Abstract
Indoleamine 2,3-dioxygenase (IDO) is the rate limiting enzyme of the kynurenine pathway that degrades L-tryptophan, but a wider range of functions have now been proposed for this enzyme, including antioxidant activity. Our previous study revealed that reduced IDO expression in the placenta induces defective feto-maternal immuno-tolerance leading to the onset of pre-eclampsia. In our present study, we assessed the effects of low placental IDO activity as an antioxidant. The placental levels of 8-hydroxy-2'-deoxy-guanosine (8-OHdG), a maker for oxidative damage to DNA, were significantly higher in pre-eclamptic than normotensive pregnancies (P < 0.05). Immunohistochemical signals of 8-OHdG were detected mainly in syncytiotrophoblasts and vascular endothelial cells, and co-localized with those for IDO. Furthermore, a significant inverse correlation was found between the IDO activity and 8-OhdG levels. These results show that oxidative stress is associated with decreased IDO activity in the pre-eclamptic placenta and suggest an impact of low IDO activity other than immune modulation in promoting the onset of this disorder.
Introduction
Pre-eclampsia is one of the most common and potentially serious pregnancy-associated disorders. It is a principal cause of maternal morbidity, accounting for almost 15 – 20% of pregnancy-related mortalities [Sibai et al. Citation2005]. However, it is not a simple complication of pregnancy, but is rather a multiple organ failure syndrome involving the liver, kidney, and lung, as well as coagulatory and neural systems. Although the prognosis of both the mother and fetus in cases of severe pre-eclampsia is poorer than generally expected, the scarcity of precise knowledge regarding the etiology of pre-eclampsia has hindered the ability of clinicians to develop etiology-based preventive and therapeutic measures [Hall et al. Citation2000]. Since the risks of maternal multi-organ dysfunction and fetal distress are high in pre-eclampsia, particularly in early onset types of this disease, it has been recommended that the fetus should be delivered at gestational weeks 32-34 in affected patients [Hall et al. Citation2000].
There is now an emerging consensus that pre-eclampsia is a complex multifactorial disease in which genetic factors, both maternal and fetal, and environmental factors are involved, although the precise mechanisms underlying this disorder have remained elusive [Roberts and Cooper Citation2001; Woodage et al. Citation2002; Cross Citation2003]. We previously demonstrated that indoleamine 2,3-dioxygenase (IDO) enzymatic activity is significantly reduced in pre-eclamptic placentas [Nishizawa et al. Citation2007]. IDO is the initial and rate limiting enzyme of the kynurenine pathway that degrades L-tryptophan in macrophages, dendritic cells, and trophoblasts in placental villi. Depletion of L-tryptophan at the microenvironment level suppresses maternal T-cells leading to immunotolerance to pregnancy-related tissues [Munn et al. Citation1998; Mellor et al. Citation2001]. The inhibition of IDO leads to pregnancy loss in an experimental mouse model system, suggesting the involvement of defective feto-maternal immunotolerance. This process may underlie the etiology of pre-eclampsia [Nishizawa et al. Citation2007].
IDO has a broad spectrum of substrate specificities for various indoleamine derivatives and catalyzes the oxidative cleavage of the indole ring of several important regulator molecules including tryptophan, serotonin, and melatonin. In addition to its role as an immune modulator via tryptophan depletion, a wide range of other functions has also been proposed for IDO. For example, IDO utilizes the superoxide anion as an oxygen source and in this context has antioxidant activity [Thomas and Stocker Citation1999]. In this regard, there is little doubt that oxidative stress is a significant contributor to the pathogenesis of pre-eclampsia [Jauniaux et al. Citation2006; Perkins Citation2006; Myatt Citation2010]. This prompted us to hypothesize that defects in the antioxidant function of IDO contribute to the etiology of various symptoms in pre-eclampsia.
The levels of 8-hydroxy-2'-deoxy-guanosine (8-OHdG), a well-known marker for oxidative damage to DNA, are often used to evaluate oxidative stress. Indeed, high levels of 8-OHdG in placental DNA or urine from pre-eclamptic women have been reported, although other studies have described no significant change [Takagi et al. Citation2004; Hung et al. Citation2010; Peter Stein et al. Citation2008]. In our current study, we examined the levels of 8-OHdG in pre-eclamptic placental tissue in the context of IDO expression to further evaluate the significance of the antioxidant function of IDO in this disorder.
Results
As reported previously, the placental IDO activity in our current sample cohort was found to be significantly reduced in the pre-eclamptic women relative to normotensive controls (A) [Nishizawa et al. Citation2007]. To study the effects of IDO as an antioxidant, we examined the levels of placental 8-OHdG, a well established marker of oxidative damage to DNA. These levels in genomic DNA from the placenta were significantly higher in pre-eclamptic (0.27 ± 0.02 ng/ml) compared with normotensive pregnancies (0.20 ± 0.05 ng/ml, P < 0.05; B). Immunohistochemical staining with anti-8-OHdG antibodies further revealed that this molecule is localized in the nuclei of syncytial trophoblasts and in the vascular endothelial cells of the placental villi both normal pregnancy and severe preeclampsia (A). However, the signal intensity was slightly higher in pre-eclamptic tissues. We additionally analyzed the localization of IDO in the placenta by immunostaining. In contrast to 8-OHdG, the signal intensities for IDO were slightly weaker in pre-eclamptic placentas compared with the normotensive controls. In both the pre-eclampsia and control samples, the IDO signals were mainly observed in the cytoplasm of endothelial cells in the chorionic villi with weaker signals detectable in syncytiotrophoblasts but no staining in the interstitial cells (B). Taken together, these results indicate that 8-OHdG and IDO co-localize at the cellular level.
Figure 1. Case-control study of pre-eclamptic and normotensive pregnancies. A) IDO enzymatic activity levels in placental tissue. B) The levels of 8-OHdG in genomic DNA from placental tissue. These data were compared for normotensive pregnancy (open bars) versus severe pre-eclampsia (grey bars). Each bar represents the mean value. Vertical bars indicate the standard deviation.
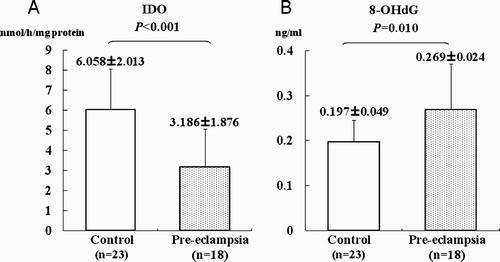
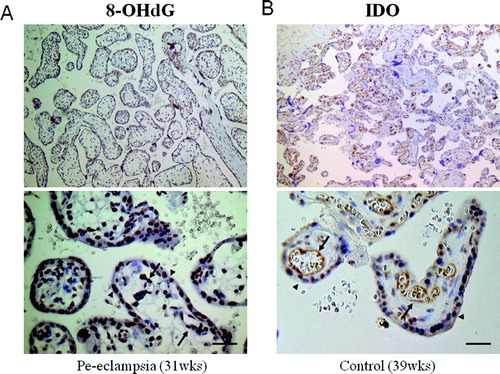
Figure 2. Immunostaining of placental tissue cross-sections. A) Immunostaining of 8-OHdG using monoclonal antibodies (31 w gestation, pre-eclampsia, original magnification x 400; scale bar, 50 µm). The signals were detected in the nuclei of vascular endothelial cells (arrows) and syncytiotrophoblasts (arrowheads) in the chorionic villi. B) Immunostaining of IDO in a placenta (39 w gestation, normotensive pregnancy, original magnification x400; scale bar, 50 µm). Prominent staining is apparent in the cytoplasm of vascular endothelial cells (arrows) as well as syncytiotrophoblasts (arrowheads) in the chorionic villi.
To examine whether higher levels of 8-OHdG indicate increased oxidization due to reduced IDO activity, this association was evaluated. A significant inverse correlation was indeed found between the levels of IDO activity and 8-OHdG (r = −0.621, P = 0.0003) (). When these analyses were performed separately for our pre-eclamptic and normotensive samples, a significant correlation was still observed (r = −0.712, P < 0.001 in normotensive pregnancies and r = −0.591, P = 0.009 in pre-eclampsia, respectively). The clinical parameters of the study groups are shown in .
Figure 3. Correlation between the 8-OHdG and IDO enzymatic activity levels in the placenta. Regression lines are shown with correlation coefficients (r) and P values. Correlations were determined for the total subject cohort (solid line) and again separately for normotensive (circles, chain line) and pre-eclamptic subjects (triangles, dashed line). A significant inverse correlation was found between the placental 8-OHdG and IDO activity for both the total cohort (r = -0.621, P = 0.0003) and the separate cohorts (r = -0.712, P < 0.001; r = -0.591, P= 0.009, respectively).
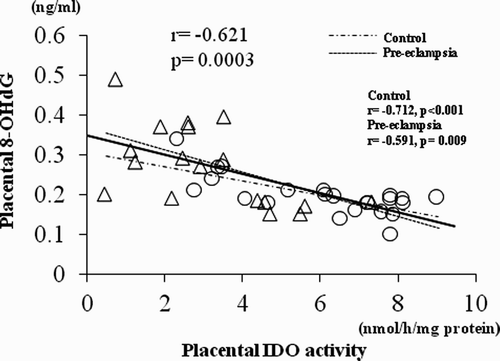
Table 1. Clinical parameters of the study groups.
Discussion
There is accumulating evidence that oxidative stress is involved in the onset of pre-eclampsia [Jauniaux et al. Citation2006; Perkins Citation2006; Myatt Citation2010]. It is also well known that dyslipidemia is a common occurrence in pre-eclampsia [Page Citation2002]. Moreover, excessive lipid peroxidation due to free radicals or reactive oxygen contributes to vascular endothelium dysfunction leading to the maternal symptoms of pre-eclampsia [Roberts and Hubel Citation1999]. However, the pathways leading to the induction of oxidative stress prior to the onset of pre-eclampsia remain unclear.
Our current data suggest that increased oxidative stress in pre-eclampsia is likely to be the result of low placental IDO activity as 8-OHdG co-localizes with IDO, and the levels of both molecules inversely correlate. When these analyses were performed separately for the pre-eclampsia and normotensive samples, significant correlations were still observed indicating that they are not simply due to a concurrence in this disease but are linked etiologically. Under normal physiological conditions during pregnancy, the placenta is protected from oxidative stress due to reactive oxygen species generated by extensive cell division and high metabolic activity [Burton et al. Citation2003; Jones et al. Citation2010]. Our present findings indicate that IDO plays a pivotal role in this antioxidant defense system in the placenta and that this is defective in pre-eclampsia.
We previously demonstrated that IDO activity is significantly reduced in pre-eclamptic placentas and that the IDO levels inversely correlate with the severity of the symptoms of pre-eclampsia [Nishizawa et al. Citation2007]. Also, in another previous study IDO inhibitors produce a pre-eclampsia-like phenotype in pregnant mice carrying allogeneic conceptuses [Nishizawa et al. Citation2008]. This suggested the possibility that reduced IDO levels may be a primary cause of pre-eclampsia. However, we have previously performed sequence analysis of the IDO gene in patients with pre-eclampsia and found no mutations or variations that could be associated with this disease [Nishizawa et al. Citation2010]. Although these genetic analyses do not support the hypothesis, our mouse model studies have shown not only increased blood pressure and proteinuria, but also detected placental lesions that are typical for human pre-eclampsia, indicating that IDO levels may play an essential role in the onset of this disorder, either as a primary or secondary cause. Hence, the diversity of maternal symptoms that appear in cases of severe pre-eclampsia may be partly related to a defective antioxidant system involving a decreased IDO activity. The mouse model might give a vital boost to this idea by showing the correlation between levels of IDO inhibition and 8-OHdG increase. Such a view might be also supported by determination of other oxidative stress markers such as nitrate/nitrite, superoxide, glutathione, or superoxide dismutase levels, although we did not find increased levels of nitrate/nitrite in pre-eclamptic placenta [Nishizawa et al. Citation2009].
Our current data should also prove to be a valuable resource for the identification of new therapeutic tools for pre-eclampsia. In this regard, a clinical trial of anti-oxidant supplements such as vitamin C and E has been performed to prevent the onset of pre-eclampsia, although the results are still somewhat controversial [Chappell et al. Citation1999; Poston et al. Citation2006]. Clinical trials of other anti-oxidants or the use of IDO activators in animal models of pre-eclampsia warrant further investigation.
Materials and Methods
Human subjects
All clinical samples were collected at the Department of Obstetrics and Gynecology, Fujita Health University, Japan. Placental biopsies were obtained from pre-eclamptic pregnancies (n = 18) and normotensive control pregnancies (n = 23). Pre-eclampsia was defined as a blood pressure of higher than 160/110 mmHg, with proteinuria of more than 2g in a 24 h collection. Normotensive control subjects were matched for maternal and gestational ages (). Pre-eclamptic women delivered newborns in the lower birth weight percentiles (Mann-Whitney U-test; P =0.002). Informed consent was obtained from each patient and this study was approved by the Ethical Review Board for Human Genome Studies at Fujita Health University (Accession numbers 43 and 87; February 23, 2005).
Placental biopsy collection
All placental biopsies were obtained following Caesarean section procedures. To avoid the effects of labor on the placental gene expression profile, only specimens from women who had not undergone labor were included. A central area of chorionic tissue was dissected, and the maternal deciduas and amnionic membranes were removed. Next, 1 cm thick sections of placental villi were dissected from the central area between the basal and chorionic plates. After vigorous washing of the maternal blood with saline, tissues were immediately frozen in liquid nitrogen and stored until use.
Determination of 8-OHdG and IDO activity
The 8-OHdG levels in placental tissue DNA were measured using a highly sensitive ELISA kit (JaICA, Fukuroi, Japan) in accordance with the manufacturer's instructions. Briefly, genomic DNA extracts from placental tissue were hydrolyzed with nuclease P1 (Wako Pure Chemical, Osaka, Japan) for 1 h, and then treated with alkaline phosphatase for a further hour. After column purification, the nucleotides were subjected to ELISA. Measurements of IDO activity were performed as previously reported [Nishizawa et al. Citation2007]. Placental tissues were also homogenized and centrifuged and the resulting supernatants were mixed with a substrate solution containing L-tryptophan (Sigma, St Louis, MO, USA), followed by incubation at 37°C for 60 min. The reaction was then stopped by incubation with 3% perchloric acid at 50°C for 30 min. The supernatants were subsequently assayed for enzymatic products by HPLC (Eicom 300 series; Eicom, Kyoto, Japan). The products detected at 60 min were used as a measure of the IDO activity values (nmol/h/mg protein).
Immunostaining
Placental samples were fixed in 10% formaldehyde overnight and then embedded in paraffin. Sections (2 µm) were collected onto silane-coated slides and dried in a conventional oven at 60°C for 24 h. Hematoxylin-eosin staining was then performed to enable histological examination. For immunostaining, samples were deparaffinized and rehydrated and endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol. For antigen retrieval, the slides were boiled in 1 mm EDTA (pH 8.0) in a pressure cooker for 10 min. Mouse monoclonal antibodies against 8-OHdG (N45.1; JaICA, Fukuroi, Japan) and against human IDO (1E7) were used [Takikawa et al. Citation1988]. The signals were detected using a peroxidase-based method with Simple Stain MAX-PO (Nichirei, Tokyo, Japan), with 3,3-diaminobenzidine as a substrate. Mouse IgG1 (Chemicon, Temecula, CA, USA) was used as the negative control. Counterstaining was performed with Mayer's hematoxylin solution.
Statistical analysis
Statistical comparisons between groups were performed using the Student t test and one-way analysis of variance. Differences were considered to be significant at P < 0.05. Data are reported as the mean ± SD for each group. Correlations were evaluated using straight line linear regression.
Abbreviations
| IDO: | = | indoleamine 2,3-dioxygenase; |
| 8-OHdG: | = | 8-hydroxy-2′-deoxy-guanosine. |
Acknowledgments
We thank Dr. Takikawa for generously providing antibodies against human IDO.
Declaration of interest: This study is supported by the JAOG Ogyaa Donation Foundation and by the Hori Information Science Promotion Foundation. The authors declare no conflicts of interest and alone are responsible for the content and writing of the paper.
References
- Burton, G.J., Hempstock, J. and Jauniaux, E. (2003) Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online 6:84–96.
- Chappell, L.C., Seed, P.T., Briley, A.L., Kelly, F.J., Lee, R., Hunt, B.J, (1999) Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet 354:810–816.
- Cross, J.C. (2003) The genetics of pre-eclampsia: a feto-placental or maternal problem? Clin Genet 64:96–103.
- Hall, D.R., Odendaal, H.J., Steyn, D.W. and Grove, D. (2000) Expectant management of early onset, severe pre-eclampsia: maternal outcome. BJOG 107:1252–1257.
- Hung, T.H., Lo, L.M., Chiu, T.H., Li, M.J., Yeh, Y.L., Chen, S.F. and Hsieh, T.T. (2010) A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod Sci 17:401–409.
- Jauniaux, E., Poston, L. and Burton, G.J. (2006) Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update 12:747–755.
- Jones, M.L., Mark, P.J., Lewis, J.L., Mori, T.A., Keelan, J.A. and Waddell, B.J. (2010) Antioxidant defenses in the rat placenta in late gestation: increased labyrinthine expression of superoxide dismutases, glutathione peroxidase 3, and uncoupling protein 2. Biol Reprod 83:254–260.
- Mellor, A.L., Sivakumar, J., Chandler, P., Smith, K., Molina, H., Mao, D. and Munn, D.H. (2001) Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol 2:64–68.
- Munn, D.H., Zhou, M., Attwood, J.T., Bondarev, I., Conway, S.J., Marshall, B., (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193.
- Myatt, L. (2010) Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 31:66–69.
- Nishizawa, H., Kato, T., Ota, S., Nishiyama, S., Pryor-Koishi, K., Suzuki, M., (2010) Genetic variation in the indoleamine 2,3-dioxygenase gene in pre-eclampsia. Am J Reprod Immunol 64:68–76.
- Nishizawa, H., Pryor-Koishi, K., Suzuki, M., Kato, T., Sekiya, T., Tada, S., (2009) Analysis of nitric oxide metabolism as a placental or maternal factor underlying the etiology of pre-eclampsia. Gynecol Obstet Invest 68:239–247.
- Nishizawa, H., Hasegawa, K., Suzuki, M., Achiwa, Y., Kato, T., Saito, K., (2008) Mouse model for allogeneic immune reaction against fetus recapitulates human pre-eclampsia. J Obstet Gynaecol Res 2008;34:1–6.
- Nishizawa, H., Hasegawa, K., Suzuki, M., Kamoshida, S., Kato, T., Saito, K., (2007) The etiological role of allogeneic fetal rejection in pre-eclampsia. Am J Reprod Immunol 58:11–20.
- Page, N.M. (2002) The endocrinology of pre-eclampsia. Clin Endocrinol 57:413–423.
- Perkins, A.V. (2006) Endogenous anti-oxidants in pregnancy and preeclampsia.'Aust N Z J Obstet Gynaecol 46:77–83.
- Peter Stein, T., Scholl, T.O., Schluter, M.D., Leskiw, M.J., Chen, X., Spur, B.W. and Rodriguez, A. (2008) Oxidative stress early in pregnancy and pregnancy outcome. Free Radic Res 42:841–848.
- Poston, L., Briley, A.L., Seed, P.T., Kelly, F.J. and Shennan, A.H. (2006) Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet 367:1145–1154.
- Roberts, J.M. and Cooper, D.W. (2001) Pathogenesis and genetics of pre-eclampsia. Lancet 357:53–56.
- Roberts, J.M. and Hubel, C.A. (1999) Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet 354:788–789.
- Sibai, B., Dekker, G. and Kupferminc, M. (2005) Pre-eclampsia. Lancet 365:785–799.
- Takagi, Y., Nikaido, T., Toki, T., Kita, N., Kanai, M., Ashida, T., (2004) Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch 444:49–55.
- Takikawa, O., Kuroiwa, T., Yamazaki, F. and Kido, R. (1988) Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem 263:2041–2048.
- Thomas, S.R. and Stocker, R. (1999) Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep 4:199–220.
- Woodage, T., Venter, J.C. and Broder, S. (2002) Application of the human genome to obstetrics and gynecology. Clin Obstet Gynecol 45:711–732.