Abstract
Bisphenol-A (BPA) is an industrial chemical and is known to act as an endocrine disrupter. This study was designed to evaluate how BPA regulates Sertoli cell (SC) signal molecules. Purified rat SCs were cultured and treated with BPA (200 µmol/l) at various time points. Western blot analysis was used to determine the activation of extracellular signal-related kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38 mitogen activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), cyclooxygenase-1,2 (COX-1, 2), estrogen receptor-α (ER-α), and androgen receptor (AR). The levels of transferrin (TF), prostaglandin E2 (PGE2), and prostaglandin F2α (PGF2α) in culture medium were quantified by ELISA. Interleukin (IL)-1β and IL-6 mRNAs were measured by quantitative real-time PCR (QRT-PCR). Compared with the control, BPA activated the phosphorylation of ERK1/2 (p-ERK1/2) through 30 min to 6 h. TF was down-regulated at 6 and 24 h. Furthermore, IL-1β was up-regulated at 30 min and IL-6 was up-regulated at 1 and 24 h. ERK activity inhibitor (PD98059, 10 µmol/l) inhibited these molecular changes. These results reveal the possibility that BPA may have adverse effects on spermatogenesis via ERK1/2.
Introduction
BPA [2,2-bis(4-hydroxyphenyl)propane] is a monomer of polycarbonate plastics and is widely used as a compound in food and drink cans [Brotons et al. Citation1995], as well as being a component of dental sealant [Olea et al. Citation1996]. It has been reported that BPA easily leaches from food packaging and dental sealants into saliva, and works as an estrogen [Brotons et al. Citation1995; Krishnan et al. Citation1993]. The actual effects of these molecules, such as BPA, on the male reproductive system have attracted tremendous attention recently, and many studies have been reported. Although controversy remains with respect to the effects of BPA on male fertility, growing evidence suggests BPA can act as a potential testicular toxicant that alters spermatogenesis [Iida et al. Citation2003; Toyama et al. Citation2004; Salian et al. Citation2009]. Spermatogenesis is a highly complex process regulated by various endocrine and paracrine/autocrine molecules. In this complex mechanism, SC synthesizes many essential factors for spermatogenesis and supports developing germ cells. Recently, there has been increasing evidence of the molecular mechanisms involved in SC paracrine/autocrine regulation in response to various molecules, such as antineoplastic reagents [Yamaguchi et al. Citation2008] and pro-inflammatory cytokines [Ishikawa et al. Citation2005; Ishikawa and Morris Citation2006a]. Iida et al. [2003] reported that BPA induced apoptosis of cultured rat SC via changes in cell structure in a dose- and time-dependent manner. Meanwhile, Salian et al. revealed that neonatal exposure of male rats to BPA impairs the fertility and expression of SC junction proteins in the testis [Salian et al. Citation2009]; however, the molecular mechanisms involved in BPA regulation of SC function, which subsequently influence developing germ cells and spermatogenesis, have not been detailed. The aim of the present study was to determine how BPA regulates SC paracrine/autocrine activities and subsequently influences developing germ cells and spermatogenesis.
Results
BPA induces activation of ERK1/2 in SC
BPA induced the activation of ERK1/2 at 30 min, 1 – 6 h after BPA treatment. The p-ERK1/2 protein doublet (44 and 42 kDa, respectively) significantly increased 1.8 – 2.0 fold compared with BPA treatment and the Ct (A and B). p-JNK or -p38 MAPK, and COX-1, COX-2 were not activated during this time course (30 min – 24 h) (data not shown). Although the toxic effects of BPA have been proposed to be mediated through binding to ER [Takayanagi et al. Citation2006], we have confirmed that BPA did not induce a change of at least ERα or AR protein levels during the time course studied (data not shown). A previous study showed that BPA significantly activated ERK and inhibited NF-κB, which is considered anti-apoptotic in PC12 cells and cortical neuronal cells but not in SC [Lee et al. Citation2007]. In neuronal cells, the ERK/NF-κB pathway may be involved in BPA-induced toxicity; however, there was no significant change in the NF-κB protein level in this study (data not shown). Treatment with BPA in the absence or presence of the ERK inhibitor, PD98059 repressed BPA-induced phosphorylated ERK1/2 protein levels at 1 – 6 h. Based on previous studies indicating that some SC secretions, including physiological and proinflammatory cytokine factors, may play a role in paracrine and autocrine regulation and the dysregulation of SC paracrine function [Ishikawa et al. Citation2005; Ishikawa and Morris Citation2006a; Citation2006b; Yamaguchi et al. Citation2008], we next examined the effect of BPA on the level of SC secretions, including cytokine expression.
Figure 1. The expression of p-ERK1/2 and ERK1/2 protein doublet (44 and 42 kDa, respectively) in rat SC cultured with BPA (200 µml/l) determined by Western blot analysis (A). Relative intensity of p-ERK1/2 protein levels in rat SC cultured with BPA in the presence or absence of specific ERK activity inhibitor, PD98059 (10 µmol/l) compared with the control (Ct), set at a value of 1 (B). *:p < 0.05; **: p < 0.01; ***:p < 0.001; significantly different from the Ct value. †: p < 0.05; ††: p < 0.01; significantly different from the increases obtained with BPA only.

BPA reduces SC TF secretion through the ERK1/2 pathway
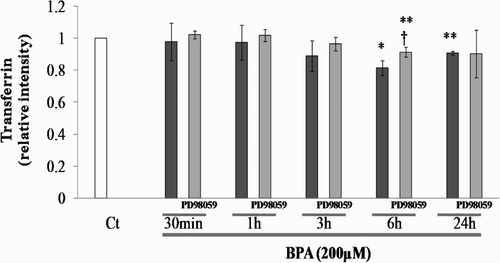
To determine the effect on the function of SC through the activation of p-ERK1/2 induced by BPA treatment, levels of SC-secreted TF were evaluated in the presence or absence of an ERK activity inhibitor. A significant reduction in the level of SC-secreted TF was observed at 6 and 24 h after BPA treatment (at 6 h: 0.81-fold, P < 0.05, at 24 h: 0.90-fold, P < 0.01) compared with BPA and vehicle (Ct) (). In the presence of the ERK inhibitor PD98059 (10 µmol/l), reduction of SC-secreted TF induced by BPA was inhibited at 6 h. From this result, it was revealed that BPA has the potential to reduce TF secretion through the ERK1/2 pathway in SC. SC PGE2 and PGF2α expression showed no significant differences after BPA treatment (data not shown).
Figure 2. Relative intensity of transferrin levels in rat SC cultured with BPA in the presence or absence of specific ERK activity inhibitor, PD98059 (10 µmol/l) compared with the control (Ct), set at a value of 1. *: p < 0.05; **: p < 0.01; significantly different from the Ct value. †: p < 0.05; significantly different from the increases obtained with BPA only.
BPA induction of IL-1β and IL-6 expression occurs in part through the ERK1/2 pathway
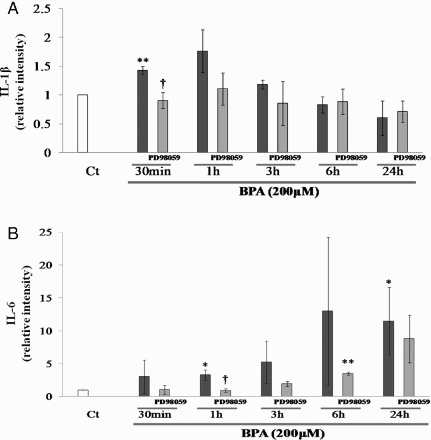
The expression of IL-1β mRNA was significantly induced by BPA 30 minutes after treatment (1.4-fold, P < 0.01, compared with BPA and the vehicle (Ct)). Although the mRNA levels remained high at 1 and 3 h after BPA treatment, there were no significant changes compared with the Ct. Meanwhile, the expression of IL-6 mRNA tended to increase from 1 h after BPA treatment. A significant increase in the corresponding level of mRNA was observed at 1 h and 24 h (3.2 and 11.5-fold: P < 0.05, compared with BPA and the Ct, respectively). To determine whether the activation of ERK1/2 was required for these changes in cytokine expression, the effect of the ERK1/2 selective activity inhibitor on BPA-mediated regulation of IL-1β and IL-6 mRNA levels was examined. PD98059 significantly inhibited, in part, BPA-stimulated increases in IL-1β mRNA. Although PD98059 tended to inhibit BPA-stimulated increases in IL-6 mRNA during this time course (30 min – 24 h). However, significant inhibition of mRNA induction was observed at 1 h after BPA treatment. From these results, changes in IL-1β and IL-6 mRNA expression are somewhat dependent on ERK1/2 activity (A and B).
Figure 3. Relative intensity of IL-1β (A) and IL-6 (B) mRNA levels in rat SC cultured with BPA in the presence or absence of specific ERK activity inhibitor, PD98059 (10 µmol/l) compared with the control (Ct), set at a value of 1. *: p < 0.05; **: p < 0.01; significantly different from the Ct value. †: p < 0.05; significantly different from the increases obtained with BPA only.
Discussion
In this study, we showed that BPA has the possibility to induce adverse effects on SC function and likely fertility. Our results show that, at least in vitro, BPA at a concentration of 200 µmol/l (45 µg/ml) seems to be harmful to SC. This likely reflects decreased SC TF secretion that parallels increased SC pro-inflammatory cytokine, such as IL-1β or IL-6, production via the ERK1/2 pathway. On first inspection the concentration (200 µmol/l) of BPA used in this study appears to be relatively high. However it is equivalent to the concentration of BPA released from plastic dental sealants in saliva (931 µg/30 ml) [Olea et al. Citation1996]. It is known that TF plays an important role in spermatogenesis by providing iron to developing germ cells and is useful as an index of SC function [Holmes et al. Citation1983; Sylvester et al. 1994]. In a previous study, a similar result was indicated by Iida et al. [2003], who showed that secretion of TF from SC decreased following exposure to BPA. However, to our knowledge, this is the first data revealing the time-dependent effect of BPA stimulation on Sertoli TF secretion. We have previously shown that an antineoplastic reagent, cis-diaminedichloroplatinium (CDDP), can similarly reduce Sertoli TF secretion through the ERK1/2 pathway [Yamaguchi et al. Citation2008]; however, in this study, the reduction of SC TF secretion by BPA was observed later and its reduction was less than previously observed using CDDP. These differences in the same signalling pathways induced by various chemicals may depend on the context of the nature of the stimuli.
ILs have been shown to play multiple interactive roles in the regulation of testicular somatic cell functions and male germ cell development [Hakovirta et al. Citation1995; Syed et al. Citation1995; Legue et al. Citation2001; Petersen et al. Citation2002; Ishikawa et al. Citation2005]. In defined stages of the rodent seminiferous cycle, the presence of residual bodies, which represent membrane-enclosed cytoplasmic contents shed by elongating spermatids during spermiogenesis, activates Sertoli cell phagocytosis and IL-1 release, events that initiate IL-6 secretion [Gerard et al. Citation1992; Syed et al. Citation1993; Syed et al. Citation1995]. This physiological stage-specific process induces SC IL-1β and IL-6 production, and also accompanies pathological testicular inflammation [Gerard et al. Citation1992; Syed et al. Citation1993; Okuda et al. Citation1994; Okuda et al. Citation1995; Syed et al. Citation1995; Cudicini et al. Citation1997]. The data presented in this communication showed that BPA also induced Sertoli cytokine secretion that might lead to inflammatory-like changes in SC. It has been shown that IL-1β induced Sertoli IL secretion is mediated by the activation of COX-2, which is the enzyme metabolizing arachidonic acid, and the phosphorylation of JNK [Ishikawa et al. Citation2005]. Similarly, our previous study showed that CDDP induced the activation of the COX-2 pathway only, which subsequently induced PGE2 and PGF2α production, and, furthermore, proceeded to stimulate IL secretion in SC. With the exception of ERK1/2, neither the activation cascade through the MAPK pathway nor arachidonic acid was observed during this time course. Thuillier et al. [2009] recently indicated that fetal BPA exposure upregulated rat testes ERK1/2 mRNA and protein, mainly in SC. However, only transient effects were observed on actual germ cell populations [Thuillier et al. 2009]. The data presented above suggest that ERK1/2 MAPK is partially involved in IL secretion in prepubertal rat SC. In summary, this study identified for the first time that BPA-ERK1/2 MAPK mediated the regulatory mechanism for TF secretion and inducible IL production in SC. Interestingly, we did not observe a change in SC ER-α and AR protein levels even though ER belongs to the family of nuclear hormone receptors that has an extended pattern of functional expression [Ordóñez-Morán and Muñoz Citation2009]. Whether ER is involved in the response to BPA is not clear. Thus to precisely establish the molecular signaling mechanism response of SC to BPA, further investigation will be necessary to fully understand the effect of BPA on male fertility.
Materials and Methods
Sertoli cell preparations
SCs were purified from the testes of 18-day-old Sprague Dawley rats purchased from Oriental Yeast Co. (Tokyo, Japan). Procedures involving the use of animals were approved and conducted according to the Guidelines for Animal Experiments at Kobe University School of Medicine. Primary cultures (≥95% pure) were maintained as described previously by Kanaki and Morris [1998]. On day 3 ex vivo, SC were rinsed twice with fresh serum- and phenol red-free culture medium and then treated with BPA (200µmol/l) (Sigma-Aldrich Co. MO, USA), which was dissolved in dimethylsulfoxide (DMSO) (Sigma-Aldrich) immediately prior to use, in the absence or presence of ERK activity inhibitor PD98059 (2’-amino-3’-methoxyflavone, 10 µmol/l) (Cayman Chemical Co., MI, USA), which was also dissolved in DMSO. At 30 min, 1, 3, 6, and 24 h following Ct, BPA, or the addition of inhibitor, whole cell lysates for protein and total RNA were isolated from each replicate. At least triplicate culture dishes were used for each drug treatment, and experiments were repeated at least twice. The mean (± SEM) of all experiments was calculated for analysis.
Protein extraction and Western blot analysis
Protein extraction and Western blot analysis were performed as described previously [Yamaguchi et al. Citation2008]. In this study, a total of 20–25 µg protein was separated by SDS-polyacrylamide gel electrophoresis using 4–20% Tris-glycine gel (Novex, CA, USA), and was electrophoretically transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, NH, USA). The membranes were probed with antibodies, as described below. Phospho-stress-activated protein kinase/JNK (p-SAPK/JNK) polyclonal antibody (pAb; 1:1000), JNK pAb (1:1000), p-ERK1/2 pAb (1:1000), ERK1/2 pAb (1:1000), phospho-p38 (p-p38) MAPK pAb (1:1000), and p38 pAb (1:1000) were obtained from Cell Signaling Technology, Inc. (MA, USA). COX-1 and COX-2 pAb (1:1000) were obtained from Cayman Chemical Co. ERα pAb (1:200), AR pAb (1:200), and NF-κB pAb (1:200) were obtained from Santa Cruz Biotechnology, Inc. (CA, USA). Monoclonal anti-β-actin antibody (1:1000) was purchased from Sigma-Aldrich Co. Blots were developed with the ECL Western blotting system (Amersham Biosciences, IL, USA) and exposed to X-ray film (FUJIFILM Medical Co., Tokyo, Japan). Densitometric analysis was performed using the personal computer version of the National Institutes of Health Image software (Scion Image; Scion Image Corp., MD, USA) after scanning. For Western blotting analyses, particular signal intensities in each lane of p-JNK, -ERK 1/2, and -p38 were normalized with those for JNK, ERK 1/2, and p38 on the same membranes, respectively. The others were normalized with those for β-actin on the same membrane and data are expressed as arbitrary units relative to the control, set at a value of “1”.
ELISA
To measure the concentration of SC-produced TF and PG levels in culture media, SC were treated with BPA (200 µmol/l), BPA with PD98059 (10 µmol/l) or matched vehicle blank controls (Ct) for 30 min, 1, 3, 6, and 24 h. At the indicated time, cell-free supernatants were transferred to sterile microcentrifuge tubes. Two 40- or 50-µl aliquots of conditioned medium were assayed using TF (Panapharm Laboratories, Kumamoto, Japan) or individual PGE2, PGF2α (Cayman Chemical) ELISA kits according to the manufacturer's instructions, respectively. The sensitivity of the assay (80% bound) was 0.63 ng/ml, 36, and 9 pg/ml for TF, PGE2, and PGF2α, respectively, and intra- and interassay coefficients of variation were less than 10% for all ELISA kits. The concentrations of TF, and PGs were determined by ELISA measured by a standard curve method using a microplate reader (model 550; Bio-Rad Laboratories, CA, USA). Data are expressed as arbitrary units relative to the control, set at a value of “1”.
Total RNA extraction and Quantitative real-time PCR analysis
Total RNA was extracted and QRT-PCR was performed as described previously [Yamaguchi et al. Citation2008]. ILs and β-actin mRNAs were detected using proprietary TaqMan primers and probes (Applied Biosystems, CA, USA). These probes and primers were used for IL-1β (assay identification number Rn 00580432_m1), IL-6 (assay identification number Rn 00561420_m1), and β-actin (assay identification number Rn 00667869_m1). Data were normalized with β-actin values and expressed as arbitrary units relative to the control, set at a value of “1”. Densitometric analyses are expressed in arbitrary units.
All results are the mean ± SEM derived from the number of different experiments. Statistical analyses were performed using t test or paired-t test, ANOVA. P values ≤ 0.05 were considered significant. We used Scheffe's procedure as a post-hoc test.
Declaration of interest: The authors declare that there is no potential conflict of interest amongst them that would prejudice the impartiality of this scientific work.
Abbreviations
| BPA: | = | Bisphenol-A |
| SC: | = | Sertoli cell |
| ERK1/2: | = | extracellular signal-related kinases 1 and 2 |
| JNK: | = | c-Jun N-terminal kinase |
| MAPK: | = | mitogen activated protein kinase |
| NF-κB: | = | nuclear factor kappa B |
| COX-1, 2: | = | cyclooxygenase-1,2 |
| ER-α: | = | estrogen receptor-α |
| AR: | = | androgen receptor |
| TF: | = | transferrin |
| PGE2: | = | prostaglandin E2 |
| PGF2α: | = | prostaglandin F2α |
| IL: | = | interleukin |
| QRT-PCR: | = | quantitative real-time PCR |
| p-ERK1/2: | = | phosphorylation of ERK1/2 |
| Ct: | = | control |
| CDDP: | = | cis-diaminedichloroplatinium |
| DMSO: | = | dimethylsulfoxide. |
References
- Brotons, J.A., Olea-Serrano, M.F., Villalobos, M., Pedraza, V. and Olea, N. (1995) Xenoestrogens released from lacquer coating in food cans. Environ Health Perspect 103:608–612.
- Cudicini, C., Kercret, H., Touzalin, A.M., Ballet, F. and Jegou, B. (1997) Vectorial production of interleukin 1 and interleukin 6 by rat Sertoli cells cultured in a dual culture compartment system. Endocrinology 138:2863–2870.
- Gerard, N., Syed, V. and Jegou, B. (1992) Lipopolysaccharide, latex beads and residual bodies are potent activators of Sertoli cell interleukin-1 alpha production. Biochem Biophys Res Commun 185:154–161.
- Hakovirta, H., Syed, V., Jegou, B. and Parvinen, M. (1995) Function of interleukin-6 as an inhibitor of meiotic DNA synthesis in the rat seminiferous epithelium. Mol Cell Endocrinol 108:193–198.
- Holmes, S.D., Bucci, L.R., Lipshultz, L.I., Smith and R.G. (1983) Transferrin binds specifically to pachytene spermatocytes. Endocrinology 113:1916–1918.
- Iida, H., Maehara, K., Doiguchi, M., Mori, T. and Yamada, F. (2003) Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod Toxicol 17:457–464.
- Ishikawa, T., Hwang, K., Lazzarino, D. and Morris, P.L. (2005) Sertoli cell expression of steroidogenic acute regulatory protein-related lipid transfer 1 and 5 domain-containing proteins and sterol regulatory element binding protein-1 are interleukin-1beta regulated by activation of c-Jun N-terminal kinase and cyclooxygenase-2 and cytokine induction. Endocrinology 146:5100–5111.
- Ishikawa, T. and Morris, P.L. (2006a) A multistep kinase-based sertoli cell autocrine-amplifying loop regulates prostaglandins, their receptors, and cytokines. Endocrinology 147:1706–1716.
- Ishikawa, T. and Morris, P.L. (2006b) Interleukin-1beta signals through a c-Jun N-terminal kinase-dependent inducible nitric oxide synthase and nitric oxide production pathway in Sertoli epithelial cells. Endocrinology 147:5424–5430.
- Kanzaki, M. and Morris, P.L. (1998) Identification and regulation of testicular interferon-gamma (IFNgamma) receptor subunits: IFNgamma enhances interferon regulatory factor-1 and interleukin-1beta converting enzyme expression. Endocrinology 139:2636–2244.
- Krishnan, A.V., Stathis, P., Permuth, S.F., Tokes, L. and Feldman, D. (1993) Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132: 2279–2286.
- Lee, Y.M., Seong, M.J., Lee, J.W., Lee, Y.K., Kim, T.M. and Nam, S.Y., (2007) Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen. J Vet Sci 8:27–38.
- Legue, F., Guitton, N., Brouazin-Jousseaume, V., Colleu-Durel, S., Nourgalieva, K. and Chenal, C. (2001) IL-6 a key cytokine in in vitro and in vivo response of Sertoli cells to external gamma irradiation. Cytokine 16:232–238.
- Okuda, Y., Sun, X.R. and Morris, P.L. (1994) Interleukin-6 (IL-6) mRNAs expressed in Leydig and Sertoli cells are regulated by cytokines, gonadotropins and neuropeptides. Endocrine 2: 617–624.
- Okuda, Y., Bardin, C.W., Hodgskin, L.R., Morris and P.L. (1995) Interleukins-1 alpha and -1 beta regulate interleukin-6 expression in Leydig and Sertoli cells. Recent Prog Horm Res 50:367–372.
- Olea, N., Pulgar, R., Perez, P., Olea-Serrano, M.F., Rivas, A. and Novillo-Fertrell, A., (1996) Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect 104:298–305.
- Ordóñez-Morán, P. and Muñoz, A. (2009) Nuclear receptors: genomic and non-genomic effects converge. Cell Cycle 8: 1675–1680.
- Petersen, C., Boitani, C., Froysa, B. and Soder, O. (2002) Interleukin-1 is a potent growth factor for immature rat sertoli cells. Mol Cell Endocrinol 186:37–47.
- Salian, S., Doshi, T. and Vanage, G. (2009) Neonatal exposure of male rats to Biphenol A impairs fertility and expression of Sertoli cell junctional proteins in the testis. Toxicology 265:56–67.
- Syed, V., Gerard, N., Kaipia, A., Bardin, C.W., Parvinen and M., Jegou, B. (1993) Identification, ontogeny, and regulation of an interleukin-6-like factor in the rat seminiferous tubule. Endocrinology 132:293–299.
- Syed, V., Stephan, J.P., Gerard, N., Legrand, A., Parvinen and M., Bardin, C.W., (1995) Residual bodies activate Sertoli cell interleukin-1 alpha (IL-1 alpha) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway Endocrinology 136:3070–3078.
- Sylvester, S.R. and Griswold, M.D. (1994) The testicular iron shuttle: a “nurse” function of the Sertoli cells. J Androl 15:381–385
- Takayanagi, S., Tokunaga, T., Liu, X., Okada, H., Matsushima, A. and Shimohigashi, Y. (2006) Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol Lett 167:95–105.
- Thuillier, R., Manku, G., Wang, Y. and Culty, M. (2009) Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells. Microscopy Research and Technique 72:773–786.
- Toyama, Y., Suzuki, F., Maekawa, M., Ito, C. and Toshimori, K. (2004) Adverse effects of bisphenol A to spermatogenesis in mice and rats. Arch Histol Cytol 67:373–381.
- Yamaguchi, K., Ishikawa, T., Kondo, Y. and Fujisawa, M. (2008) Cisplatin regulates Sertoli cell expression of transferring and Interleukins. Mol Cell Endocrinol 283:68–75.