Abstract
Evidently, children born after intracytoplasmic sperm injection (ICSI) are at an increased risk of having sex chromosomal abnormalities. Here we evaluate the change in methods used for prenatal diagnostics in patients having ICSI with epididymal or testicular sperm from the introduction of the procedure in 1995 until December 2007. Four hundred and fifty pregnancies resulted in the birth of 553 children. Of the Danish subpopulation 115 (34.2%) received nuchal translucency examination (NT) and 43 (12.8%) received invasive prenatal diagnostics (IPD). IPD was carried out in 11 out of 23 couples (48%) during the period 1995–1998. Since 2002, less than 10% chose to receive IPD. Twenty-one (57%) of 37 Danish women 37–44 years of age underwent IPD compared to only 22 (7.4%) of the 299 women less than 37 years of age (p < 0.001). Conversely, since 1999 the use of NT has gradually increased to a frequency of 88.9% in 2007. The partners of vasectomized men had significantly more often NT performed compared to those of non-vasectomized men. IPD were not otherwise associated with the etiology of azoospermia. This study documents a shift in prenatal diagnostics from IPD to NT for testicular sperm aspiration/percutaneous epididymal sperm aspiration (TESA/PESA) couples.
Introduction
Since the Danish national follow-up study of testicular sperm aspiration/percutaneous epididymal sperm aspiration (TESA/PESA) children [Fedder et al. Citation2007] was planned, several studies have suggested an increased occurrence of de novo chromosomal abnormalities in children born after intracytoplasmic sperm injection (ICSI) using ejaculated sperm - even after correction for maternal age and paternal chromosomal abnormalities [Bonduelle et al. Citation2002]. When this treatment was introduced in the middle of the 1990s, patients pregnant after ICSI with epididymal or testicular sperm were recommended to have amniocentesis or chorionic villus biopsy, due to the immaturity of these germ cells. However, many couples with a long history of infertility are reluctant to go through an invasive procedure. Therefore, nuchal translucency examination (NT), which was introduced a few years later [Nicolaides Citation2004] and since 2004, implemented in all Danish obstetrical departments as a component in first-trimester combined screening for Down syndrome [The Danish National Board of Health Citation2004], is preferred over invasive procedures by many couples.
However, first trimester combined screening (NT combined with the serum markers pregnancy associated plasma protein-A (PAPP-A) and free β-human chorionic gonadotrophin (β-hCG)) [Nicolaides Citation2004] seems to be a less reliable procedure in identifying fetuses having Downs syndrome in IVF- and ICSI-pregnancies [Gjerris et al. Citation2009]. Furthermore, chromosomal abnormalities not detectable by NT or serum markers, e.g., sex chromosomal abnormalities, seem to occur with an increased frequency in children born after ICSI [Bonduelle et al. Citation2002].
The objective of this study was to document and evaluate a possible change in methods used for prenatal diagnostics in patients having ICSI with epididymal/testicular sperm from the introduction of the procedure in Denmark in 1995 until December 2007. The frequencies of invasive and non-invasive procedures were evaluated according to maternal age and etiology of male infertility (azoospermia/aspermia). The study was not designed to make conclusions about the efficiency of IPD versus NT. Limited power in the present dataset hampers definitive generalization.
Results
Four hundred and fifty couples answered the questionnaire until December 31st 2007, giving a response rate of 96.5%. The 450 pregnancies resulted in the birth of 553 children: 349 singletons, 198 twins, and 6 triplets, respectively. Overall, examination of nuchal translucency (NT) was performed in 120 (27%) of the 450 pregnancies, while invasive diagnostics (IPD = amniocentesis or chorionic villus sampling (CVS)) was performed in 50 (11%). In five of the cases (including one from Norway) CVS was done due to a high-risk estimate for trisomy 21 based on NT, double test, and female age. A total of 165 (37%) of the couples giving birth to live children after TESA/PESA treatment received prenatal diagnostics.
One hundred and fourteen (25%) of the couples came from Norway (n = 112), Sweden (n = 1), or the Faroe Islands (n = 1), and of these only five had a NT and seven had IPD. Due to the very low level of prenatal diagnostics – and particularly NT – the non-Danish patients were excluded from further analysis. In total, 154 Danish patients were included (111 NT, 39 IPD, 4 NT + IPD).
During the period 1995-1998, IPD was carried out for 47.8% (11 of 23) of Danish couples having prenatal diagnostics (). Since 1998, the percentage of patients receiving IPD has gradually decreased to levels of 3.7%-10.3% in the period 2002 to 2007. From 1999 an increasing proportion has received NT, reaching a level of 88.9% in 2007 ().
Figure 1. Percent of 336 Danish couples having invasive prenatal diagnostics (amniocentesis or chorionic villus biopsy) (red bars) or nuchal translucency examination (blue bars) after ICSI with epididymal or testicular sperm in the period 1995-2007.
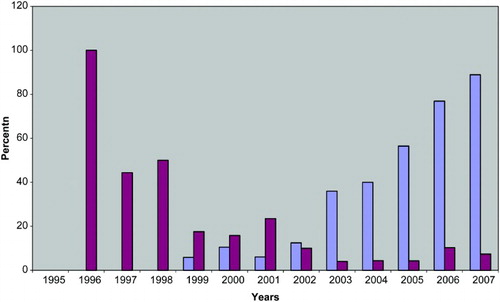
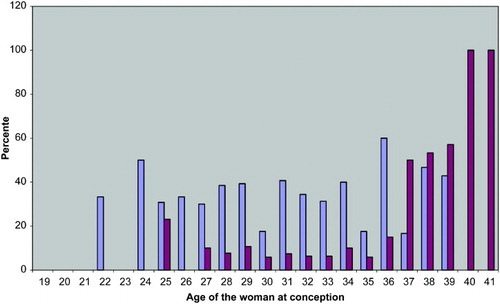
The proportion of patients receiving NT appears to be independent of age in women younger than 40 years of age (). Conversely, as shows, IPD was more often carried out for women 37 years of age or older. Thus, 21 (57%) of 37 Danish 37-41 year old women received IPD, while only 22 (7.4%) of the 299 women less than 37 years of age received IPD (p < 0.001, χ2-test). No woman younger than 25 years of age received IPD.
Figure 2. Percent of 336 Danish couples having invasive prenatal diagnostics (amniocentesis or chorionic villus biopsy)(red bars) or nuchal translucency examination (blue bars) in relation to the age of the woman at conception.
Partners of vasectomized men received NT significantly more often (p < 0.02), and partners of men with azoospermia without known etiology received NT significantly less often compared to the rest of the patient population (p < 0.05). NT was not related to the etiology of azoospermia in any other case (). IPD could not be related to the etiology of the male factor ().
Table 1. Percentages of 336 Danish couples having nuchal translucency examination (NTE) and invasive prenatal diagnostics (IPD) (amniocentesis or chorionic villus biopsy), respectively, arranged according to the etiology of azoospermia in the male partner. Levels of significance for each etiology group were calculated by comparison with the sum of the other etiology groups.
Except for one balanced de novo 1/3–translocation detected by amniocentesis, no chromosomal abnormalities were observed in this study. The frequencies of NT and IPD did not differ significantly in singleton versus twin pregnancies.
Discussion
The initial aim of this study was to evaluate the use of prenatal diagnostics for all TESA/PESA couples (Danes and non-Danes) treated in Denmark. However, as couples from neighboring countries received NT in very few cases, we decided to exclude non-Danish citizens. In Sweden, a significant effort has been made to document the usefulness of NT in screening for Down syndrome [Saltvedt et al. Citation2005], and NT is offered routinely in Sweden as it is in Denmark. In Norway however, where the majority of the excluded couples came from, NT is not routinely available. Here ultrasonography is offered routinely only in weeks 17-19, unless otherwise medically indicated. Excess ultrasonographic examinations are not publicly financed [Social- og helsedirektoratet 2005]. Additional prenatal diagnostics are offered routinely only to women 38 years of age or older at delivery [Elster Citation2004].
This study on prenatal diagnostics in TESA/PESA couples, focusing on NT, amniocentesis, and CVS during the period 1995-2007, clearly shows that during this period an increasing number of TESA/PESA couples choose NT, while the use of IPD was decreasing except for women of older ages. These data are consistent with the Danish background population, where at least 90% receive NT (data received from local obstetrical databases using software from Astraia, version 17.74; www.iol.gr/en/astraia). Furthermore, the utilization of IPD are similar to a recent register study showing a decrease in IPD by 55% in Denmark during the period 1996-2006 [Vestergaard et al. Citation2009].
IPD is mostly performed in women 37 years of age or older. For this age group, the risk of giving birth to a child with a chromosomal abnormality is higher [DeVore Citation2001] and these older women may essentially prefer an exact diagnosis rather than a probability for having a chromosomal abnormality. Recent studies show that first trimester risk assessment is very sensitive – particularly when combining ultrasonographic and biochemical parameters. In prospective studies including more than 200,000 pregnancies and 871 fetuses with trisomy 21, NT could identify 76.8% of these fetuses with trisomy 21, and the false-positive rate was 4.2% (positivity defined as calculated risk of trisomy 21 > 1:300). When combining NT with other ultrasonographic markers (e.g., fetal growth restriction, tachycardia, abnormal flow in the ductus venosus, megacystis, exomphalos, and single umbilical artery) and biochemistry (maternal serum free-β-hCG and PAPP-A), 97% of trisomy 21 fetuses could be identified, with a false-positive rate of only 5% [Nicolaides Citation2004]. However, an American study including 182,669 NT from 327 centers showed that many centers have lower maximum and median NT values, suggesting a systematic undermeasurement of NT [Evans et al. Citation2010]. According to this finding the rate for Down syndrome births in Colorado, for women older than 35 years of age, has risen significantly from 1989 to 2005 [Henry et al. Citation2008]. Additionally, Gjerris et al. [2009] found lower median NT values in IVF and ICSI pregnancies compared with controls. At the same time a significantly increased false-positive rate of trisomy 21 fetuses in IVF- and ICSI-pregnancies compared with controls by first-trimester screening using NT combined with the serum markers PAPP-A and β-hCG (9.0% vs. 6.0%), suggesting that NT combined with the double test might be less accurate in predicting trisomy 21 in TESA/PESA pregnancies [Gjerris et al. Citation2009].
All pregnant women in this study received ultrasonography in week 7-9 and many women also later in pregnancy (with or without NT or malformation ultrasonography). However, it is often difficult for people to answer (in the questionnaire) exactly what type of ultrasonography examination they received. Furthermore, in the present study it was not possible to obtain complete data according to supplementary biochemical data such as the double test.
In Denmark patients may choose to have a legal abortion until a gestational age of 12 weeks. CVS can be carried out in the first trimester, and is therefore often more attractive than amniocentesis, which is performed in the second trimester. Similarly, first-trimester prenatal diagnostic screening including ultrasonography and biochemistry is preferable, since it allows patients to choose legal abortion if the child suffers from a chromosomal abnormality.
This study reports just one genetic abnormality in 154 examinations (111 NT, 39 IPD, 4 NT + IPD). No genetic abnormalities were reported for the Norwegian children. However, the present study is biased since it only includes couples that have actually given birth. Couples who have chosen legal abortion due to prenatally diagnosed chromosomal abnormalities are not included in this study. Thus, the material is useful for evaluation of changes in the pattern of prenatal diagnostics for this patient group, but insufficient to evaluate frequencies of chromosomal abnormalities. As described in the introduction it has not been the aim of this study to make conclusions about accuracy of IPD versus NT.
The practice of offering prenatal diagnostic involves great responsibility, and the diagnosis must be correct, as the consequence might be that the couple decides to have an abortion. Doctors performing prenatal diagnostics must be able to offer comprehensive counselling. Obviously it is not optimal that the couple has to make pivotal decisions based on a risk estimate (given by NT and/or biochemical analysis combined with age) rather than an absolute diagnosis. If the risk for trisomy 21 is high (1:300), it is recommended to supplement with IPD in order to have a karyotype. In this study 4 out of 115 cases had a high risk estimate prompting a CVS, which, however, in all cases showed normal conditions.
In conclusion, this study documents that TESA/PESA patients have shifted in their choice of prenatal diagnostic methods from invasive procedures such as amniocentesis and chorionic villus biopsy to NT first trimester screening during the period 1995-2007. Although most Danish fertility clinics recommend IPD for patients treated with ICSI using testicular or epididymal sperm, the couples choose to have the same kind of prenatal diagnostics as other pregnant women. IPD is most often chosen by women 37 years of age or older. Besides a slightly increased frequency of NT in couples where the man is vasectomized, the frequency of NT or IPD could not be related to the etiology of azoospermia/aspermia.
Given the lower reliability of NT (combined with serum markers) in the detection of Down syndrome fetuses in IVF- and ICSI-pregnancies [Gjerris et al. Citation2009] and a higher frequency of sex chromosomal abnormalities in ICSI children, one could express concerns for the trend of IPD deselection by these couples.
Materials and Methods
A consecutive cohort of women giving birth to living children after ICSI treatment with epididymal or testicular sperm in Denmark were previously included in a follow-up study [Fedder et al. Citation2007]. In addition to data from the respective fertility clinics in Denmark, data have been obtained from questionnaires filled by the couples three months or later after birth. The questionnaires concerned the etiology of azoospermia, the pregnancy, birth, and health of the child. Moreover, the questionnaires included information regarding prenatal diagnostics such as amniocentesis, CVS, and ultrasound examinations. As NT was not yet introduced when the questionnaire was designed, all information about NT was retrospectively collected from all obstetrical departments and specialists involved in the pregnancies (32 in Denmark, 34 in Norway, one in Sweden, and one in the Faroe Islands). The couples had in advance, in the questionnaire, given assent to obtain further medical information if necessary. Couples receiving testicular sperm aspiration/extraction (TESA/E) and PESA are treated as one group due to material size.
When the conditions were fulfilled, the χ2-test (with Yates correction) was used to evaluate whether the differences between respective etiological groups and the rest of the patient material were significant. Otherwise, Fishers exact test was used.
Abbreviations
| ICSI: | = | intracytoplasmic sperm injection |
| TESA: | = | testicular sperm aspiration |
| PESA: | = | percutaneous epididymal sperm aspiration |
| NT: | = | nuchal translucency examination |
| IPD: | = | invasive prenatal diagnostics |
| CVS: | = | chorionic villus sampling |
| PAPP-A: | = | pregnancy associated plasma protein |
| β-hCG: | = | β-human chorionic gonadotrophin. |
Acknowledgments
The authors wish to thank the following colleagues all of whom contributed important data.
Colleagues from the following fertility clinics in Denmark:
Skejby Hospital (Erik Ernst), Herlev Hospital (Hjördis Mikkelsen, Trine Lemvigh and Hanne Udengaard), Ballerup Fertilitetsklinik (Peter Lundström), Nordica Fertility Clinic (Mette Munk), Horsens Fertilitetsklinik (Gerhardt Børlum), Holbæk Fertilitetsklinik (Torben Philipsen) and Trianglen (Jorgen Grinsted)
Colleagues from the following obstetrical departments:
DENMARK:
Gentofte Hospital (Annamari Nikkilä), Roskilde Hospital (Lennart Isager-Sally), Sygehus Lillebælt (Kolding, Fredericia), Rigshospitalet, Copenhagen (Anne Loft), Aalborg Sygehus (Bjørn Pedersen), Hjørring Sygehus (Bjørn Petersen), Hvidovre Hospital (Finn Stener Jørgensen), Herlev Hospital (Hanne Udengaard), Glostrup Hospital (Helle Zingenberg), Nordsjællands Hospital, Hillerød (Tina Sejersbøl Wagner), Regionshospitalet Randers (Karin Roed Meyer), Næstved Sygehus (Susanne Pouplier), Sydvestjysk Sygehus, Esbjerg and Grindsted (Hans Ole Daugaard), Regionshospitalet Horsens (Marianne Christiansen), Svendborg Sygehus (Merete Skov), Sygehus Thy-Mors, Thisted (Richard Farlie), Skejby Sygehus (Niels Uldbjerg), Regionshospitalet Viborg (Lars Schierup), Sygehus Sønderjylland, Sønderborg (Pia Pallesen), Storstrømmens Sygehus, Nykøbing Falster (Jens Christian Prien-Larsen), Bornholms Hospital (Hans Grundsell), Sygehus Sønderjylland, Haderslev (Hanne Christensen), Slagelse Sygehus (Jens Ole Fall), Regionshospitalet Silkeborg (Mette Heinel Frederiksen), Hospitalsenheden Vest, Herning, Holstebro (Carsten Byrjalsen)
NORWAY:
Rikshospitalet, Oslo (Peter Fedorcsak), Haukeland Sykehus, Bergen (Synnøve Lian Johnsen), Stavanger Sentralsykehus, St.Olavs Hospital, Trondheim, Lillehammer Sykehus, Kristiansand Sykehus, Dr. Ernst Faber Swenson, Kristansand, Dr. Johan Bergh, Hafrsfjord, Sentralsykehuset i Rogaland, Stavanger (Torbjørn Moe Eggebø), Frederikstad Sentralsykehus, Akershus Universitetssykehus, Oslo (Seth Granberg), Dr. Merethe Blakstad, Oslo, Vest Agder Sentralsykehus, Aust Agder Sentralsykehus, Nordfjord Sykehus, Elverum Sentralsykehus, Sykehuset Innlandet, Gjøvik (Anja Døssland Holstad), Sykehuset Asker og Bærum (Kjartan Moe), Vestfold Sentralsykehus (Dr. Knut Urdal), Aker Sykehus, Oslo, Orkdal Sjukehus, Orhanger, Ullevål Universitetssykehus (Bjørn Busund), Sykehuset Telemark (Eirik Eliassen), Kongsberg Sykehus, Harstad Sykehus, Sentralsykehuset Østfold, Frederikstad, Sentralsykehuset Møre/Romsedal/Ålesund, Haugesund Sykehus (Torolf Holst-Larsen), Fylkesjukehuset i Kristiansund (Ellen Jendal), Lister Sykehus, Flekkefjord, Sentralsjukehuset Buskerud (Marieke Claessen), Stora Fylkesjukehus
THE FAROE ISLANDS:
Torshavn Sygehus
SWEDEN:
Blekingesjukhuset, Karlshamn (Per Buchhave)
Declaration of Interest: The authors have no financial or personal conflicts of interest. Collection of supplementary information from the obstetrical departments and all data work was performed by JF. The authors are responsible for the content and JF, AL, and MDK for the writing of the paper.
References
- Bonduelle, M., Van Assche, E., Joris, H., Keymolen, K., Devroey, P., Van Steirteghem, A., (2002) Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod 10:2600–2614.
- DeVore, G.R. (2001) The genetic sonogram: its use in the detection of chromosomal abnormalities in fetuses of women of advanced maternal age. Prenat Diag 21:40–45.
- Elster, J. (2004) Vilkår for fosterdiagnostikk. Genialt 1:10–13 (www.bioteknologinemnda.no).
- Evans, M.I., Krantz, D.A., Hallahan, T.W., Sherwin, J.E. (2010) Undermeasurement of nuchal translucencies: Implications for screening. Obstet Gynecol 116:815–818.
- Fedder, J., Gabrielsen, A., Humaidan, P., Erb, K., Ernst, E., Loft, A. (2007) Malformation rate and sex ratio in 412 children conceived with epididymal or testicular sperm. Hum Reprod 22:1080–1085.
- Gjerris, A.C., Loft, A., Pinborg, A., Christiansen, M., Tabor, A. (2009) First-trimester screening markers are altered in pregnancies conceived after IVF/ICSI. Ultrasound Obstet Gynecol 33:8–17.
- Henry, G.P., Britt, D.W., Evans, M.I. (2008) Screening advances and diagnostic choice: the problem of residual risk. Fetal Diagn Ther 23:308–315.
- Nicolaides, K.H. (2004) Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol 191:45–67.
- Saltvedt, S., Almström, H., Kublickas, M., Valentin, L., Bottinga, R., Bui, T-H., (2005) Screening for Down syndrome based on maternal age or fetal xnuchal translucency: a randomized controlled trial in 39572 pregnancies. Ultrasound Obstet Gynecol 25:537–545.
- Social- og helsedirektoratet (2005) Vejledende retningslinjer for bruk av ultralyd i svangerskapet. Bruk av ultralyd i den alminnelige svangerskapsomsorgen og i forbindelse med diagnostikk; www.bioteknologinemnda.no
- The Danish National Board of Health (2004) Guidelines for prenatal diagnosis. Information, risk assessment, counselling and diagnosis (in Danish. No abstract available). The National Board of Health, Copenhagen, Denmark.
- Vestergaard, C.H.F., Lidegaard, Ø., Tabor, A. (2009) Invasive prenatal diagnostic practice in Denmark 1996 to 2006. Acta Obstet Gynecol Scand 88:362–365.