normal and pathological human placentas exist. Tissue specimens of normal, preeclamptic, hemolysis, elevated liver enzymes, low platelets (HELLP), and intrauterine growth restriction (IUGR) pregnancies (n = 24) were obtained at the conclusion of a cesarean section. Normal and pathological placental tissues were analyzed by an immunohistochemical staining reaction with a specific antibody against this novel inhibin-betaC subunit. Overall, expression of the inhibin-betaC subunit could be demonstrated in normal and pathological placental tissue. The immunoreactive score (IRS) for inhibin-betaC did not show any significant differences between normal, preeclamptic, HELLP, and IUGR tissue in extravillous trophoblast and syncytiotrophoblast cells. Immunolabelling of this novel inhibin-βC protein in normal and pathological placental tissue was demonstrated, although no differences in the staining intensity could be observed. Therefore, the inhibin-βC isoform might not primarily be involved in the pathogenesis of these pregnancy-associated disorders. The functional role of this novel inhibin-betaC subunit in normal and pathological human placenta is still quite unclear and should thus be further investigated.
Introduction
Preeclampsia, hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, and fetal intrauterine growth restriction (IUGR) still remain among the major causes for maternal and neonatal morbidity and mortality [Haram et al. Citation2009; Kanasaki and Kalluri Citation2009]. Both preeclampsia and HELLP syndrome are multisystemic disorders during pregnancy and the precise pathology still remains unclear [Haram et al. Citation2009; Kanasaki and Kalluri Citation2009]. The incidence of preeclampsia is estimated at approximately 5 to 7% of pregnancies [Witlin and Sibai Citation1998], while the assumed maternal mortality from HELLP is estimated at 1% with a perinatal mortality rate from 7 to 22% [Hepburn and Schade Citation2008]. Additionally, small fetuses due to IUGR are at higher risk for poor perinatal and long-term outcome [Baschat Citation2004], being associated with an increased risk of heart diseases, hypertension, and diabetes [Barker Citation2004]. Therefore, a fast and trustworthy identification of these conditions is of major importance to improve maternal and perinatal outcome [Askie et al. Citation2007].
Insufficient placental implantation and a failure of adequate trophoblast invasion into spiral arteries is thought to be extremely important in the pathogenesis of preeclampsia, HELLP-syndrome, and IUGR [Lim et al. Citation1997; Madazli et al. Citation2000]. In pathological pregnancies an imbalance between angiogenetic factors is thought to result in systemic vasospasm and subsequent endothelial damage [Kanasaki and Kalluri Citation2009]. Moreover, additional markers including inhibins and activins, a subgroup of the TGF-β superfamily [Vale et al. Citation2004; Xia and Schneyer Citation2009] that are produced by placental tissue [Jones et al. Citation2006b] and vascular endothelial cells [Tannetta et al. Citation2003], have been identified to be involved in the pathogenesis in preeclampsia. Members of the TGFβ superfamily are involved in cytotrophoblast fusion and syncytialization [Debieve et al. Citation2000; Jones et al. Citation2006a; Citation2006b], regulation of placental steroidogenesis [Petraglia et al. Citation1991; Luo et al. Citation2002], and local and systemic immunomodulatory functions [Casey and MacDonald Citation1996; Florio et al. Citation2004; Phillips et al. Citation2009]. Moreover, local produced inhibin and activin is thought to be of major importance in the establishment of a viable pregnancy [Florio et al. Citation2004; Jones et al. Citation2006a; Citation2006b]. Since the serum concentrations of inhibins and activins are found to be higher in maternal serum of preeclamptic patients compared to normal pregnancy [Muttukrishna et al. Citation1997], endocrine markers for preeclampsia and adverse pregnancy outcome [Muttukrishna et al. Citation1997; Dugoff et al. Citation2005; Grill et al. Citation2009] as well as a predictive value of these molecules has been suggested [Muttukrishna et al. Citation2000].
Within the inhibin/activin subgroup, one α-subunit and four β-subunit isoforms (βA, βB, βC, and βE) have been identified in mammals [Hötten et al. Citation1995; Fang et al. Citation1996; Hashimoto et al. Citation2002; Vale et al. Citation2004; Xia and Schneyer Citation2009]. These β-subunits can either form activins by dimerization with a second β-subunit, or, alternatively, inhibins, by pairing with an α-subunit. Thus, depending on the subunit combination, there are two forms of inhibin (A: α-βA and B: α-βB) and three isoforms of activin (namely, activin A: βA-βA, activin B: βB-βB, and activin AB: βA-βB). Many investigations have focused on the more widely expressed βA and βB subunits. In comparison the functions of the novel βC and βE isoforms remain to be defined. Only limited data on histological expression of the inhibin-βC in normal and pathological placental tissue is available. The inhibin βC protein was primarily expressed in human liver and prostate tissue [Mellor et al. Citation2000]. Recently it has been demonstrated that inhibin-βC can also be synthesized in human endometrium [Kimmich et al. 2010; Käufl et al. 2010a; 2010b], placenta tissue and choriocarcinoma cell lines [Casagrandi et al. Citation2003; Weissenbacher et al. Citation2010].
Specific monoclonal antibodies against this inhibin-subunit have been available for just a short period of time. Systematic investigation of normal and pathologic placental tissue has yet to be performed. The putative expression of inhibin-β subunits is of extreme importance, since activin signaling might be a promising target for therapeutic interventions [Tsuchida et al. Citation2009]. Therefore, the aim of the present study was to evaluate the synthesis of the novel inhibin-βC subunit in normal and pathological human placental tissue.
Results
The βC-subunit antibody was first tested by using appropriate positive controls including normal human liver specimens as previously described [Kimmich et al. Citation2010; Weissenbacher et al. Citation2010]. A positive staining reaction for inhibin-βC expression could be demonstrated for normal human liver tissue, confirming previous results (data not shown) [Lau et al. Citation2000; Hashimoto et al. Citation2002; CitationKimmich et al. 2009; Weissenbacher et al. Citation2010]. Having established specificity, the assay was then utilized to characterize subject characteristics. Tissue samples were obtained from patients diagnosed with preeclampsia (n = 8), HELLP (n = 5), IUGR (n = 5), and normal pregnancies (n = 6) after delivery through cesarean section ().
Table 1. Clinical details of the patients and newborns with preeclampsia, HELLP syndrome, IUGR, and the normal control group of well characterized group of patients [Mylonas et al. 2006a; 2006b; Schiessl et al. 2005; 2006]. Data represent mean ± standard deviation.
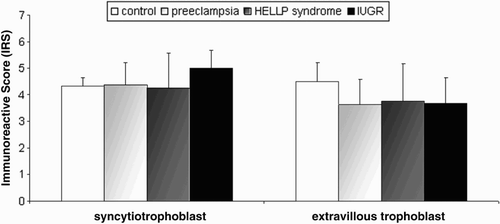
In normal placental tissues, inhibin-βC was primarily expressed in syncytiotrophoblast cells (), while immunostaining in extravillous trophoblast cells was weaker (). While a diffuse cytoplasmatic immunohistochemical staining could be observed in syncytiotrophoblast cells obtained from normal, preeclamptic, and HELLP pregnancies (), a subjective increase of the immunolabelling was observed in IUGR pregnancies (). In extravillous trophoblast cells diffuse immunolabelling could also be demonstrated in all analyzed placental tissue (), with a subjective stronger staining in normal placental tissue (). The immunoreactive score (IRS) for inhibin-βC did not demonstrate any significant differences in all analyzed normal and pathological placental tissues (p > 0.05) ().
Figure 1. Immunohistochemical staining reaction of inhibin-βC in placental syncytiotrophoblast cells. Syncytiotrophoblast cells demonstrated a positive cytoplasmatic staining intensity for inhibin-βC antibody in normal ( A; lens 20, top left panel), preeclamptic ( B; lens 40, top right panel), HELLP (C; lens 40, lower left panel), and IUGR (D; lens 40, lower right panel) placental tissue.
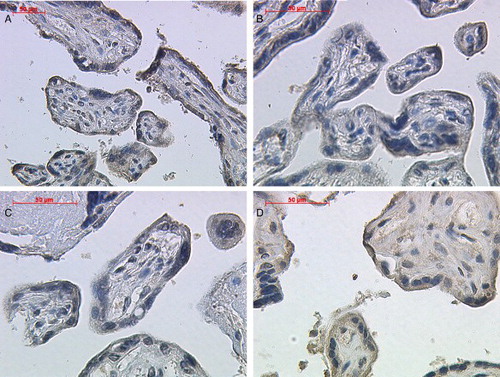
Figure 2. Immunohistochemical staining reaction of inhibin-βC in placental extravillous trophoblast cells. Extravillous trophoblast cells demonstrated a positive cytoplasmatic staining intensity for inhibin-βC antibody in normal (A; lens 20, upper left panel), preeclamptic (B; lens 20, upper right panel), HELLP (C; lens 20, lower left panel), and IUGR (D; lens 40, lower right panel) placental tissue.
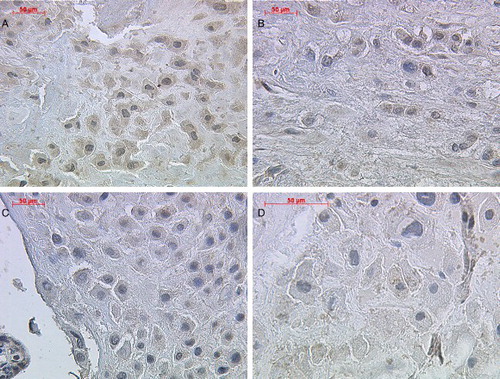
Figure 3. Immunohistochemical evaluation of the inhibin-βC subunits in normal, preeclamptic, and HELLP placenta tissue. The immunoreactive score for inhibin-βC did not show any significant differences between normal, preeclamptic, HELLP, or IUGR placental tissue in syncytiotrophoblast cells and extravillous trophoblast. Data represent mean ± SEM. Significance was assumed at p < 0.05.
Discussion
Preeclampsia, HELLP syndrome, and IUGR are major causes for maternal and neonatal morbidity and mortality [Haram et al. Citation2009; Kanasaki and Kalluri Citation2009] and a rapid, early, and reliable diagnosis is of extreme clinical importance to prevent an adverse maternal and perinatal outcome [Askie et al. Citation2007]. In these pregnancy-associated diseases, trophoblast invasion is restrained and remodeling of the spiral arteries is limited, resulting in reduced uteroplacental perfusion [Lim et al. Citation1997; Madazli et al. Citation2000]. Since human placenta expresses inhibin and activin subunits [Petraglia et al. Citation1991; Casagrandi et al. Citation2003; Mylonas et al. Citation2006a; Citation2006b] and being the predominant source of maternal circulating inhibin and activin [Florio et al. Citation2001], these molecules are very intriguing as possible prognostic serological markers. These assumptions are underlined by the fact that the inhibin-subunits as well as the increased serum levels of inhibins and activins have been associated with preeclampsia [Muttukrishna et al. Citation1997; Dugoff et al. Citation2005; Grill et al. Citation2009], HELLP syndrome [Seufert et al. Citation2004; Mylonas et al. Citation2006b], and in pregnancies complicated with IUGR [Keelan et al. Citation2002; Mylonas et al. Citation2006a].
The expression of inhibin-α, βA, βB, and βC subunits, follistatin, betaglycan, and activin receptor genes were demonstrated in placental tissue from both uncomplicated term pregnancies and term pregnancies with preeclampsia [Casagrandi et al. Citation2003]. However, inhibin-βC mRNA did not demonstrate any differences between normal and preeclamptic tissue [Casagrandi et al. Citation2003]. In this preliminary study we demonstrated immunohistochemical expression of this novel inhibin-βC protein in normal and pathological placental tissue. However, no differences in the staining intensity could be observed. Therefore, the inhibin-βC isoform is probably not involved in the pathogenesis of preeclampsia, HELLP syndrome, and IUGR and therefore it might not constitute an adequate and sufficient predictive marker for these pregnancy-associated diseases. These observations may reflect either the antibody used or the low number of cases analyzed. Nevertheless, the expression pattern of this subunit is different compared to the other β-subunits [Mylonas et al. Citation2006a; Citation2006b], suggesting a different function than the other β-isoforms in normal and pathological placental tissue.
Inhibin-βC was primarily detected in hepatocytes [Lau et al. Citation2000; Hashimoto et al. Citation2002], being implicated in the regulation of liver cell growth as demonstrated by an inhibin-βC mRNA down regulation after partial hepatectomy in rats [Esquela et al. Citation1997]. In human reproduction, this subunit is believed not to be a significant regulator of activin bioactivity, since no abnormalities or malformations have been observed in inhibin-βC knockout mice [Lau et al. Citation2000]. However, there might be functional redundancy with other TGF-β factors [Gold et al. Citation2009]. An antagonistic and regulative role for activin A bioactivity has been recently proposed [Mellor et al. Citation2000; Citation2003; Gold et al. Citation2009]. Interestingly, it was demonstrated that activin C (βC-βC) did not activate activin A (βA-βA) - responsive promoters, and it was suggested that the βC subunit regulates the levels of bioactive activin A (βA-βA) through the formation of signaling incompetent activin AC heterodimers [Mellor et al. Citation2003; Butler et al. Citation2005; Gold et al. Citation2009].
Although the precise role of this subunit is not elucidated yet, several possible functions have been suggested. Ectopic expression of inhibin/activin βC subunit induced apoptosis in human (HepG2, Hep3B) and rat (H4, EC3) hepatoma cells [Chabicovsky et al. Citation2003; Vejda et al. Citation2003]. In an immortalized mouse hepatocyte cell line (AML12) and primary rat hepatocytes, activin βC increased the rate of DNA synthesis [Wada et al. Citation2005]. Moreover, the βC-subunit was identified as an autocrine growth modulator in liver regeneration, leading to mitosis in a subset of hepatocytes [Gold et al. Citation2005]. This still remains to be defined in human placenta. The function of the βC-isoform is further complicated by the fact that the formation of homodimeric activin C (βC-βC) as well as heterodimeric activins AC (βA-βC), BC (βB-βC), CE (βC-βE), and inhibin C (α-C) have been demonstrated [Mellor et al. Citation2000; Ushiro et al. Citation2006]. It is still not clear, which of these homodimers/heterodimers exert a biological function. Moreover, due to the lack of appropriate immunoassays, it is still not possible to determine if dimeric proteins are secreted into the circulation.
In conclusion, we have demonstrated an immunohistochemical expression of the inhibin-βC protein in normal and pathological placental tissue, although no differences in the staining intensity could be observed. Therefore, the inhibin-βC isoform would subsequently not primarily be involved in the pathogenesis of preeclampsia, HELLP syndrome, and IUGR and would subsequently not constitute an adequate predictive marker for these pregnancy-associated diseases. The functional role of the inhibin-βC subunit in normal and pathological human placenta is still unclear and warrants further investigation.
Material and methods
Tissue samples
Placental tissues were obtained from 24 placentas of women giving birth at the 1st Department of Obstetrics and Gynecology of the Ludwig-Maximilians-University Munich after delivery through cesarean section of a well-characterized group of patients [Mylonas et al. Citation2006a; Citation2006b; Schiessl et al. Citation2005; Citation2006]. Tissue samples were obtained from patients diagnosed with preeclampsia (n = 8), HELLP (n = 5), IUGR (n = 5), and normal pregnancies (n = 6) (Table 1). The study had the approval of the local ethical committee of the Ludwig-Maximilians-University Munich, Germany (No. 158/00) and informed consent from the patients was obtained.
Although no sufficient data on the precise role of TGFβ or inhibin/activin during labor are available, the link between interleukin and inhibin/activin synthesis [Okuma et al. Citation2005] suggests a potential involvement in the cascade of actions during labor [Muttukrishna et al. Citation1997]. Therefore, tissue specimens of normal pregnancies were obtained at the conclusion of an elective cesarean section for breech presentation during the 38th week of gestation to avoid any influencing factors due to the physiological stress during normal delivery [Mylonas et al. Citation2006a; Citation2006b]. Placental tissue specimens of pregnancies complicated by preeclampsia, HELLP syndrome, or IUGR were also obtained at the conclusion of cesarean section for pathological umbilical Doppler waves, pathological fetal cardiotocography (CTG), or increasing maternal symptoms [Mylonas et al. Citation2006a; Citation2006b].
Immunohistochemistry
Immunohistochemistry was performed using a combination of pressure cooker heating and the standard streptavidin-biotin-peroxidase complex by using the goat-IgG-Vectastain Elite ABC kit (Vector Laboratories, Burlingame, California, USA) as previously described [Kimmich et al. Citation2010; Weissenbacher et al. 2010; Käufl et al. 2010a; Citation2010b]. Briefly, paraffin-fixed tissue sections were dewaxed using xylol for 15 min and rehydrated in 100% of ethanol twice. Endogenous peroxidase activity was quenched by immersion in 3% hydrogen peroxide (Merck, Darmstadt, Germany) in methanol for 20 min. After washing slides were subjected to antigen retrieval for 5 min in a pressure cooker using sodium citrate buffer (pH 6.0), containing 0.1 M citric acid and 0.1 M sodium citrate in distilled water. After cooling to room temperature, sections were washed twice in phosphate-buffered saline (PBS). Non-specific binding was blocked by incubating the sections with Ultra-V-Block (Lab Vision, Fremont, California, USA) for 45 min at room temperature. Sections were then incubated at 4°C over night with the inhibin-βC polyclonal goat antibody (R&D Systems, Wiesbaden, Germany) at a dilution of 1:50 in Ultra-V-Block (Lab Vision) as previously described [CitationKimmich et al. 2009]. After washing with PBS, sections were incubated with biotinylated secondary anti-rabbit antibody (provided by Vector Laboratories) for 30 min at room temperature. After incubation with the avidin-biotin peroxidase complex (diluted in 10 ml PBS; Vector Laboratories) for 30 min and repeated washing steps with PBS, visualization was performed with ABC substrate buffer (Vectastain Elite ABC kit, Vector Laboratories) and chromogenic 3,3′-diaminobenzidine (DAB; Dako, Glostrup, Denmark) at 1mg/ml concentration for 2 min. Sections were then counterstained with Mayer's acidic hematoxylin and dehydrated in an ascending series of alcohol (50-98%). After xylol treatment, sections were mounted. Negative controls were performed by replacing the primary antibody with normal goat IgG or rabbit IgG as isotype control in the same dilution compared to the primary antibody, respectively. Immunohistochemical staining was performed using human liver tissue as an appropriate positive control. Positive cells showed a brownish color and negative controls as well as unstained cells were blue.
Statistical analysis
The intensity and distribution patterns of specific inhibin/activin-subunit immunohistochemical cytoplasmatic staining was evaluated by two blinded, independent observers, including a gynecological pathologist (J.M.), using a semi-quantitative score as previously described and used to assess the expression pattern of inhibin/activin subunits [Mylonas et al. Citation2004; Citation2006a; Citation2006b; Citation2009]. The IRS was calculated by multiplication of optical staining intensity (graded as 0 = no, 1 = weak, 2 = moderate, and 3 = strong staining) and the percentage of positive stained cells (0 = no staining, 1 = < 10% of the cells, 2 = 11–50% of the cells, 3 = 51–80% of the cells, and 4 = > 81% of the cells). Sections were examined using a Leica (Solms, Germany) photomicroscope. The IRS of inhibin-βC immunohistochemical expression levels were compared using the non-parametric Mann-Whitney U test. Significance of differences was assumed at p ≤ 0.05 at the two-sided test.
Abbreviations
| HELLP: | = | hemolysis, elevated liver enzymes, low platelets |
| IUGR: | = | intrauterine growth restriction |
| IRS: | = | immunoreactive score. |
Acknowledgments
We would like to thank Mrs. I. Krienke, Mrs. S. Kunze, Mrs S. Schulze, and Mrs. I. Wiest for their excellent work with placental samples. Moreover, we express our gratitude to Mrs. C. Kuhn and Dr. U. Jeschke for their help with the manuscript. This study was partially supported by the FöFoLe program of the Ludwig-Maximilians-University Munich (297/03), the Friedrich-Baur-Institute Munich and the Weigland Stipendium Program of the Ludwig-Maximilians-University Munich to I. M.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Askie, L.M., Duley, L., Henderson-Smart, D.J., Stewart, L.A., Group, P.C. (2007) Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 369(9575):1791–1798.
- Barker, D.J. (2004) The developmental origins of chronic adult disease. Acta Paediatr Suppl 93(446):26–33.
- Baschat, A.A. (2004) Fetal responses to placental insufficiency: an update. BJOG 111:1031–1041-.
- Butler, C.M., Gold, E.J., Risbridger, G.P. (2005) Should activin betaC be more than a fading snapshot in the activin/TGFbeta family album? Cytokine Growth Factor Rev 16(4–5):377–385.
- Casagrandi, D., Bearfield, C., Geary, J., Redman, C.W., Muttukrishna, S. (2003) Inhibin, activin, follistatin, activin receptors and beta-glycan gene expression in the placental tissue of patients with pre-eclampsia. Mol Hum Reprod 9(4):199–203.
- Casey, M.L., MacDonald, P.C. (1996) The endothelin-parathyroid hormone-related protein vasoactive peptide system in human endometrium: modulation by transforming growth factor-beta. Hum Reprod 11( Suppl 2):62–82.
- Chabicovsky, M., Herkner, K., Rossmanith, W. (2003) Overexpression of activin beta(C) or activin beta(E) in the mouse liver inhibits regenerative deoxyribonucleic acid synthesis of hepatic cells. Endocrinology 144(8):3497–3504.
- Debieve, F., Pampfer, S., Thomas, K. (2000) Inhibin and activin production and subunit expression in human placental cells cultured in vitro. Mol Hum Reprod 6(8):743–749.
- Dugoff, L., Hobbins, J.C., Malone, F.D., Vidaver, J., Sullivan, L., Canick, J.A., (2005) Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol 106(2):260–267.
- Esquela, A.F., Zimmers, T.A., Koniaris, L.G., Sitzmann, J.V., Lee, S.J. (1997) Transient down-regulation of inhibin-betaC expression following partial hepatectomy. Biochem Biophys Res Commun 235(3):553–556.
- Fang, J., Yin, W., Smiley, E., Wang, S.Q., Bonadio, J. (1996) Molecular cloning of the mouse activin beta E subunit gene. Biochem Biophys Res Commun 228(3):669–674.
- Florio, P., Cobellis, L., Luisi, S., Ciarmela, P., Severi, F.M., Bocchi, C., Petraglia, F. (2001) Changes in inhibins and activin secretion in healthy and pathological pregnancies. Mol Cell Endocrinol 180(1–2):123–130.
- Florio, P., Luisi, S., Ciarmela, P., Severi, F.M., Bocchi, C., Petraglia, F. (2004) Inhibins and activins in pregnancy. Mol Cell Endocrinol 225(1–2):93–100.
- Gold, E., Jetly, N., O'Bryan, M.K., Meachem, S., Srinivasan, D., Behuria, S., (2009) Activin C antagonizes activin A in vitro and overexpression leads to pathologies in vivo. Am J Pathol 174(1):184–195.
- Gold, E.J., Zhang, X., Wheatley, A.M., Mellor, S.L., Cranfield, M., Risbridger, G.P., (2005) betaA- and betaC-activin, follistatin, activin receptor mRNA and betaC-activin peptide expression during rat liver regeneration. J Mol Endocrinol 34(2):505–515.
- Grill, S., Rusterholz, C., Zanetti-Dallenbach, R., Tercanli, S., Holzgreve, W., Hahn, S., Lapaire, O. (2009) Potential markers of preeclampsia–a review. Reprod Biol Endocrinol 7:70.
- Haram, K., Svendsen, E., Abildgaard, U. (2009) The HELLP syndrome: clinical issues and management. A Review. BMC Pregnancy Childbirth 9: 8.
- Hashimoto, O., Tsuchida, K., Ushiro, Y., Hosoi, Y., Hoshi, N., Sugino, H., Hasegawa, Y. (2002) cDNA cloning and expression of human activin betaE subunit. Mol Cell Endocrinol 194(1–2):117–122.
- Hepburn, I.S., Schade, R.R. (2008) Pregnancy-associated liver disorders. Dig Dis Sci 53(9):2334–2358.
- Hötten, G., Neidhardt, H., Schneider, C., Pohl, J. (1995) Cloning of a new member of the TGF-beta family: a putative new activin beta C chain. Biochem Biophys Res Commun 206(2):608–613.
- Jones, R.L., Findlay, J.K., Salamonsen, L.A. (2006a) The role of activins during decidualisation of human endometrium. Aust N Z J Obstet Gynaecol 46(3):245–249.
- Jones, R.L., Stoikos, C., Findlay, J.K., Salamonsen, L.A (2006b) TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 132(2):217–232.
- Käufl, S.D., Kuhn, C., Kunze, S., Shabani, N., Brüning, A., Friese, K., Mylonas, I. (2010b) Inhibin/activin-beta C does not represent a prognostic parameter in human endometrial cancer. Arch Gynecol Obstet doi: 10.1007/s00404-010-1614-y ahead of print.
- Käufl, S.D., Makovitzky, J., Kuhn, C., Kunze, S., Jeschke, U., Mylonas, I. (2010a) Inhibin/activin-beta C subunit in human endometrial adenocarcinomas and HEC-1a adenocacinoma cell line. In Vivo 24(5):695–698.
- Kanasaki, K., Kalluri, R. (2009) The biology of preeclampsia. Kidney Int 76(8):831–837.
- Keelan, J.A., Taylor, R., Schellenberg, J.C., Groome, N.P., Mitchell, M.D., North, R.A. (2002) Serum activin A, inhibin A, and follistatin concentrations in preeclampsia or small for gestational age pregnancies. Obstet Gynecol 99(2):267–274.
- Kimmich, T., Bruning, A., Kaufl, S.D., Makovitzky, J., Kuhn, C., Jeschke, U., (2010) Inhibin/activin-betaC and -betaE subunits in the Ishikawa human endometrial adenocarcinoma cell line. Arch Gynecol Obstet 282(2):185–191.
- Lau, A.L., Kumar, T.R., Nishimori, K., Bonadio, J., Matzuk, M.M. (2000) Activin betaC and betaE genes are not essential for mouse liver growth, differentiation, and regeneration. Mol Cell Biol 20(16):6127–6137.
- Lim, K.H., Zhou, Y., Janatpour, M., McMaster, M., Bass, K., Chun, S.H. (1997) Human cytotrophoblast differentiation/invasion is abnormal in preeclampsia. Am J Pathol 151:1809 –1818.
- Luo, S., Yu, H., Wu, D., Peng, C. (2002) Transforming growth factor-beta1 inhibits steroidogenesis in human trophoblast cells. Mol Hum Reprod 8(4):318–325.
- Madazli, R., Budak, E., Calay, Z., Aksu, M.F. (2000) Correlation between placental bed biopsy findings, vascular cell adhesion molecule and fibronectin levels in pre-eclampsia. BJOG 107(4):514–518.
- Mellor, S.L., Ball, E.M., O'Connor, A.E., Ethier, J.F., Cranfield, M., Schmitt, J.F., (2003) Activin betaC-subunit heterodimers provide a new mechanism of regulating activin levels in the prostate. Endocrinology 144(10):4410–4419.
- Mellor, S.L., Cranfield, M., Ries, R., Pedersen, J., Cancilla, B., de Kretser, D., (2000) Localization of activin beta(A)-, beta(B)-, and beta(C)-subunits in humanprostate and evidence for formation of new activin heterodimers of beta(C)-subunit. J Clin Endocrinol Metab 85(12):4851–4858.
- Muttukrishna, S., Knight, P.G., Groome, N.P., Redman, C.W., Ledger, W.L. (1997) Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet 349(9061):1285–1288.
- Muttukrishna, S., North, R.A., Morris, J., Schellenberg, J.C., Taylor, R.S., Asselin, J., (2000) Serum inhibin A and activin A are elevated prior to the onset of pre-eclampsia. Hum Reprod 15(7):1640–1645.
- Mylonas, I., Jeschke, U., Wiest, I., Hoeing, A., Vogl, J., Shabani, N., (2004) Inhibin/activin subunits alpha, beta-A and beta-B are differentially expressed in normal human endometrium throughout the menstrual cycle. Histochem Cell Biol 122(5):461–471.
- Mylonas, I., Schiessl, B., Jeschke, U., Vogl, J., Makrigiannakis, A., Kuhn, C., (2006a) Expression of inhibin/activin subunits alpha (-alpha), beta A (-beta (A)) and beta B (-beta (B)) in placental tissue of normal and intrauterine growth restricted (IUGR) pregnancies. J Mol Histol 37(1–2):43–52.
- Mylonas, I., Schiessl, B., Jeschke, U. Vogl, J., Makrigiannakis, A., Kuhn, C., (2006b) Expression of inhibin/activin subunits alpha (-alpha), betaA (-betaA), and betaB (-betaB) in placental tissue of normal, preeclamptic and HELLP pregnancies. Endocr Pathol 17(1):19–34.
- Mylonas, I., Worbs, S., Shabani, N., Kuhn, C., Kunze, S., Schulze, S., (2009) Inhibin-alpha subunit is an independent prognostic parameter in human endometrial carcinomas: analysis of inhibin/activin-alpha, -betaA and -betaB subunits in 302 cases. Eur J Cancer 45(7):1304–1314.
- Okuma, Y., Saito, K., O'Connor, A.E., Phillips, D.J, de Kretser, D.M., Hedger, M.P. (2005) Reciprocal regulation of activin A and inhibin B by interleukin-1 (IL-1) and follicle-stimulating hormone (FSH) in rat Sertoli cells in vitro. J Endocrinol 185(1):99–110.
- Petraglia, F., Garuti, G.C., Calza, L., Roberts, V., Giardino, L., Genazzani, A.R., (1991) Inhibin subunits in human placenta: localization and messenger ribonucleic acid levels during pregnancy. Am J Obstet Gynecol 165(3):750–758.
- Phillips, D.J., de Kretser, D.M., Hedger, M.P. (2009) Activin and related proteins in inflammation: not just interested bystanders. Cytokine Growth Factor Rev 20(2):153–164.
- Schiessl, B., Mylonas, I., Hantschmann, P., Kuhn, C., Schulze, S., Kunze, S., (2005) Expression of endothelial NO synthase, inducible NO synthase, and estrogen receptors alpha and beta in placental tissue of normal, preeclamptic, and intrauterine growth-restricted pregnancies. J Histochem Cytochem 53(12):1441–1449.
- Schiessl, B., Mylonas, I., Kuhn, C., Kunze, S., Schulze, S., Friese, K., Jeschke, U. (2006) Expression of estrogen receptor-alpha, estrogen receptor-beta and placental endothelial and inducible NO synthase in intrauterine growth-restricted and normal placentals. Arch Med Res 37(8):967–975.
- Seufert, R., Neubert, S., Tanner, B., Schaffrath, M., Pollow, K., Kölbl, H. (2004) Inhibins and Activin A in hypertensive Disorders of Pregnancy and HELLP-Syndrome. Zentralbl Gynakol 126(3):148–153.
- Tannetta, D.S., Muttukrishna, S., Groome, N.P., Redman, C.W., Sargent, I.L. (2003) Endothelial cells and peripheral blood mononuclear cells are a potential source of extraplacental activin a in preeclampsia. J Clin Endocrinol Metab 88(12):5995–6001.
- Tsuchida, K., Nakatani, M., Hitachi, K., Uezumi, A., Sunada, Y., Ageta, H., Inokuchi, K. (2009) Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal 7:15.
- Ushiro, Y., Hashimoto, O., Seki, M., Hachiya, A., Shoji, H., Hasegawa, Y. (2006) Analysis of the Function of Activin beta(C) Subunit Using Recombinant Protein. J Reprod Dev 52(4):487–495.
- Vale, W., Wiater, E., Gray, P., Harrison, C., Bilezikjian, L., Choe, S. (2004) Activins and inhibins and their signaling. Ann N Y Acad Sci 1038:142–147.
- Vejda, S., Erlach, N., Peter, B., Drucker, C., Rossmanith, W., Pohl, J., (2003) Expression of activins C and E induces apoptosis in human and rat hepatoma cells. Carcinogenesis 24(11): 1801–1809.
- Wada, W., Medina, J., Hasegawa, Y., Kuwano, H., Kojima, I. (2005) Adenovirus-mediated overexpression of the activin betaC subunit accelerates liver regeneration in partially hepatectomized rats. J Hepatol 43(5):823–828.
- Weissenbacher, T., Brüning, A., Kimmich, T., Makovitzky, J., Gingelmaier, A., Mylonas, I. (2010) Immunohistochemical labeling of the inhibin/activin betaC subunit in normal human placental tissue and chorionic carcinoma cell lines. J Histochem Cytochem 58(8):751–757.
- Witlin, A.G., Sibai, B.M. (1998) Magnesium sulfate therapy in preeclampsia and eclampsia. Obstet Gynecol 92:883–889.
- Xia, Y., Schneyer, A.L. (2009) The biology of activin: recent advances in structure, regulation and function. J Endocrinol 202(1):1–12.