Abstract
Di-n-butyl phthalate (DBP) is one of the most abundantly produced endocrine disruptors that leaches out from polyvinyl chloride plastics and can cause hypospadias in male rats during maternal exposure. The objective of this study was to first explore the roles of Wnt/β-catenin pathway in the fetal rat genital tubercle (GT) following in-utero exposure to DBP. Timed-pregnant rats were given DBP by gastric intubation at a dose of 750 mg/kg body weight (bw)/day from gestation day (GD) 14 to GD18 to establish a rat model of hypospadias. On GD19, genital tubercle down-regulation of β-catenin, Phospho-GSK-3β, and up-regulation of GSK-3β (glycogen synthase kinase-3β), NFκB in fetal male rats was observed by western blot analysis. β-catenin was located in the urethral plate epithelium (UPE). Immunochemistry showed that the relative expression of β-catenin decreased in the DBP-treated fetal rat GT compared to the normal control. These findings, for the first time, indicate that DBP may affect the development of GT by down-regulating the Wnt/β-catenin pathway in fetal male rats.
INTRODUCTION
Hypospadias is one of the most common disorders of sexual development fueling interest in understanding this response during organogenesis of the external genitalia. This developmental anomaly, in which the urethral meatus is located at the ventral (lower) side of the penis, occurs in approximately 1 of 125 - 200 live male human births [Gallentine et al. Citation2001; Wang and Baskin et al. 2008] and its incidence is rising [Boisen et al. Citation2005, Yang et al. Citation2007]. Although the combined effects of genetic and environmental factors have been suggested to play an important role in hypospadias [Kalfa et al. Citation2009; Manson and Carr Citation2003], the precise etiopathogenesis and molecular mechanism of this malformation remains largely unknown.
Di-n-butyl phthalate (DBP) resides in our environment as a product of manufacturing [Pan et al. Citation2006] that may cause adverse effects in the male reproductive system [Foster Citation2006; Martino-Andrade and Chahoud Citation2010; Huang et al. Citation2009; Rozati et al. Citation2000]. Results from recent epidemiological studies indicate associations of phthalate exposure with a number of human reproductive disorders including reduced anogenital distance (AGD) in male infants [Swan et al. Citation2005] and poor semen quality in adult males [Duty et al. Citation2003; Duty et al. Citation2004; Hauser et al. Citation2006]. It has been reported that in utero exposure of rats to DBP can provide a useful animal model for studying the human testicular dysgenesis syndrome (TDS), a condition characterized by cryptorchidism, hypospadias, low sperm counts, and testicular cancer [Fisher et al. Citation2003]. In a previous study our group successfully established a reproducible rat model of hypospadias by maternal exposure to DBP at 750 mg/kg bw/day during later pregnancy [Zhang et al. Citation2007; Jiang et al. Citation2007] and we also have utilized the technique of proteomic analysis to compare the differential expression of proteins in testis between fetal control and DBP-treated rats at GD19. However, a close examination of developmental alterations and protein expression during the mesenchymal-to-epithelial transformation in the genital tubercle (GT), the precursor of the penis in males and the clitoris in females, induced by maternal exposure to DBP had yet to be detailed.
Recent remarkable advances in molecular approaches have provided an insight into the molecular genetics of external genitalia formation and have contributed to identifying causative and risk factors for hypospadias [Kojima et al. Citation2010]. It was first reported that fibroblast growth factor (Fgf) was a key element orchestrating GT development, with the role of Sonic hedgehog (Shh) in external genitalia formation subsequently demonstrated [Haraguchi et al. Citation2000; Haraguchi et al. Citation2001]. Up to now, various studies have shown that the initial GT outgrowth and patterning requires the coordinated output of several growth factors, including Shh, Wnt, bone morphogenetic protein (Bmp), and Fgf [Miyagawa et al. 2009; Beleza-Meireles et al. Citation2007; Seifert et al. Citation2009]. It was subsequently shown that DBP disrupted the expression of Shh, Bmp, and Fgf in the GT of newborn hypospadiac rats [Zhu et al. Citation2009]. However, the role of the Wnt/β-catenin pathway, an essential masculine effecter for GT development [Miyagawa et al. 2009], has not been clearly elucidated. Therefore, in this study, a rat model of hypospadias was established by gavaging pregnant Sprague–Dawley rats with DBP at a dose of 750 mg/kg bw/day during late gestation (GD14-GD18). The developmental abnormalities and the protein expression including Wnt/β-catenin and NFκB pathway in the GT of fetal DBP-treated male rats were evaluated to explore the mechanism.
RESULTS
Reproductive malformations of male offspring
On GD19, five pregnant rats of each group were sacrificed by carbon dioxide asphyxiation. On the day of delivery, a total of thirty-two and twenty-nine male pups were recovered from the control and DBP-treated litters, respectively. Of these, a total of thirty-two and thirty-one male pups from DBP-treated and control litters, respectively, were used in this study. Reproductive parameters of the litters are summarized in . A significantly decreased ratio of AGD/bw was observed in DBP-treated groups (P < 0.05). The gross malformation of hypospadias in DBP-treated rats was difficult to recognize until postnatal day (PND)60, when the male pups became sexually mature (). At this time, twenty-nine male pups were recovered from the five DBP-treated litters, including twelve hypospadiac rats and thirteen rats with cryptorchidism. In the control group, thirty-two male pups were observed from the five litters at PND60. Neither hypospadias nor cryptorchidism was found (). In comparison, the urethral meatus located ventrally at the base of the external genitalia with wider separation of the prepuce and the AGD (distance from the external genitalia to the anus) of the hypospadiac rats was decreased compared to that of the controls. Furthermore, in DBP-treated males, the hypospadiac rats usually presented with cryptorchidism. Both are consistent with the incidence of 41.3% and 45.7%, respectively, as previously reported [Jiang et al. Citation2007].
Figure 1. Typical gross images of hypospadias and control in adult rats (PND60). Gross images of the external genitalia of adult offsprings: B) hypospadiac rat. A and C) female and male control rats, respectively. Arrow represents the urethral meatus. The AGD (distance from the external genitalia to the anus) of the hypospadiac rats was remarkably decreased compared to that of the control males.
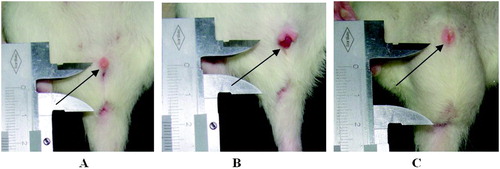
Table 1. Reproductive parameters in male offspring exposed prenatally to DBP.
Western blotting analysis of Wnt/β-catenin and NFκB pathway
Using the method of western blotting, we confirmed differential expression of Wnt/β-catenin pathway in GT between DBP-treated and control rats (). The protein expression of β-catenin in GD19 male GT of DBP-treated rats significantly decreased compared with control males (P < 0.05, A), while no significant difference was observed between DBP-treated and control GD19 females. Moreover, protein expression of Phospho-GSK-3β also decreased, which was associated with the increased expression of GSK-3β and NFκB in the DBP-treated groups (P < 0.05, B-D).
Figure 2. Quantitative changes in protein expression in the genital tubercle (GT) of fetal rats at GD19 induced by DBP (n = 3). A) Relative expression of β-catenin in the GT of fetal male and fetal female. From left to right: fetal control male, fetal DBP-treated male, fetal control female, and fetal DBP-treated female. B) Relative expression of Phospho-GSK-3β in fetal control male and fetal DBP-treated male. C) Relative expression of GSK-3β in fetal control male and fetal DBP-treated male. D) Relative expression of NF-κB in fetal control male and fetal DBP-treated male. *Significantly different from controls (P < 0.05).
Immunohistochemical and histological analysis of fetal DBP-treated genitalia
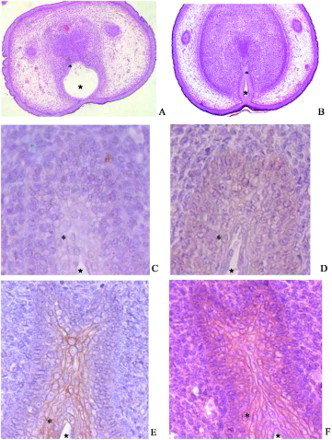
Cell-specific expression of β-catenin protein in GD19 rat GT, was determined by H&E (A,B) and immunohistochemical localization (C-F). There was a strong staining of β-catenin in the urethral plate epithelium (UPE; E,F) in control male GT. A relatively faint but positive signal was detected in the DBP-treated group (C,D). These observations are in agreement with the western blotting analysis described above.
Figure 3. Spatial expression of GD19 GTβ-catenin. Histological (A and B) and immunohistochemical (C-F) detection of fetal GT at GD19. Staining of β-catenin was mainly present in the cytoplasm of urethral plate epithelium (UPE) of control male (E and F). *: urethral plate epithelium (UPE). ★: urethra. Compared to the control male (E and F), relatively faint positive signal was detectable in DBP-treated group (C and D). These observations also verified the results of the western blotting analysis mentioned previously. Magnification: A, B × 10; C – F × 40.
Discussion
The molecular mechanism of hypospadias, one of the most frequent birth defects caused by the disorders in the formation of GT, is still not entirely clear [Kalfa et al. Citation2009; Manson and Carr Citation2003]. In the present study we successfully established a rat model of hypospadias following in-utero exposure to DBP during GD14 – GD18. The canonical Wnt signaling pathway of Wnt/β-catenin was down-regulated in association with the up-regulation of the NF-κB signaling pathway. This may provide a new perspective to the molecular mechanism of this malfunction induced by DBP. Recently, a loss- and gain-of-function mutant mouse for β-catenin presented a severe model of hypospadias [Lin et al. Citation2008], demonstrating a requirement of β-catenin during the early phase of GT development. Moreover, genetic interactions of the androgen and Wnt/β-catenin pathway were also confirmed during reproductive organ development. This suggests that β-catenin plays a significant role in the formation of male external genitalia [Miyagawa et al. 2009]. Previously, we observed a significant decrease of serum testosterone in hypospadiac male rats induced by maternal exposure to DBP. This was consistent with the results from other similar animal studies [Jiang et al. Citation2007]. β-catenin has been reported to play an important role in cancer [Choi et al. Citation2010; Wang et al. Citation2010; Teng et al. Citation2010] and osteocyte development [Santos et al. Citation2010]. We have observed that the Wnt/β-catenin pathway in the UPE was down-regulated in GT of fetal male rats following maternal exposure to DBP. It is essential for the GT formation. Interestingly, compared with the DBP-treated GD19 female control, β-catenin was not differentially expressed. Combined with the role of β-catenin in the masculinization of mouse external genitalia [Miyagawa et al. 2009], the DBP disruption of β-catenin likely also reflects its role in the masculinization of the GT.
GSK-3β is a protein-serine kinase, which acts as an inhibitor of Wnt signaling during embryonic development and cell proliferation in mammals [Shakoori et al. Citation2005; Dale Citation1998]. In an alternative mode of regulation, the interaction between GSK-3β and its substrate β-catenin is controlled by Wnt signaling through altering a multiprotein scaffolding complex which provides the context for phosphorylation of β-catenin by GSK-3β [Liu et al. Citation2005; Cadigan and Nusse Citation1997]. As was shown in Xenopus oocytes treated with activators of G proteins [Najafi Citation2009] phosphorylation of GSK-3β will lead to the stabilization of β-catenin and the up-regulation of the canonical Wnt signaling pathway. We observed that the expression of the GSK-3β protein was up-regulated, associated with the down-regulation of β-catenin and Phospho-GSK-3β in DBP-treated fetal rats. This suggests that DBP may affect the development of GT by down-regulating the Wnt/β-catenin pathway in fetal male rats.
NF-κB is a major factor that plays a significant role in apoptosis, cancer development, cell proliferation, and differentiation [Bours et al. Citation2000; Karin Citation2006]. β-catenin can interact with NFκB, to reduce NF-κB DNA binding, transactivation, and target gene expression [Deng et al. Citation2002]. In addition, GSK-3β may regulate transcription of NFκB in embryonic development [Hoeflich et al. Citation2000]. Furthermore, studies in prostate cancer have indicated that the Wnt/GSK-3β/β-catenin and NF-κB signaling pathways could cooperate to regulate the proliferation and survival of prostate cancer cells by affecting the activity of androgen receptor (AR), a key factor in androgen-independent as well as androgen-dependent tumors [Yang et al. Citation2002; de la Taille et al. Citation2003; Wang et al. Citation2007]. We have observed the differential expression of both Wnt/ GSK-3β/β-catenin and NF-κB signaling pathway in fetal male GT treated with DBP. The level of the NF-κB protein was up-regulated, which indicated that DBP might increase NF-κB expression through attenuating Wnt/ GSK-3β/β-catenin signaling pathway.
In this study a rat model of hypospadias by maternal exposure to DBP was successfully established and the protein expression profiles of Wnt/β-catenin and NFκB signaling pathway were evaluated. Compared with our previous findings [Zhang et al. Citation2007; Jiang et al. Citation2007], the current results demonstrated that DBP could affect the development of GT by down-regulating the Wnt/β-catenin pathway and up-regulating the NF-κB pathway in fetal male rats. The results of this research describing the cellular location of β-catenin and the protein expression of Wnt/β-catenin pathways, provides the possibility to affect the reproductive toxicity of DBP through their regulation in GT tissues. The developmental malformation induced by DBP is a complex process [Scarano et al. Citation2009] and further studies using the DBP model that we have developed could be used to address the molecular mechanisms of cryptorchidism and crosstalk among the signaling pathways we have identified. This would further our understanding and clarify the underlying mechanisms induced by environmental contaminants.
MATERIALS AND METHODS
Animals
This study was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Sprague-Dawley rats were housed in the laboratory animal center of Nanjing Medical University under controlled temperature (23 ± 1°C), light (12/12 h light dark cycle), humidity (55 ± 5%), and received food and water following the Guide for the Care and Use of Laboratory Animals. During the time of proestrus, virgin female rats (weighing 260 ± 15g) were mated overnight with fertile male rats. The day sperm was found in the vagina of the mated female, was considered gestation day 0 (GD0). Twenty successfully mated females were randomly distributed into 2 groups and housed individually. The body weight of female rats was recorded daily.
Treatment
The pregnant rats were treated daily by gavage with DBP (99.5% pure, Sigma Chemical Co., St. Louis, MO, USA) at a dosage of 750 mg/kg bw/day during late gestation (GD14 – GD18), as was shown in our previous study, this dosage could establish a rat model of hypospadias [Zhang et al. Citation2007; Jiang et al. Citation2007]. Corn oil (99.5% pure, Shanghai Solvent Factory, Shanghai, China) was utilized to dissolve DBP and the volume of each dose was adjusted to 5 ml/kg according to daily body weight. Only corn oil was given to control pregnant rats and all drug solutions were prepared daily before administration. On GD19, ten pregnant rats (five of each group) were sacrificed by carbon dioxide asphyxiation, then live pups were counted and the sex was determined. GT samples of both DBP-treated and control (male and female) fetus were either immediately snap-frozen for protein extraction or fixed in buffered formalin for immunohistochemical analysis. In total 15 male samples (three per litter) from each group were selected for protein extraction and the rest were for immunohistochemical and histological analysis. GT samples isolated from embryos were pooled to obtain sufficient material. On the day of delivery, live pups from each litter were counted and their sex was determined. Moreover, the male pups were weighed during the newborn stage and the AGD of male pups was determined to calculate the ratio of AGD/bw. Upon sexual maturity at PND60, the phenotype of the malformation induced by DBP was easy to recognize. The male pups were examined for TDS including hypospadias and cryptorchidism.
Western blotting
Protein extracts were prepared by solubilizing the proteins from the GT tissues with lysis buffer (2M thiourea, 7M urea, 2% (v/v) IPG buffer, pH 3–10, 2% (w/v) DTT, 4% (w/v) CHAPS) in the presence -1mM phenylmethylsulfonyl fluoride (PMSF) according to standard procedures as previously described [Zhang et al. Citation2007]. The GT proteins were detected with specific antibodies against β-catenin (polyclonal, abcam Inc.), phospho-GSK-3β (polyclonal, Cell Signaling Technology Inc.), GSK-3β (polyclonal, Cell Signaling Technology Inc.), and NFκB (polyclonal, abcam Inc.). At the same time, anti-β actin antibody and anti-β tubulin antibodies were used to verify the amount and integrity of the proteins. Blots were developed using a horseradish peroxidase (HRP)-conjugated secondary antibody (Beijing ZhongShan Biotechnology Co., Ltd., Beijing, China), an ECL kit (Amersham Biosciences, Buckinghamshire, England), and AlphaImager (FluorChem5500, Alpha Innotech, San Leandro, CA, USA). ImageMaster software (Amersham Biosciences, Version 5.0) was used to analyze the expression values derived from these blots. The relative expression of β-catenin and NFκB were calculated as the ratio of their average pixel intensity to that of the β-actin loading control. The intensity of GSK-3β and Phospho-GSK-3β were normalized against that of β-tubulin. Three repeated experiments were performed independently.
Histology and Immunohistochemistry
Fetal GT were fixed in 10% neutral buffered formalin and dehydrated through ethanol, embedded in paraffin, and 5 µm serial sections were prepared. Hematoxylin and Eosin (H&E) staining was processed using standard procedures as previously described (Zhang et al. Citation2007; Jiang et al. Citation2007; Zhu et al. Citation2009). For immunohistochemistry, sections were incubated in 1% hydrogen peroxide, washed in PBS and non-specific protein binding was blocked with goat serum (Beijing ZhongShan Biotechnology Co.). The sections were incubated at a 1:400 dilution of anti-β-catenin antibody and then reacted with HRP conjugated secondary antibody (Beijing ZhongShan Biotechnology Co.). Immunoreactive sites were visualized brown with diaminobenzidine and mounted for bright field or differential interference contrast microscopy. The negative controls were incubated with dilution lacking primary antibody and were otherwise subject to all the immunohistochemical procedures.
Statistical Analysis
All statistical values were presented as means ± standard deviation (S.D.). The results of western blotting were analyzed by ImageMaster software (Version 5.0). Comparisons between two experimental groups were performed using the t test. Chi-square (χ2) was used to compare data including the ratio of hypospadias and cryptorchidism. STATA software version 9 (STATA Corp., College Station, TX, USA) was used to analyze the experimental data, as was demonstrated in our previous study (Zhang et al. Citation2007; Zhu et al. Citation2009). Differences were considered significant if P < 0.05.
Abbreviations
| DBP: | = | di-n-butyl phthalate |
| bw: | = | body weight |
| GD: | = | gestation day |
| PND: | = | postnatal day |
| GT: | = | genital tubercle |
| TDS: | = | testicular dysgenesis syndrome |
| GSK-3β: | = | glycogen synthase kinase-3β |
| BMP: | = | bone morphogenetic protein |
| Fgf: | = | fibroblast growth factor |
| Shh: | = | Sonic hedgehog |
| HRP: | = | horseradish peroxidase |
| H&E: | = | hematoxylin and eosin |
| SD: | = | standard deviation |
| UPE: | = | urethral plate epithelium |
| PMSF: | = | phenylmethylsulfonyl fluoride |
| AGD: | = | anogenital distance |
Acknowledgments
We would like to thank Professor J.H. Sha (Laboratory of Reproductive Medicine, Nanjing Medical University, Nanjing, China) for providing us with an excellent laboratory.
Declaration of Interest: The research was supported by grants from the National Natural Science Foundation of China (No. 30872596). The authors declare that there is no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Beleza-Meireles, A., Lundberg, F., Lagerstedt, K., Zhou, X., Omrani, D., Frisén, L., Nordenskjöld, A. (2007) FGFR2, FGF8, FGF10 and BMP7 as candidate genes for hypospadias. Eur J Hum Genet 15:405–410.
- Boisen, K.A, Chellakooty, M., Schmidt, I.M, Kai, C.M., Damgaard, I.N., Suomi, A.M., (2005) Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at three months of age. J Clin Endocrinol Metab 90:4041–4046.
- Bours, V., Bentires-Alj, M., Hellin, A.C., Viatour, P., Robe, P., Delhalle, S., (2000) Nuclear factor-kappaB, cancer, and apoptosis. Biochem Pharmacol 60:1085–1089.
- Cadigan, K.M., Nusse, R. (1997) Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305.
- Choi, H., Gwak, J., Cho, M., Ryu, M.J., Lee, J.H., Kim, S.K., (2010) Murrayafoline A attenuates the Wnt/beta-catenin pathway by promoting the degradation of intracellular beta-catenin proteins. Biochem Biophys Res Commun 391:915–920.
- Dale, T.C. (1998). Signal transduction by the Wnt family of ligands. Biochem J 329:209–223.
- de la Taille, A., Rubin, M.A, Chen, M.W, Vacherot, F, de Medina, S.G, Burchardt, M, (2003) Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res 9:1801–1807.
- Deng, J., Miller, S.A., Wang, H.Y., Xia, W., Wen, Y., Zhou, B.P., (2002) beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell 2:323–334.
- Duty, S.M., Calafat, A.M., Silva, M.J., Brock, J.W., Ryan, L., Chen, Z., (2004) The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl 25:293–302.
- Duty, S.M, Silva, M.J, Barr, D.B, Brock, J.W., Ryan, L., Chen, Z., (2003) Phthalate exposure and human semen parameters. Epidemiology 14:269–277.
- Fisher, J.S., Macpherson, S., Marchetti, N., Sharpe, R.M. (2003) Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod 18:1383–1394.
- Foster, P.M. (2006) Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl 29:181–185.
- Gallentine, M.L., Morey, A.F., Thompson, I.M. (2001) Hypospadias: a contemporary epidemiologic assessment. Urology 57:788–790.
- Haraguchi, R., Suzuki, K., Murakami, R., Sakai, M., Kamikawa, M., Kengaku, M., (2000) Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development 127:2471–2479.
- Haraguchi, R., Mo, R., Hui, C., Motoyama, J., Makino, S., Shiroishi, T., (2001) Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128:4241–4250.
- Hauser, R., Meeker, J.D., Duty, S., Silva, M.J., Calafat, A.M. (2006) Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 17:682–691.
- Hoeflich, K.P., Luo, J., Rubie, E.A., Tsao, M.S., Jin, O., Woodgett, J.R. (2000) Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406:86–90.
- Huang, P.C., Kuo, P.L., Chou, Y.Y., Lin, S.J., Lee, C.C. (2009) Association between prenatal exposure to phthalates and the health of newborns. Environ Int 35:14–20.
- Jiang, J., Ma, L., Yuan, L., Wang, X., Zhang, W. (2007) Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butyl phthalate(DBP). Toxicology 232:286–293.
- Kalfa, N., Philibert, P., Sultan, C. (2009) Is hypospadias a genetic, endocrine or environmental disease, or still an unexplained malformation? Int J Androl 32:187–197.
- Karin, M. (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441:431–436.
- Kojima, Y., Kohri, K., Hayashi, Y. (2010) Genetic pathway of external genitalia formation and molecular etiology of hypospadias. J Pediatr Urol 6:346–354.
- Lin, C., Yin, Y., Long, F., Ma, L. (2008) Tissue-specific requirements of beta-catenin in external genitalia development. Development 135:2815–2825.
- Liu, X., Rubin, J.S., Kimmel, A.R. (2005) Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol 15:1989–1997.
- Manson, J.M., Carr, M.C. (2003) Molecular epidemiology of hypospadias: review of genetic and environmental risk factors. Birth Defects Res (Part A) 67:825–836.
- Martino-Andrade, A.J., Chahoud, I. (2010) Reproductive toxicity of phthalate esters. Mol Nutr Food Res 54:148–157.
- Miyagawa, S., Moon, A., Haraguchi, R. (2009) Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development 136:3969–3978.
- Miyagawa, S., Satoh, Y., Haraguchi, R., Suzuki, K., Iguchi, T., Taketo, M.M., (2009) Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol Endocrinol 23:871–880.
- Najafi, S.M. (2009) Activators of G proteins inhibit GSK-3beta and stabilize beta-Catenin in Xenopus oocytes. Biochem Biophys Res Commun 382:365–369.
- Pan, G., Hanaoka, T., Yoshimura, M. (2006) Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a crosssectional study in China. Environ Health Perspect 114:1643–1648.
- Rozati, R., Reddy, P.P., Reddanna, P., Mujtaba, R. (2000) Xenoesterogens and male infertility: myth or reality? Asian J Androl 2:263–269.
- Santos, A., Bakker, A.D., Zandieh-Doulabi, B., de Blieck-Hogervorst, J.M., Klein-Nulend, J. (2010) Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun 391:364–369.
- Scarano, W.R, Toledo, F.C, Guerra, M.T, de Campos, S.G., Júnior, L.A., Felisbino, S.L., (2009) Long-term effects of developmental exposure to di-n-butyl-phthalate (DBP) on rat prostate: proliferative and inflammatory disorders and a possible role of androgens. Toxicology 262:215–223.
- Seifert, A.W., Bouldin, C.M., Choi, K.S., Harfe, B.D., Cohn, M.J. (2009) Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development 136:3949–3957.
- Shakoori, A., Ougolkov, A., Yu, Z.W., Zhang, B., Modarressi, M.H., Billadeau, D.D., (2005) Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun 334:1365–1373.
- Swan, S.H., Main, K.M., Liu, F., Stewart, S.L., Kruse, R.L., Calafat, A.M., (2005) Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 113:1056–1061.
- Teng, Y., Wang, X., Wang, Y., Ma, D. (2010) Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun 392:373–379.
- Wang, M.H., Baskin, L.S. (2008) Endocrine disruptors, genital development, and hypospadias. J Androl 29:499–505.
- Wang, Y., Kreisberg, J.I., Ghosh, P.M. (2007) Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets 7:591–604.
- Wang, Y., Krivtsov, A.V., Sinha, A.U., North, T.E., Goessling, W., Feng, Z., (2010) The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 327:1650–1653.
- Yang, F., Li, X., Sharma, M. Sasaki, C.Y., Longo, D.L., Lim, B., Sun, Z. (2002) Linking beta–catenin to androgen-signaling pathway. J Biol Chem 277:11336–11344.
- Yang, W., Carmichael, S.L., Shaw, G.M. (2007) Congenital malformations co-occurring with hypospadias in California, 1983–1997. Am J Med Genet 143A:2627–2634.
- Zhang, W., Shen, H., Ma, L., Shen, B., Xu, Z., Wang, X. (2007) Differential expression of peroxiredoxin 6 in fetal rat testis following in utero exposure to di(nbutyl) phthalate. Toxicology. 240:86–95.
- Zhu, Y.J., Jiang, J.T., Ma, L., Zhang, J., Hong, Y., Liao, K., (2009) Molecular and toxicologic research in newborn hypospadiac male rats following in utero exposure to di-n-butyl phthalate (DBP). Toxicology 260:120–125.
