Abstract
The site specific action of the calcium channel blocker diltiazem in blocking prostaglandin synthesis and hence causing blastocyst implantation failure has been previously described. Based on this understanding it was important to learn if this pathway was under the control of the fine balance in estradiol-progesterone (E2-P4) milieu, considered to be of the utmost significance for effective implantation. In the current study the circulating E2-P4 levels were monitored on the first 6 d of pregnancy at various time points using sensitive chemiluminescence based assays. Next, diltiazem was administered intra-luminally into the uterus at 10-20 h prior to implantation as this time has been previously implicated to be when the best anti-implantation activity of diltiazem can be observed. Following this, the E2-P4 in peripheral circulation was again monitored. On d 6 (post implantation) the implantation sites were observed in the uterus of both diltiazem treated and untreated groups using Chicago blue dye and correlated to the hormonal activity. The levels of both estradiol and progesterone were very similar in both untreated and diltiazem treated groups during and post implantation. However complete implantation failure was noted in the diltiazem treated group whereas prominent implantation sites were observed in the untreated animals. Thus, the previously reported inhibition of blastocyst implantation cascade by calcium channel blockers during the ‘implantation window’ seems to be an independent mechanism interfering with uterine receptivity without any direct estrogen-progesterone control and further studies to understand its regulation need to be performed.
Keywords:
Introduction
The hormonally primed uterine milieu and the mature blastocyst interact at a molecular level via a complex mechanism to result in implantation. Lack of synchronization in this process leads to implantation failure. These processes are coordinated by multiple signals including those from steroid hormones. The preovulatory increase in 17β-estradiol secretion stimulating the proliferation and differentiation of uterine epithelium is one of the primary events leading to successful implantation. Similarly, differentiation of stromal cells is due to a continuous production of progesterone by the corpus luteum. Further downstream actions are controlled by peptide hormones, growth factors, and cytokines.
It has been previously shown that implantation can be induced in the ovarectomized pregnant rat or mouse by a single injection of estrogen [Cochrane and Meyer Citation1957; Bloch Citation1958; Smith and Biggers Citation1968; Hedlund and Nilsson Citation1971], and can also be initiated in the ovarectomized pregnant rat by administration of small but frequent doses of estrogen and progesterone [DeFeo Citation1963; Zeilmaker Citation1963]. Finn and Martin [1969] postulated that progesterone and estrogen secretion begins about 48 h after mating and continued (possibly increasing) until after implantation initiates. Shaikh [1971] confirmed that a preimplantation increase of estrogen occurs on d 4 of pregnancy in the rat. Mouse is known to be very sensitive to exogenous estrogen, requiring as little as 5 to 7 ng to elicit a pro-implantation effect [Finn and Martin Citation1969].
Previous experiments have shown that the calcium channel played a significant role in blastocyst implantation and that intra-uterine delivery of calcium channel blockers like diltiazem at about 10-12 h prior to implantation causes implantation failure [Banerjee et al. Citation2009]. Thus the inflammatory cascade responsible for prostaglandin synthesis activated by the rate limiting mobilization of cPLA2 was shown to be under the control of the rise in intracellular calcium levels just prior to the onset of implantation. It became important to understand if this calcium channel regulated pathway was acting independently or was somehow coordinated with the estrogen surge often implicated as the most important aspect defining the implantation event. Hence this study was undertaken to confirm the cross-talk, if any, between the estrogen surge and the calcium influx both essentially very important pre-implantation requirements.
Results and discussion
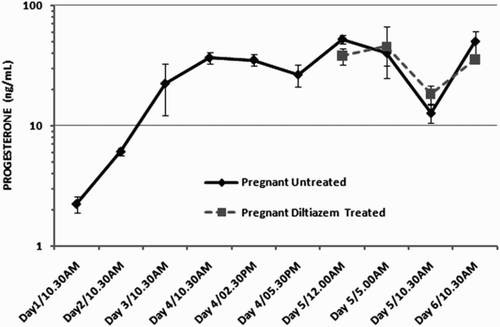
The use of Chicago blue dye confirmed that implantation was proceeding normally in the untreated animals () whereas implantation was completely inhibited in the diltiazem treated animals and no implantation sites were observed as previously reported [Banerjee et al. Citation2009]. The levels of 17β-estradiol and progesterone were similar to earlier reported values for the initial days of pregnancy in mice (). A peak in estradiol was seen on d 4, 10:30 a.m. which continued until d 4, 2:30 p.m. and reduced by 5:30 p.m. Progesterone levels were almost unchanged except for a minor decrease right after implantation ().
Figure 1. Implantation sites in the normal mouse uterus. Chicago blue dye stained implantation sites in untreated normal mouse uterus on d 6, 10:30 a.m. Equally spaced, normal appearing implants are observed.
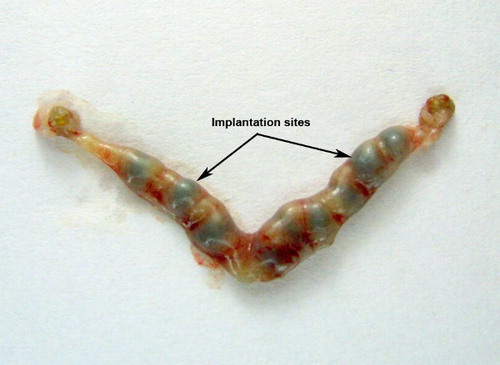
Figure 2. The level of estradiol in pregnant mice. Circulating estradiol levels in pregnant untreated mice on days 1-6 of early pregnancy. Circulating estradiol levels in pregnant mice on days 5 and 6 that were treated with 25 µg of diltiazem.
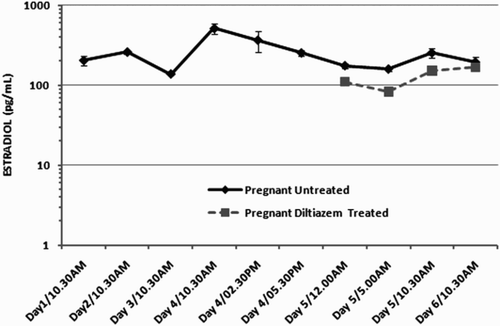
Figure 3. The level of progesterone in pregnant mice. Circulating progesterone levels in pregnant mice on days 1-6 of early pregnancy. Circulating progesterone levels in pregnant mice on days 5 and 6 that were treated with 25 µg of diltiazem.
The peripheral plasma concentration of estradiol in mice during pregnancy [McCormack and Greenwald Citation1974a] is believed to rarely exceed 60 pg/ml. However in our study the levels were almost always greater than 100 pg/ml. This can be attributed to the sensitive analytical systems that are available. Peripheral levels of 17ß-estradiol were low on d 4 of pregnancy, and the peak observed between 10:30 a.m. and 2:30 p.m., although significantly different from the other times sampled, did not constitute a marked ‘surge'. Therefore, even though the 'surge' is not dramatic it is of physiological consequence with respect to implantation.
In mouse, many of the uterine changes depend on a transient rise in estradiol that occurs between 09:00 a.m. and 12:00 p.m. on d 4 and persists for about 24 h [McCormack and Greenwald Citation1974b]. The central role of estradiol in triggering these events is indicated by their failure to occur in ovarectomized animals and their subsequent induction when such animals are injected with estradiol [Ma et al. Citation2003; Paria et al. Citation1998; Citation2002]. Nonetheless, the pathway linking estradiol to these events is in most cases not well defined, although its ability to regulate expression of many growth factors and cytokines is well established [Das et al. Citation2000; Smith et al. Citation1997; Cheng et al. Citation2002; Chen 2000]. Thus estrogen is essential for implantation in the progesterone primed mouse uterus. It has been noted that the period from 10:00 a.m. to 12:00 p.m. on d 4 appears to be critical; before this time, insufficient estrogen is secreted, in most instances, to initiate implantation [McCormack 1974a]. This was also reflected in our study.
In vivo administration of diltiazem about 12 h prior to implantation (approximated at 5:00 a.m., d 5) did not show any interference with the normal systemic E2-P4 levels when compared to the normal circulating levels during the first week of pregnancy in mice ( and ). Thus the surge in estrogen had indeed passed when calcium channels were inhibited prior to implantation and in no way did the use of the calcium channel blocker diltiazem interfere with the circulating hormones. Thus it would be apparent that the failure in implantation observed upon the injection of diltiazem 12 h prior to implantation is not due to its effect on the E2-P4 mediated pathway but is probably under a separate regulatory control.
Accordingly, the previously reported inhibition of blastocyst implantation cascade by calcium channel blockers during the ‘implantation window’ seems to be an independent mechanism interfering with uterine receptivity without any direct estrogen-progesterone control [Banerjee et al. Citation2009]. This makes it essential now to understand the alternate mechanism controlling calcium channel regulation during blastocyst implantation and is the subject of further research.
Materials and methods
Animals
Mature inbred female mice (Swiss albino, 2-3 months old) were used for all the studies (n = 6/group). Animals were housed 3 per cage in polypropylene cages with autoclaved rice husk as bedding material. The general environmental conditions were strictly controlled in the colony, such as 10% air exhaust in the air conditioning unit, relative humidity 60 ± 5%, temperature 27 ± 3°C and a light:dark regimen of 14:10 h. Amrut certified rodent diet (Maharashtra Chakan Oil Mills Ltd., India) and tap water (boiled and cooled to room temperature) were provided ad libitum to all the animals. Animal handling techniques were performed in accordance with GLP and all experimental protocols were approved by the IAEC, constituted by the Ministry of Social Justice and Empowerment, Government of India, prior to initiation of the experiments.
Only those females showing a regular 4-5 d estrous cycle were used. Vaginal smears were examined daily between 10:00–10:30 a.m. [Stockard and Papanicolaou Citation1917]. Those found in proestrus or P2E stage were placed in a cage with a male of proven fertility for mating. The next morning at 10:00 a.m., female mice were checked for the presence of a vaginal plug or the presence of sperms in a vaginal smear. The finding of either was taken to be indicative of d 1 of pregnancy. Pregnant animals were segregated randomly into two groups.
Luminal delivery of diltiazem in the uterus
In one group of pregnant female mice on d 4 of pregnancy, at 5:00 p.m. both uterine horns of the animals were injected with the test drug (diltiazem in 0.9% normal saline; 25 µg/ animal) through a ventral incision under ketamine:xylazine anesthesia (80:10mg/kg) [Banerjee et al. Citation2009].
Evaluation of the circulating 17β-estradiol and progesterone levels in the pregnant and diltiazem treated pregnant mice
Blood was withdrawn from both treated and untreated groups of pregnant female mice through the retro orbital sinus under light ether anaesthesia as per the following schedule: Days 1–6, 10:30 a.m., Day 4: 5:00 p.m., Day 5: 12:00 a.m. and 5:00 a.m. Blood samples were centrifuged at 2500 rpm for 10 min, serum was separated and stored at −20°C until analysis. Estimation of 17β-estradiol and progesterone was performed using the chemiluminescence assay system (ADVIA-Centaur chemistry analyser, Siemens Medical Solutions Diagnostics, USA) using ACS Centaur E2 6 assay system.
Visualization of implantation sites in pregnant uterus using Chicago blue vital dye
Chicago blue dye (0.1ml of 1%) was injected intravenously through the tail vein followed by euthanasia after 5 min. in CO2. Intact uterus was excised in normal saline, adhering fat was cleaned and the tissue was taken for photography.
Statistical analysis
All results are presented as mean ± SEM. Statistical analyses were performed using Student's unpaired t-test and p < 0.05 was considered significant.
Abbreviations
| cPLA2: | = | cytosolic phospholipase A2 |
| GLP: | = | Good Laboratory Practice |
| IAEC: | = | Institutional Animal House Ethics Committee |
| P2E: | = | proestrus to estrus |
| E2-P4: | = | estradiol-progesterone |
Declaration of Interest: The authors express their gratitude towards the Industries Commissionerate, Government of Gujarat, India for their financial support towards this project. No conflicts of interest exist. The authors alone are responsible for the content and writing of the paper.
References
- Banerjee, A., Padh, H. and Nivsarkar, M. (2009) Role of calcium channel in blastocyst implantation: A novel contraceptive target. J Basic Clin Physiol Pharmacol 20:43–53.
- Bloch, S. (1958) Experimental research on the hormonal basis of implantation in mammals. Experientia 14:447–449.
- Chen, J.R., Cheng, J.G., Shatzer, T., Sewell, L., Hernandez, L. and Stewart, C.L. (2000) Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 141:4365–4372.
- Cheng, J.G., Rodriguez, C.I. and Stewart, C.L. (2002) Control of uterine receptivity and embryo implantation by steroid hormone regulation of LiF production and LiF receptor activity: towards a molecular understanding of “the window of implantation.” Rev Endocr Metab Disord 3:119–126.
- Cochrane, R.L. and Meyer, R.K. (1957) Delayed nidation in the rat induced by progesterone. Proc Soc Exp Biol Med 96:155.
- Das, S.K., Tan, J., Raja, S., Halder, J., Paria, B.C. and Dey, S.K. (2000) Estrogen targets genes involved in protein processing, calcium homeostasis Wnt signaling in the mouse uterus independent of estrogen receptor-alpha and -beta. J Biol Chem 275:28834–28842.
- DeFeo, V.J. (1963) Temporal aspects of uterine sensitivity in the pseudopregnant or pregnant rat. Endocrinology 72:305–316.
- Finn, C.A. and Martin, L. (1969) Hormone secretion during early pregnancy in the mouse. J Endocr 45:57–65.
- Hedlund, K. and Nilsson, O. (1971) Hormonal requirements for the uterine attachment reaction and blastocyst implantation in the mouse, hamster and guinea-pig. J Reprod Fert 26:267–269.
- Ma, W.G., Song, H., Das, S.K., Paria, B.C. and Dey, S.K. (2003) Estrogen is a critical determinant that specifies the duration of the window of the uterine receptivity for implantation. Proc Natl Acad Sci USA 100:2963–2968.
- McCormack, J.T. and Greenwald, G.S. (1974a) Progesterone and estradiol-17ß concentrations in peripheral plasma during pregnancy in the mouse. J Endocr 62:101–107.
- McCormack, J.T. and Greenwald, G.S. (1974b) Evidence for a preimplantation rise in estradiol-17ß on day 4 of pregnancy in the mouse. J Reprod Fert 41:297–301.
- Paria, B.C., Lim, H., Wang, X.N., Liehr, J., Das, S.K. and Dey, S.K. (1998) Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse. Endocrinology 139:5235–5236.
- Paria, B.C., Reese, J., Das, S.K. and Dey, S.K. (2002) Deciphering the cross-talk of implantation: advantages and challenges. Science 296:2185–2188.
- Shaikh, A.A. (1971) Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod 5:297–307.
- Smith, S.E., French, M.M., Julian, J., Paria, B.C. and Dey, S.K., Carson, D.D. (1997) Expression of heparin sulfate proteoglycan (perlecan) in the mouse blastocyst is regulated during normal and delayed implantation. Dev Biol 184:38–47.
- Smith, D.M. and Biggers, J.D. (1968) The oestrogen requirement for implantation and the effect of its dose on the implantation response in the mouse. J Endocr 41:1–9.
- Stockard, C.R. and Papanicolaou, G.N. (1917) The existence of a typical oestrous cycle in the guinea pig with a study of its biochemical and physiological changes. Am J Anat 22:225–283.
- Zeilmaker, G.H. (1963) Experimental studies on the effect of ovarectomy and hypophysectomy on blastocyst implantation in the rat. Acta Endocrinologica 44:355–366.