Abstract
Male infertility is a common and complex pathology affecting0about 7% of men of reproductive age. Given its complexity, the underlying etiology for male infertility is often unknown. A growing amount of evidence suggests genomic instability may be an important factor in some cases of male factor infertility. While some specific manifestations of genomic instability, such as increased sperm aneuploidy rates and increased somatic translocations and inversions in infertile men, are well established, other facets of genomic instability associated with male infertility have not been thoroughly investigated. A limited body of recent work has identified a potential association between microsatellite instability and spermatogenic failure. In addition, mutations in mismatch repair and tumor suppressor genes, which could potentially lead to genomic instability, have been identified in some infertile men and animal models. In addition, results of two epidemiologic studies suggest spermatogenic defects might be just one aspect of a more systemic problem, possibly due to increased genomic instability. In this review we discuss well-established links between genomic instability and male infertility, as well as some of the emerging but less established data to support this relationship. We also propose some important areas of future research toward a more complete understanding of the underlying mechanisms for male infertility.
Introduction
Infertility is a widespread problem affecting approximately 15% of couples worldwide. In about half of the cases, infertility is associated with problems with the male partner or a combination of both partners. Despite its prevalence and a considerable amount of research focused on identifying the root causes of male infertility, approximately half of male infertility cases are classified as idiopathic [Dohle et al. Citation2005]. Identification of the underlying causes of male infertility is a critical step toward improving diagnosis and treatment of fertility disorders.
One challenge in identifying etiologic factors leading to male infertility is the wide clinical spectrum of the associated pathology. Male infertility may arise from endocrine, genetic, or epigenetic changes that result in aberrant sperm production, or any of a number of sperm function problems that include abnormal morphology, reduced motility, loss of capacity to bind to, penetrate, or activate the oocyte, etc. Male factor infertility may be associated with a normal sperm count (defined as > 20 million sperm per ml), reduced sperm count (oligozoospermia), or complete absence of mature sperm cells in the ejaculate (azoospermia). Further, azoospermia can result from a complete absence of testicular sperm at any stage (e.g., Sertoli cell only syndrome), arrested sperm development at any stage of spermatogenesis (maturation arrest), hypospermatogenesis in which small numbers of sperm are present in the testis but not present in the ejaculate, or essentially normal spermatogenesis coupled with a lack of ability of sperm passage from the testes (obstructive azoospermia).
Spermatogenesis is clearly a very complex process requiring the proper function and transcriptional and translational timing of a host of genes [Iguchi et al. Citation2006]. Testicular gene expression analysis in pre- and post-pubertal mice has given some indication of the incredible complexity of spermatogenesis with over 1,600 genes whose expression increased at the time of meiotic onset, approximately 20% of which are expressed exclusively in the testes [Schultz et al. Citation2003]. The large and growing number of gene knockout animal models with an infertility phenotype, including monumental efforts such as the Jackson Labs mutagenesis project (reprogenomics.jax.org) also offers some insight into the complexity of the network of genes required for normal spermatogenesis and fertility [Tamowski et al. Citation2010; Yatsenko et al. Citation2010]. The information gleaned from animal models that display infertility phenotypes provides a foundation for better characterizing the etiology of male infertility in humans.
Well-established genetic causes of male infertility include microdeletions on the q-arm of the Y chromosome (Yq), which commonly occur in severely oligozoospermic and azoospermic men [Li et al. Citation2008]. In addition, Klinefelter's syndrome results in severe oligozoospermia or azoospermia, and chromosomal translocations and inversions are observed significantly more frequently in infertile men than in the general population [Martin Citation2008a]. Obstructive azoospermia due to congenital bilateral absence of vas deferens is commonly a result of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [Radpour et al. Citation2008]. While these factors are well characterized and enable diagnosis of the underlying cause of male infertility in some men, these factors account for fewer than half of cases [Dohle et al. Citation2002].
Many studies have evaluated the associations between single nucleotide polymorphisms (SNPs) or gene mutations and male infertility with many significant associations being reported; however, to date no SNP with reported associations has been conclusively demonstrated to be causal of infertility [Aston and Carrell Citation2009; Aston et al. Citation2010; Krausz and Giachini Citation2007; Nuti and Krausz Citation2008; Tuttelmann et al. Citation2007]. It is likely that some of the SNPs identified will prove to confer some risk for male infertility, but sufficiently large and well-designed studies have yet to be undertaken to this end.
With an underlying genetic cause remaining elusive in the majority of male infertility cases, and in light of the tremendous efforts to identify causal variants, it appears increasingly unlikely that a single genetic defect is responsible for the infertility pathology in a significant proportion of patients. In addition, increasing evidence is emerging to support the notion that male infertility phenotypes are not limited to spermatogenic defects, but rather that spermatogenic defects may be just one manifestation of a more systemic problem [Jensen et al. Citation2009; Salonia et al. Citation2009; Wohlfahrt-Veje et al. Citation2009]. The occurrence of genomic instability associated with male infertility is receiving increasing scrutiny in light of a growing body of evidence to support this notion, however, given the paucity of studies specifically addressing the involvement of genomic instability in male infertility and the small sample sizes of relevant studies, future research in this area is certainly warranted. In this review we discuss the data that support the involvement of genomic instability in some cases of male infertility, along with the potential implications of these data. We also address important future areas of research that will be necessary to better characterize the involvement of genomic instability in male infertility.
Genomic instability and male infertility
Accumulating evidence suggests that genomic instability may play a role in some cases of male infertility, with disrupted spermatogenesis being just one manifestation of a multifactorial condition. Genomic instability, a common feature of most cancers [Negrini et al. Citation2010], is broadly classified into chromosomal instability (CIN) and microsatellite instability (MSI) [Charames and Bapat Citation2003]. The majority of cancers involve CIN characterized by gross chromosomal anomalies including aneuploidy and changes in chromosome structure, such as large translocations or inversions [Thompson et al. Citation2010]. Microsatellite instability involves changes in the length of repetitive microsatellite regions and is a feature of a smaller proportion of cancer types than CIN [de la Chapelle Citation2003]. Microsatellite instability is typically associated with a mutator phenotype, characterized by increased base-pair mutation rates in cells.
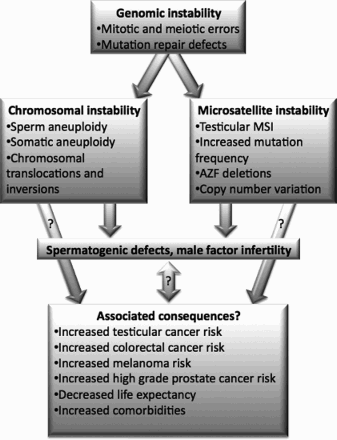
Male infertility research has revealed evidence to support the idea that spermatogenic defects are often not limited to testicular dysfunction. Researchers have noted increased urogenital disorders in infertile men, increased incidences of several types of cancer, as well as overall reduced general health in infertile men as a whole, as manifested by decreased life expectancy and increased morbidity [Jensen et al. Citation2009; Salonia et al. Citation2009]. In addition, increased sperm aneuploidy rates, and incidence of translocations and Y chromosome microdeletions are well documented in infertile men. A limited amount of data suggests MSI as well as increased mutation rates might be other important etiologic factors in some classes of male infertility. illustrates a proposed model for the potential role of genomic instability in male infertility as discussed below. Importantly, coincidence of male infertility and the various forms of genomic instability observed does not necessarily imply a causal link between the two. The molecular bases for the manifestations of genomic instability observed in infertile men have generally not been identified. A great deal of future work will be required to identify the sources of genomic instability in some infertile men and to determine whether the increased cancer incidence, increased comorbidities, and decreased life expectancy observed in infertile men are due in part to DNA replication defects associated with genomic instability or whether the two are entirely unrelated.
Figure 1. Proposed model for the involvement of genomic instability in male infertility. Two broad classes of genomic instability include CIN and MSI. Evidence for the involvement of CIN in male infertility include the observations of increased incidence of somatic and germline aneuploidy rates and increased chromosomal translocations and inversions in infertile men. Evidences for the role of MSI in male infertility include the established importance of AZF deletions, data indicating increased MSI in testicular tissue from idiopathic azoospermic men, increased testicular tissue mutation rates, and the involvement of CNVs in male infertility. Potential consequences of genomic instability on infertile men are included in the lower tier. Importantly, the link between these factors and CIN and MSI is theoretical and has not been demonstrated experimentally. Abbreviations: CIN: chromosome instability; CNV: copy number variation; MSI: microsatellite instability.
Chromosome instability and male infertility
Chromosomal abnormalities are a well-established signature in a significant proportion of male infertility cases [O'Flynn O'Brien et al. Citation2010; Walsh et al. Citation2009]. Chromosomal abnormalities associated with male infertility can be found constitutively (e.g., somatic translocations, inversions, XXY), or they can arise through meiotic errors and be found specifically in the sperm.
An early study evaluating the chromosomal constitution of 1,599 unselected men attending a sub-fertility clinic found chromosomal abnormalities in 2.2% of patients, a frequency almost five times higher than that seen in the general population [Chandley et al. Citation1975]. A subsequent study focusing on severe male factor infertility evaluated 496 azoospermic and oligozoospermic men. In this cohort of patients displaying severe spermatogenic defects, chromosomal abnormalities were identified in 14.1% of azoospermic and 5.1% of oligozoospermic men [Retief et al. Citation1984].
Peschka et al. [1999] performed cytogenetic analysis in 781 couples undergoing intracytoplasmic sperm injection (ICSI) treatment for infertility. Male factor infertility was the indication for ICSI in 80.9% of the cases, and 19.1% of cases included both male factor and female infertility. Strikingly, karyotypic abnormalities including aneuploidies, mosaisicm, translocations, inversions, fragile sites on autosomes, and chromosomal gaps, breaks, or exchanges were identified in 13% of these men. An additional 22% of men evaluated displayed single cell aberrations, the clinical significance of which is unclear at present [Peschka et al. Citation1999]. More recently, a survey by Vincent et al. of 2,651 infertile men identified somatic cytogenetic abnormalities in 7.7% of that population, with frequency increasing with decreasing sperm count [Vincent et al. Citation2002]. Subsequently, a smaller study that evaluated the chromosomal status of 42 azoospermic and 46 severely oligozoospermic men yielded similar findings. In this study, constitutional chromosomal abnormalities were observed in 14.3% of azoospermic and 6.5% of severely oligozoospermic men [Nagvenkar et al. Citation2005].
While volumes of data exist to demonstrate the increased incidence of somatic chromosomal abnormalities in infertile men, and especially in men with severe spermatogenic defects, fewer studies have evaluated the incidence of sperm aneuploidies in infertile men and controls. However, various recent reports indicate increased frequency of sperm chromosome aneuploidies among infertile men [Martin Citation2008b; Tempest Citation2011; Zhou et al. Citation2011], and the sperm aneuploidy rate may be even higher in carriers of somatic chromosomal abnormalities [Perrin et al. Citation2011; Wong et al. Citation2008].
While the underlying cause for increased chromosomal aberrations in infertile men is unknown, clearly CIN is an important feature of some cases of male infertility. Future studies should seek to determine the mechanisms by which these abnormalities arise.
Microsatellite instability and male infertility
Microsatellites are short stretches of DNA composed of one to six nucleotide tandem repeats. MSI is characterized by expansion or contraction of these regions due to the gain or loss of one or more microsatellite repeats [de la Chapelle Citation2003] and is an important marker for several types of cancers arising as a result of defective DNA repair mechanisms. MSI typically results from mutations in DNA mismatch repair (MMR) genes, but sometimes occurs through epigenetic silencing of MMR genes [Veigl et al. Citation1998]. Either mechanism results in over 100-fold increases in DNA mutation rates [Eshleman et al. Citation1995].
Animal knockout models have consistently demonstrated the importance of DNA stability and repair genes in spermatogenesis. Mice deficient in postmeiotic segregation increased 2 (Pms2) and MutL homolog 1 (Mlh1) genes both exhibit MSI and infertility with a meiotic arrest phenotype [Baker et al. Citation1995; Baker et al. Citation1996]. Similarly, exonuclease 1−/− (Exo1) mice are prone to MSI, increased mutation rates, increased tumor susceptibility, and sterility due to meiotic defects [Wei et al. Citation2003]. Like Mlh1-null mice, zebrafish mlh1-null mutants are sterile and display spermatogenic arrest at metaphase I [Feitsma et al. Citation2007]. Mlh3, which localizes to meiotic chromosomes and aids in Mlh1 binding, while not required for mismatch repair, is a critical component in the meiotic pathway, as spermatocytes in Mlh3−/− mice undergo apoptosis at metaphase I [Lipkin et al. Citation2002]. In addition, disruption of other genes involved in the maintenance of DNA stability and DNA repair pathways including histone H2AX, ubiquitin specific peptidase 1 (Usp1), poly(ADP-ribose) polymerase 1 (Parp1), poly(ADP-ribose) glycohydrolase (Parg), and tumor protein p73 result in similar phenotypes exhibiting MSI or CIN as well as phenotypes ranging from sub-fertility to sterility [Tomasini et al. Citation2008].
The potential involvement of MMR and meiotic recombination gene mutations in male infertility has been proposed by several groups [Mukherjee et al. Citation2010; Sanderson et al. Citation2008]. Evaluation of MLH3 gene sequence in men displaying spermatogenic arrest at the primary spermatocyte stage identified missense mutations in four out of thirteen patients [Ferras et al. Citation2007]. In agreement with the mouse model, MSI was not detected in individuals harboring these mutations [Ferras et al. Citation2007]. In another study, polymorphisms in MSH5 and MLH3 mismatch repair genes were reported to be associated with idiopathic azoospermia and severe oligozoospermia [Xu et al. Citation2010]. In addition, transcript levels of several MMR genes were evaluated in the testis of 13 patients with spermatogenic failure, and a significant decrease in expression of the majority of genes analyzed was noted in azoospermic men compared with controls [Terribas et al. Citation2010].
A mechanistic link between testicular cancer and male infertility has not yet been established, but importantly MSI is a prominent prognostic feature of some testicular germ cell tumors [Faulkner and Friedlander Citation2000; Huddart et al. Citation1995]. In addition, the degree of instability exhibited by germ cell tumors as well as the level of mismatch repair activity are predictive of clinical outcome in testicular germ cell tumors [Honecker et al. Citation2009; Velasco et al. Citation2008; Velasco et al. Citation2004]. The observed association and the potential for a common mechanism between testicular cancer and male infertility as well other urogenital malformations gave rise to the theory of a testicular dysgenesis syndrome [Sharpe and Skakkebaek Citation1993; Skakkebaek et al. Citation2001].
While MSI is a well-established signature for several cancers, its potential involvement in male infertility has received much less attention. Relatively high rates of microsatellite [Bacon et al. Citation2001] and minisatellite [Jeffreys et al. Citation1994] mutations have been detected in sub-populations of the male germline using small pool PCR in studies that did not evaluate the male infertility phenotype, indicating the male germline may be inherently less stable than other cell types. A single study has evaluated the incidence of MSI in azoospermic men with different spermatogenic defects [Maduro et al. Citation2003]. In this study, seven microsatellite regions were compared between blood and testis DNA from men with maturation arrest (n = 10), Sertoli cell only syndrome (n = 19), hypospermatogenesis (n = 12), and from vasectomized men with normal testis histology (n = 20). While some degree of instability was noted in all of the groups, significantly more MSI was found in Sertoli cell only patients than in controls [Maduro et al. Citation2003]. Further, immunohistochemical examination to evaluate testicular, somatic, and germline expression levels of DNA mismatch repair proteins revealed a higher expression level of DNA mismatch repair proteins in Sertoli cell only patients compared with controls [Maduro et al. Citation2003]. This relatively small study provides impetus for larger studies to evaluate the role of MSI in spermatogenic disorders.
A single study evaluated the frequency of point mutations within a polymorphic microsatellite (D19S49) in testis tissue from seven men with meiotic arrest and five controls (men with obstructive azoospermia, but otherwise normal spermatogenesis). Following PCR of the microsatellite region, PCR products were cloned, and 25 colonies from each sample were picked for sequencing. This small study found a significantly increased frequency of point mutations in testis DNA from men with meiotic arrest compared with controls [Nudell et al. Citation2000]. Analysis of the same region in blood DNA from the same individuals found no evidence for mutations, indicating that the mutations most likely arose during meiosis and likely as a result of defective DNA repair mechanisms, as the mutation frequencies were similar to those found in Mlh1-/- mice [Baker et al. Citation1996].
Our group recently completed a pilot genome-wide SNP association study of male factor infertility utilizing the Illumina HumanCNV370-duo oligonucleotide array [Aston and Carrell Citation2009]. In an effort to evaluate mutation rates genome-wide, we determined the frequency of occurrence of minor alleles in azoospermic (n = 40), severe oligozoospermic (n = 52), and normospermic (n = 80) men as a proxy for the direct evaluation of mutation rates. With this analysis we found a slight but significant increase in minor alleles in the azoospermic group compared with controls [Aston and Carrell Citation2009]. While not a direct measure of genomic instability, the increased frequency of minor alleles observed in infertile men compared with controls may be due to a decrease in DNA replication fidelity in those men. Additional studies to evaluate the occurrence of MSI in infertile men compared with controls will be important in determining whether DNA mismatch repair defects and MSI are important factors in male infertility.
Copy number variants (CNVs) and male infertility
Copy number variants, defined as duplications or deletions in the genome comprising at least one kilobase of genomic DNA, have historically been an under-appreciated form of genetic variation in terms of their contribution to phenotypic variability and disease [Lee and Scherer Citation2010; Stankiewicz and Lupski Citation2010]. A deluge of genome-wide association studies over the past several years, as well as some more recent ultra-high density array and whole genome sequencing studies have offered insight into the massive amount of inter-individual genomic variation involving CNVs as well as the importance of CNVs in a large number of diseases [Stankiewicz and Lupski Citation2010].
A classic and simple example of CNVs involved in male infertility is microdeletions in the azoospermia factor (AZF) locus of Yq [Jobling Citation2008; Krausz et al. Citation2011; Skaletsky et al. Citation2003]. Yq deletions in connection with azoospermia were first described nearly four decades ago [Neu et al. Citation1973; Tiepolo and Zuffardi Citation1976]. The highly repetitive nature of the AZF region of the Y chromosome makes it prone to frequent rearrangements, often resulting in complete or partial AZF deletions [Jobling and Tyler-Smith Citation2003; Vogt Citation2004]. The region is composed of three sub-regions: AZFa, AZFb, and AZFc [Repping et al. Citation2002; Vogt et al. Citation1996]. AZFc is the most commonly deleted of the three sub-regions, and AZFc deletions result in azoospermia or less frequently in severe oligozoospermia. Deletions that include the AZFa and/or AZFb regions almost invariably result in azoospermia. In all, AZF deletions are the causative factor in approximately 5-20% of infertile men, with frequencies varying widely based on the class of male infertility as well as ethnicity, making these deletions the most common known genetic cause of spermatogenic defects [Krausz and Degl'Innocenti Citation2006; Krausz et al. Citation2003; Vogt Citation2004]. A primary mechanism by which AZF deletions arise as a result of meiotic errors involving non-allelic homologous recombination (NAHR) of repeat sequences is well established, however NAHR does not account for all AZF deletions [Repping et al. Citation2002]. Moreover, the relative frequency of de novo AZF deletions in fertile and infertile men has not been evaluated, so it is not known whether deletions occur at increased rates in infertile men as a result of meiotic checkpoint or repair deficiencies, or whether they occur at similar rates between fertile and infertile men.
While it has been suggested that the evaluation of CNVs in connection with male factor infertility represents an important new avenue of study [Aston and Carrell Citation2009; Visser et al. Citation2009], few studies to date have evaluated the involvement of CNVs in spermatogenic defects [Hansen et al. Citation2010; Jorgez et al. Citation2011; Tuttelmann et al. Citation2011].
In a recent study, Jorgez et al. screened 87 infertile men with AZF deletions and 35 controls for CNVs by array comparative genomic hybridization (aCGH) or CNV-Taqman assays. CNVs were identified in the pseudoautosomal regions (PAR) of X and Y in all 13 men displaying both an abnormal karyotype and AZF deletions, while PAR CNVs were present in 7/74 AZF-deleted men with a normal karyotype [Jorgez et al. Citation2011]. PAR deletions, which occur at a relatively high frequency in men with AZF deletions, could contribute to recombination errors in ICSI offspring. Additional studies will be necessary to better characterize the functional consequences of PAR deletions.
Recently a moderately sized genome-wide study employing aCGH to identify CNVs associated with severe oligozoospermia and Sertoli cell only syndrome, was performed. The study identified fourteen recurrent CNVs present exclusively in patient groups as well as several other CNVs present significantly more frequently in infertile men than controls [Tuttelmann et al. Citation2011]. While the number of deletions per individual was negatively correlated with sperm count in control men, comparable numbers of CNVs per individual were found between groups [Tuttelmann et al. Citation2011]. Follow-up studies to evaluate the importance of the patient-specific CNVs identified are warranted.
Given the obvious importance of the Y chromosome in spermatogenesis, and its inherent genetic instability, several other variable regions in addition to AZF have been evaluated for copy number variation. The testis-specific protein Y-encoded 1 (TSPY1) is a copy number polymorphic gene located on the p arm of the Y chromosome and is present in a highly variable number of copies ranging from 20-73 [Nickkholgh et al. Citation2009]. The gene is expressed primarily in spermatogonia and primary spermatocytes, and its function is unknown. Three groups have evaluated the association between the number of TSPY1 repeats and spermatogenic status. The first study reported significantly higher TSPY1 copy number in infertile men compared with controls [Vodicka et al. Citation2007] while the second study found no association between copy number and fertility status [Nickkholgh et al. Citation2009]. The most recent, and most comprehensive study, evaluated the variability of TSPY1 copy number between and within Y chromosome haplogroups and the association with infertility. With careful phenotypic selection of 154 idiopathic infertile men and 130 normospermic controls, the authors found a significant positive correlation between TSPY1 copy number and sperm count [Giachini et al. Citation2009]. The differences in findings among studies could reflect differences in ethnicities between or within study groups, or possibly differences in selection criteria for cases and controls. This series of studies emphasizes the necessity of careful study design with particular attention to ethnic homogeneity. Additional TSPY copy number studies similar in design to the study by Giachini et al. [2009] should be performed to more fully characterize the functional impact of the gene on fertility status.
Another copy number polymorphic region that has received considerable attention in the context of male infertility is located in the androgen receptor (AR) gene. The AR contains a polymorphic region consisting of variable numbers of copies of the CAG trinucleotide. It has been proposed that increasing copies associate with impaired spermatogenesis, but results are conflicting [Yong et al. Citation2003].
The deleted in azoospermia (DAZ) gene located within the AZFc region of the Y chromosome, like TSPY1, is a multi-copy gene and is apparently required for successful completion of the later stages of meiosis. It is typically present in four copies, however, duplications and deletions of some or all copies of the gene have been reported [de Vries et al. Citation2002; Giachini et al. Citation2008; Lin et al. Citation2007]. Evaluation of DAZ copy number in fertile and infertile men has shown that reduced copy number does correlate with reduced fertility [de Vries et al. Citation2002; Giachini et al. Citation2008; Repping et al. Citation2003; Visser et al. Citation2009; Writzl et al. Citation2005; Yang et al. Citation2010], but a deletion leaving two copies of the gene is compatible with normal spermatogenesis [de Vries et al. Citation2002].
Other examples of studies evaluating the involvement of CNVs in male infertility include a recent report by Gohring et al. in which a 6.7 Mb duplication of 11q24.2q25 was reported in an azoospermic man with hypogonadism [Gohring et al. Citation2008]. In addition, Hansen et al. evaluated the contribution of gene copy number in the sperm protein associated with the nucleus on the X chromosome (SPANX) gene cluster to male infertility. The SPANX genes are expressed throughout spermatogenesis, and the gene family is known to be prone to variation. While evidence for variation in copy number of the gene cluster was observed in the study, no correlation between copy number and infertility was found [Hansen et al. Citation2010].
While several specific CNVs have been implicated in male infertility, genome-wide CNV studies in the context of male infertility are scarce. Several groups have noted an overall increased number of CNVs or mutations in patients with other complex diseases including schizophrenia [Stefansson et al. Citation2008; Walsh et al. Citation2008a], hypertriglyceridemia [Johansen et al. Citation2010], and autism spectrum disorders [Pinto et al. Citation2010]. Evaluation of the CNV burden in infertile men will be another important measure of the involvement of genomic instability in male infertility.
While CNVs certainly play a role in male infertility, the extent of their involvement is not yet known. AZF deletions currently represent the best-characterized and most frequent known genetic cause for spermatogenic failure. Closer scrutiny of the involvement of CNVs in male infertility on a genome-wide scale will be necessary to identify other copy number variable regions important in spermatogenesis.
Potential consequences of genomic instability in infertile men
Observational as well as experimental evidence on several fronts implicates genomic instability in some cases of male infertility. These include reports of reduced general health, increases in comorbidities, and birth defects in infertile men, and increased incidences of some types of cancer. In addition, while AZF deletions are a well-documented cause of spermatogenic defects in a significant proportion of infertile men, it is not yet known whether these deletions arise as a result of increased genomic instability in some men or simply by a random event equally likely to occur irrespective of the genetic background of the individual.
Health characteristics of infertile men
Two recent studies have evaluated the general health of infertile men compared with controls by evaluating the associations between various semen parameters and life expectancy in one study [Jensen et al. Citation2009], and by measuring comorbidities associated with male infertility in the other study [Salonia et al. Citation2009].
A Danish cohort study evaluated semen parameters in 43,277 non-azoospermic men who were referred for infertility problems over a period of almost four decades from 1963 to 2001. The researchers found a significant negative correlation between sperm motility and mortality rate. Similarly, a significant trend was noted between a decreasing percentage of morphologically normal sperm and reduced life expectancy [Jensen et al. Citation2009]. These trends remained significant when adjustments were made for lifestyle factors and when men who developed testicular cancer following referral for infertility were excluded from analysis. The decreased life expectancy in the infertile group was found to be due to a variety of different diseases with infectious and vascular diseases being the most over-represented causes of death among men with reduced sperm count [Jensen et al. Citation2009]. The link between decreased life expectancy and male infertility is unclear, but it supports the notion of male infertility being a systemic rather than an isolated condition.
A prospective study evaluating the general health of infertile versus fertile men obtained similar results [Salonia et al. Citation2009]. In this study 344 infertile men and 293 age-comparable fertile men were recruited, and general health was assessed using the Charlson Comorbidity Index. Results of the study indicated infertile men were generally less healthy than fertile controls even after adjusting for body mass index, age, and educational status. While the relatively small number of study participants limits the conclusions that can be drawn, it is noteworthy that vascular and pulmonary diseases were more common in infertile men compared with controls [Salonia et al. Citation2009].
While the data are limited, these two studies offer compelling evidence that even in a diverse group of infertile men, general health and consequently life expectancy are significantly lower than in controls. There is no direct evidence to link these observations with genomic instability, however future studies are certainly warranted to validate these initial findings and to identify the underlying cause for the observed differences. In addition, larger prospective studies with improved statistical power will be better able to identify commonalities in affected men, which will hopefully enable the identification of disrupted pathways that might link infertility with other comorbidities.
Male infertility and cancer
Genomic instability, most frequently in the form of CIN and occasionally associated with MSI, is a signature feature of most types of cancer. If genomic instability is an important etiology of some cases of male infertility, it would be expected that cancer risk be concomitantly elevated in genomic instability-associated male infertility cases. As discussed previously, testicular cancer incidence is significantly higher (three-fold or more) in infertile men compared with the general population [Hotaling and Walsh Citation2009]. While the incidences of other types of cancer in infertile men have not been thoroughly evaluated, there are a few reports that indicate other types of cancer might occur more frequently in infertile men compared with controls.
A cross analysis between men attending infertility centers and cancer registry data found men with male factor infertility were subsequently diagnosed with colorectal cancer, melanoma, and prostate cancer significantly more frequently than age-matched men from the general population [Walsh et al. Citation2008b]. Conversely, a recent cohort study of prostate cancer risk evaluating 445 prostate cancer cases actually found significantly reduced prostate cancer risk in infertile men compared with biological fathers, presumably due to generally lower steroidogenic capacity in infertile men, with steroidogenesis being an important contributing factor to prostate cancer development [Ruhayel et al. Citation2010]. Earlier studies also found increased risk of prostate cancer associated with increased number of children fathered, but these studies did not directly assess fertility status [Giwercman et al. Citation2005; Jorgensen et al. Citation2008]. Another recent study found that while infertile men compared with the general population had similar risks for developing prostate cancer, the risks of high grade prostate cancer were significantly greater in infertile men [Walsh et al. Citation2010]. Future studies evaluating the incidence of all types of cancer in infertile men as well as the involvement of genomic instability in these cases will be important in determining whether genomic instability associated with some classes of male infertility is an important risk factor for cancer development.
Future work and conclusions
In addition to genetics, cancer studies have demonstrated that epigenetic factors including changes in DNA methylation and histone modifications can give rise to genomic instability through the silencing of DNA replication and repair genes [Aguilera and Gomez-Gonzalez Citation2008]. The epigenetic landscape of sperm has received increasing attention in recent years [Brykczynska et al. Citation2010; Hammoud et al. Citation2009a], and sperm epigenetic aberrations associated with spermatogenic defects have been described by several groups [Boissonnas et al. Citation2010; Filipponi and Feil Citation2009; Hammoud et al. Citation2011; Hammoud et al. Citation2009b; Houshdaran et al. Citation2007; Kobayashi et al. Citation2007; Marques et al. Citation2004; Marques et al. Citation2008; Nanassy and Carrell Citation2011; Navarro-Costa et al. Citation2010; Poplinski et al. Citation2010; Sato et al. Citation2011]. A thorough investigation of the involvement of altered epigenetic marks associated with male infertility, and specifically in the context of genomic instability, will be another important avenue of study in the coming years.
Given the large proportion of male factor infertility cases that have no known underlying etiology, as well as the potential for compound health problems associated with male infertility, there is clearly a great deal of research that remains to be done. Understanding the different etiologies for infertility is critical in order to provide the best counseling and treatment for infertile couples.
New and emerging tools will be instrumental in more fully characterizing the genetic and epigenetic basis for male infertility. Next generation sequencing is becoming a cost-effective means to directly evaluate mutation rates and copy number variations at single base-resolution. Larger repositories of DNA derived from comprehensively phenotyped infertile men will enable sufficiently powered studies aimed at identifying the etiology of male infertility.
With the advent of assisted reproductive technologies (ART), including in vitro fertilization and intracytoplasmic sperm injection, severely oligozoospermic men are now able to produce offspring. With the ever-increasing use of ART in society it is becoming more important that we gain a sound understanding of the underlying cause associated with each case of male infertility. Given the significant potential for other negative health consequences associated with genomic instability, a renewed focus on the involvement of genomic instability in male infertility, and an understanding of the possible consequences of passing a genome potentially prone to instability to the next generation is necessary. Close monitoring of offspring is also warranted.
Another important question that remains to be addressed is whether the conditions discussed in this review are causative of male infertility, or rather are associated in a syndromic fashion; i.e., infertility is one aspect of a much broader and more complex phenotype. This will continue to be an important question to be addressed in future studies in order to better characterize the nature of male infertility, to improve diagnostic capabilities, and ultimately to improve patient care.
Abbreviations
| aCGH: | = | array comparative genomic hybridization |
| AZF: | = | azoospermia factor |
| CFTR: | = | cystic fibrosis transmembrane conductance regulator |
| CIN: | = | chromosome instability |
| CNV: | = | copy number variant |
| DAZ: | = | deleted in azoospermia |
| Exo1: | = | exonuclease 1 |
| ICSI: | = | intracytoplasmic sperm injection |
| Mlh1/3: | = | MutL homolog 1/3 |
| MMR: | = | mismatch repair |
| MSI: | = | microsatellite instability |
| PAR: | = | pseudoautosomal region |
| Parg: | = | poly(ADP-ribose) glycohydrolase |
| Parp1: | = | poly(ADP-ribose) polymerase 1 |
| Pms2: | = | postmeiotic segregation increased 2 |
| SNP: | = | single nucleotide polymorphism |
| SPANX: | = | sperm protein associated with the nucleus on the X chromosome |
| TSPY1: | = | testis-specific protein Y-encoded 1 |
| Usp1: | = | ubiquitin specific peptidase 1 |
| Yq: | = | long arm of the Y chromosome. |
Acknowledgment
The authors would like to thank Dr. Christopher Somers for review of the manuscript prior to submission.
Declaration of Interest: The authors report no declarations of interest.
References
- Aguilera, A. and Gomez-Gonzalez, B. (2008) Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet 9:204–217.
- Aston, K.I., Carrell, D.T. (2009) Genome-wide study of single-nucleotide polymorphisms associated with azoospermia and severe oligozoospermia. J Androl 30:711–725.
- Aston, K.I., Krausz, C., Laface, I., Ruiz-Castane, E., Carrell, D.T. (2010) Evaluation of 172 candidate polymorphisms for association with oligozoospermia or azoospermia in a large cohort of men of European descent. Hum Reprod. 25(6):1383–97.
- Bacon, A.L., Dunlop, M.G., Farrington, S.M. (2001) Hypermutability at a poly(A/T) tract in the human germline. Nucleic Acids Res 29:4405–4413.
- Baker, S.M., Bronner, C.E., Zhang, L., Plug, A.W., Robatzek, M., Warren, G., (1995) Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell 82:309–319.
- Baker, S.M., Plug, A.W., Prolla, T.A., Bronner, C.E., Harris, A.C., Yao, X., (1996) Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet 13:336–342.
- Boissonnas, C.C., Abdalaoui, H.E., Haelewyn, V., Fauque, P., Dupont, J.M., Gut, I., (2010) Specific epigenetic alterations of IGF2-H19 locus in spermatozoa from infertile men. Eur J Hum Genet 18:73–80.
- Brykczynska, U., Hisano, M., Erkek, S., Ramos, L., Oakeley, E.J., Roloff, T.C., (2010) Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 17(6):679–87.
- Chandley, A.C., Edmond, P., Christie, S., Gowans, L., Fletcher, J., Frackiewicz, A., (1975) Cytogenetics and infertility in man. I. Karyotype and seminal analysis: results of a five-year survey of men attending a subfertility clinic. Ann Hum Genet 39:231–254.
- Charames, G.S., Bapat, B. (2003) Genomic instability and cancer. Curr Mol Med 3:589–596.
- de la Chapelle, A. (2003) Microsatellite instability. N Engl J Med 349:209–210.
- de Vries, J.W., Hoffer, M.J., Repping, S., Hoovers, J.M., Leschot, N.J., van der Veen, F. (2002) Reduced copy number of DAZ genes in subfertile and infertile men. Fertil Steril 77:68–75.
- Dohle, G.R., Colpi, G.M., Hargreave, T.B., Papp, G.K., Jungwirth, A., Weidner, W. (2005) EAU guidelines on male infertility. Eur Urol 48:703–711.
- Dohle, G.R., Halley, D.J., Van Hemel, J.O., van den Ouwel, A.M., Pieters, M.H., Weber, R.F., (2002) Genetic risk factors in infertile men with severe oligozoospermia and azoospermia. Hum Reprod 17:13–16.
- Eshleman, J.R., Lang, E.Z., Bowerfind, G.K., Parsons, R., Vogelstein, B., Willson, J.K., (1995) Increased mutation rate at the hprt locus accompanies microsatellite instability in colon cancer. Oncogene 10:33–37.
- Faulkner, S.W., Friedlander, M.L. (2000) Microsatellite instability in germ cell tumors of the testis and ovary. Gynecol Oncol 79:38–43.
- Feitsma, H., Leal, M.C., Moens, P.B., Cuppen, E., Schulz, R.W. (2007) Mlh1 deficiency in zebrafish results in male sterility and aneuploid as well as triploid progeny in females. Genetics 175:1561–1569.
- Ferras, C., Zhou, X.L., Sousa, M., Lindblom, A., Barros, A. (2007) DNA mismatch repair gene hMLH3 variants in meiotic arrest. Fertil Steril 88:1681–1684.
- Filipponi, D., Feil, R. (2009) Perturbation of genomic imprinting in oligozoospermia. Epigenetics 4:27–30.
- Giachini, C., Laface, I., Guarducci, E., Balercia, G., Forti, G., Krausz, C. (2008) Partial AZFc deletions and duplications: clinical correlates in the Italian population. Hum Genet 124:399–410.
- Giachini, C., Nuti, F., Turner, D.J., Laface, I., Xue, Y., Daguin, F., (2009) TSPY1 copy number variation influences spermatogenesis and shows differences among Y lineages. J Clin Endocrinol Metab 94:4016–4022.
- Giwercman, A., Richiardi, L., Kaijser, M., Ekbom, A., Akre, O. (2005) Reduced risk of prostate cancer in men who are childless as compared to those who have fathered a child: a population based case-control study. Int J Cancer 115:994–997.
- Gohring, I., Blumlein, H.M., Hoyer, J., Ekici, A.B., Trautmann, U., Rauch, A. (2008) 6.7 Mb interstitial duplication in chromosome band 11q24.2q25 associated with infertility, minor dysmorphic features and normal psychomotor development. Eur J Med Genet 51:666–671.
- Hammoud, S.S., Nix, D.A., Hammoud, A.O., Gibson, M., Cairns, B.R., Carrell, D.T. (2011) Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod.
- Hammoud, S.S., Nix, D.A., Zhang, H., Purwar, J., Carrell, D.T., Cairns, B.R. (2009a) Distinctive chromatin in human sperm packages genes for embryo development. Nature 460:473–478.
- Hammoud, S.S., Purwar, J., Pflueger, C., Cairns, B.R., Carrell, D.T. (2009b) Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil Steril 94(5):1728–33.
- Hansen, S., Eichler, E.E., Fullerton, S.M., Carrell, D. (2010) SPANX gene variation in fertile and infertile males. Syst Biol Reprod Med 55:18–26.
- Honecker, F., Wermann, H., Mayer, F., Gillis, A.J., Stoop, H., van Gurp, R.J., (2009) Microsatellite instability, mismatch repair deficiency, and BRAF mutation in treatment-resistant germ cell tumors. J Clin Oncol 27:2129–2136.
- Hotaling, J.M., Walsh, T.J. (2009) Male infertility: a risk factor for testicular cancer. Nat Rev Urol 6:550–556.
- Houshdaran, S., Cortessis, V.K., Siegmund, K., Yang, A., Laird, P.W., Sokol, R.Z. (2007) Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS One 2:e1289.
- Huddart, R.A., Wooster, R., Horwich, A., Cooper, C.S. (1995) Microsatellite instability in human testicular germ cell tumours. Br J Cancer 72:642–645.
- Iguchi, N., Tobias, J.W., Hecht, N.B. (2006) Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc Natl Acad Sci USA 103:7712–7717.
- Jeffreys, A.J., Tamaki, K., MacLeod, A., Monckton, D.G., Neil, D.L., Armour, J.A. (1994) Complex gene conversion events in germline mutation at human minisatellites. Nat Genet 6:136–145.
- Jensen, T.K., Jacobsen, R., Christensen, K., Nielsen, N.C., Bostofte, E. (2009) Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol 170:559–565.
- Jobling, M.A. (2008) Copy number variation on the human Y chromosome. Cytogenet Genome Res 123:253–262.
- Jobling, M.A., Tyler-Smith, C. (2003) The human Y chromosome: an evolutionary marker comes of age. Nat Rev Genet 4:598–612.
- Johansen, C.T., Wang, J., Lanktree, M.B., Cao, H., McIntyre, A.D., Ban, M.R., (2010) Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet 42:684–687.
- Jorgensen, K.T., Pedersen, B.V., Johansen, C., Frisch, M. (2008) Fatherhood status and prostate cancer risk. Cancer 112:919–923.
- Jorgez, C.J., Weedin, J.W., Sahin, A., Tannour-Louet, M., Han, S., Bournat, J.C., (2011) Aberrations in pseudoautosomal regions (PARs) found in infertile men with Y-chromosome microdeletions. J Clin Endocrinol Metab 96:E674–679.
- Kobayashi, H., Sato, A., Otsu, E., Hiura, H., Tomatsu, C., Utsunomiya, T., (2007) Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum Mol Genet 16:2542–2551.
- Krausz, C., Chianese, C., Giachini, C., Guarducci, E., Laface, I., Forti, G. (2011) The Y chromosome-linked copy number variations and male fertility. J Endocrinol Invest 34:376–382.
- Krausz, C., Degl'Innocenti, S. (2006) Y chromosome and male infertility: update, 2006. Front Biosci 11:3049–3061.
- Krausz, C., Forti, G., McElreavey, K. (2003) The Y chromosome and male fertility and infertility. Int J Androl 26:70–75.
- Krausz, C., Giachini, C. (2007) Genetic risk factors in male infertility. Arch Androl 53:125–133.
- Lee, C., Scherer, S.W. (2010) The clinical context of copy number variation in the human genome. Expert Rev Mol Med 12:e8.
- Li, Z., Haines, C.J., Han, Y. (2008) “Micro-deletions” of the human Y chromosome and their relationship with male infertility. J Genet Genomics 35:193–199.
- Lin, Y.W., Hsu, L.C., Kuo, P.L., Huang, W.J., Chiang, H.S., Yeh, S.D., (2007) Partial duplication at AZFc on the Y chromosome is a risk factor for impaired spermatogenesis in Han Chinese in Taiwan. Hum Mutat 28:486–494.
- Lipkin, S.M., Moens, P.B., Wang, V., Lenzi, M., Shanmugarajah, D., Gilgeous, A., (2002) Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet 31:385–390.
- Maduro, M.R., Casella, R., Kim, E., Levy, N., Niederberger, C., Lipshultz, L.I., (2003) Microsatellite instability and defects in mismatch repair proteins: a new aetiology for Sertoli cell-only syndrome. Mol Hum Reprod 9:61–68.
- Marques, C.J., Carvalho, F., Sousa, M., Barros, A. (2004) Genomic imprinting in disruptive spermatogenesis. Lancet 363:1700–1702.
- Marques, C.J., Costa, P., Vaz, B., Carvalho, F., Fernandes, S., Barros, A., (2008) Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol Hum Reprod 14:67–74.
- Martin, R.H. (2008a) Cytogenetic determinants of male fertility. Hum Reprod Update 14:379–390.
- Martin, R.H. (2008b) Meiotic errors in human oogenesis and spermatogenesis. Reprod Biomed Online 16:523–531.
- Mukherjee, S., Ridgeway, A.D., Lamb, D.J. (2010) DNA mismatch repair and infertility. Curr Opin Urol 20:525–532.
- Nagvenkar, P., Desai, K., Hinduja, I., Zaveri, K. (2005) Chromosomal studies in infertile men with oligozoospermia & non-obstructive azoospermia. Indian J Med Res 122:34–42.
- Nanassy, L., Carrell, D.T. (2011) Abnormal methylation of the promoter of CREM is broadly associated with male factor infertility and poor sperm quality but is improved in sperm selected by density gradient centrifugation. Fertil Steril 95:2310–2314.
- Navarro-Costa, P., Nogueira, P., Carvalho, M., Leal, F., Cordeiro, I., Calhaz-Jorge, C., (2010) Incorrect DNA methylation of the DAZL promoter CpG island associates with defective human sperm. Hum Reprod 25:2647–2654.
- Negrini, S., Gorgoulis, V.G., Halazonetis, T.D. (2010) Genomic instability–an evolving hallmark of cancer. Nat Rev Mol Cell Biol 11:220–228.
- Neu, R.L., Barlow, M.J., Gardner, L.I. (1973) Communications and commentaries: A 46,XYq- male with aspermia. Fertil Steril 24:811–813.
- Nickkholgh, B., Noordam, M.J., Hovingh, S.E., van Pelt, A.M., van der Veen, F., Repping, S. (2009) Y chromosome TSPY copy numbers and semen quality. Fertil Steril 94:1744–1747.
- Nudell, D., Castillo, M., Turek, P.J., Pera, R.R. (2000) Increased frequency of mutations in DNA from infertile men with meiotic arrest. Hum Reprod 15:1289–1294.
- Nuti, F., Krausz, C. (2008) Gene polymorphisms/mutations relevant to abnormal spermatogenesis. Reprod Biomed Online 16:504–513.
- O'Flynn O'Brien, K.L., Varghese, A.C., Agarwal, A. (2010) The genetic causes of male factor infertility: a review. Fertil Steril 93:1–12.
- Perrin, A., Basinko, A., Douet-Guilbert, N., Gueganic, N., Le Bris, M.J., Amice, V., (2011) Aneuploidy and DNA fragmentation in sperm of carriers of a constitutional chromosomal abnormality. Cytogenet Genome Res 133:100–106.
- Peschka, B., Leygraaf, J., Van der Ven, K., Montag, M., Schartmann, B., Schubert, R., (1999) Type and frequency of chromosome aberrations in 781 couples undergoing intracytoplasmic sperm injection. Hum Reprod 14:2257–2263.
- Pinto, D., Pagnamenta, A.T., Klei, L., Anney, R., Merico, D., Regan, R., (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466:368–372.
- Poplinski, A., Tuttelmann, F., Kanber, D., Horsthemke, B., Gromoll, J. (2010) Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int J Androl. 33(4):642–649.
- Radpour, R., Gourabi, H., Dizaj, A.V., Holzgreve, W., Zhong, X.Y. (2008) Genetic investigations of CFTR mutations in congenital absence of vas deferens, uterus, and vagina as a cause of infertility. J Androl 29:506–513.
- Repping, S., Skaletsky, H., Brown, L., van Daalen, S.K., Korver, C.M., Pyntikova, T., (2003) Polymorphism for a 1.6-Mb deletion of the human Y chromosome persists through balance between recurrent mutation and haploid selection. Nat Genet 35:247–251.
- Repping, S., Skaletsky, H., Lange, J., Silber, S., Van Der Veen, F., Oates, R.D., (2002) Recombination between palindromes P5 and P1 on the human Y chromosome causes massive deletions and spermatogenic failure. Am J Hum Genet 71:906–922.
- Retief, A.E., Van Zyl, J.A., Menkveld, R., Fox, M.F., Kotze, G.M., Brusnicky, J. (1984) Chromosome studies in 496 infertile males with a sperm count below 10 million/ml. Hum Genet 66:162–164.
- Ruhayel, Y., Giwercman, A., Ulmert, D., Rylander, L., Bjartell, A., Manjer, J., (2010) Male infertility and prostate cancer risk: a nested case-control study. Cancer Causes Control. 2010 Oct;21(10):1635–43.
- Salonia, A., Matloob, R., Gallina, A., Abdollah, F., Sacca, A., Briganti, A., (2009) Are Infertile Men Less Healthy than Fertile Men? Results of a Prospective Case-Control Survey. Eur Urol. 56(6):1025–1031
- Sanderson, M.L., Hassold, T.J., Carrell, D.T. (2008) Proteins involved in meiotic recombination: a role in male infertility? Syst Biol Reprod Med 54:57–74.
- Sato, A., Hiura, H., Okae, H., Miyauchi, N., Abe, Y., Utsunomiya, T., (2011) Assessing loss of imprint methylation in sperm from subfertile men using novel methylation polymerase chain reaction Luminex analysis. Fertil Steril 95(1):129–34.
- Schultz, N., Hamra, F.K., Garbers, D.L. (2003) A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA 100:12201–12206.
- Sharpe, R.M., Skakkebaek, N.E. (1993) Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341:1392–1395.
- Skakkebaek, N.E., Rajpert-De Meyts, E., Main, K.M. (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978.
- Skaletsky, H., Kuroda-Kawaguchi, T., Minx, P.J., Cordum, H.S., Hillier, L., Brown, L.G., (2003) The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 423:825–837.
- Stankiewicz, P., Lupski, J.R. (2010) Structural variation in the human genome and its role in disease. Annu Rev Med 61:437–455.
- Stefansson, H., Rujescu, D., Cichon, S., Pietilainen, O.P., Ingason, A., Steinberg, S., (2008) Large recurrent microdeletions associated with schizophrenia. Nature 455:232–236.
- Tamowski, S., Aston, K.I., Carrell, D.T. (2010) The use of transgenic mouse models in the study of male infertility. Syst Biol Reprod Med 56:260–273.
- Tempest, H.G. (2011) Meiotic recombination errors, the origin of sperm aneuploidy and clinical recommendations. Syst Biol Reprod Med 57:93–101.
- Terribas, E., Bonache, S., Garcia-Arevalo, M., Sanchez, J., Franco, E., Bassas, L., (2010) Changes in the expression profile of the meiosis-involved mismatch repair genes in impaired human spermatogenesis. J Androl 31:346–357.
- Thompson, S.L., Bakhoum, S.F., Compton, D.A. (2010) Mechanisms of chromosomal instability. Curr Biol 20:R285–295.
- Tiepolo, L., Zuffardi, O. (1976) Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet 34:119–124.
- Tomasini, R., Tsuchihara, K., Wilhelm, M., Fujitani, M., Rufini, A., Cheung, C.C., (2008) TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev 22:2677–2691.
- Tuttelmann, F., Rajpert-De Meyts, E., Nieschlag, E., Simoni, M. (2007) Gene polymorphisms and male infertility–a meta-analysis and literature review. Reprod Biomed Online 15:643–658.
- Tuttelmann, F., Simoni, M., Kliesch, S., Ledig, S., Dworniczak, B., Wieacker, P., (2011) Copy number variants in patients with severe oligozoospermia and sertoli-cell-only syndrome. PLoS One 6:e19426.
- Veigl, M.L., Kasturi, L., Olechnowicz, J., Ma, A.H., Lutterbaugh, J.D., Periyasamy, S., (1998) Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci USA 95:8698–8702.
- Velasco, A., Corvalan, A., Wistuba, II, Riquelme, E., Chuaqui, R., Majerson, A., (2008) Mismatch repair expression in testicular cancer predicts recurrence and survival. Int J Cancer 122:1774–1777.
- Velasco, A., Riquelme, E., Schultz, M., Wistuba, II, Villarroel, L., Koh, M.S., (2004) Microsatellite instability and loss of heterozygosity have distinct prognostic value for testicular germ cell tumor recurrence. Cancer Biol Ther 3:1152–1158; discussion 1159–1161.
- Vincent, M.C., Daudin, M., De, M.P., Massat, G., Mieusset, R., Pontonnier, F., (2002) Cytogenetic investigations of infertile men with low sperm counts: a 25-year experience. J Androl 23:18–22; discussion 44–45.
- Visser, L., Westerveld, G.H., Korver, C.M., van Daalen, S.K., Hovingh, S.E., Rozen, S., (2009) Y chromosome gr/gr deletions are a risk factor for low semen quality. Hum Reprod 24:2667–2673.
- Vodicka, R., Vrtel, R., Dusek, L., Singh, A.R., Krizova, K., Svacinova, V., (2007) TSPY gene copy number as a potential new risk factor for male infertility. Reprod Biomed Online 14:579–587.
- Vogt, P.H. (2004) Genomic heterogeneity and instability of the AZF locus on the human Y chromosome. Mol Cell Endocrinol 224:1–9.
- Vogt, P.H., Edelmann, A., Kirsch, S., Henegariu, O., Hirschmann, P., Kiesewetter, F., (1996) Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet 5:933–943.
- Walsh, T., McClellan, J.M., McCarthy, S.E., Addington, A.M., Pierce, S.B., Cooper, G.M., (2008a) Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 320:539–543.
- Walsh, T.J., Croughan, M.S., Schembri, M., Smith, J.F., Chan, J.M., Turek, P.J. (2008b) Infertile men may have increased risk for non-germ cell cancers: data from 51,318 infertile couples. J Urol 179:654.
- Walsh, T.J., Pera, R.R., Turek, P.J. (2009) The genetics of male infertility. Semin Reprod Med 27:124–136.
- Walsh, T.J., Schembri, M., Turek, P.J., Chan, J.M., Carroll, P.R., Smith, J.F., (2010) Increased risk of high-grade prostate cancer among infertile men. Cancer 116:2140–2147.
- Wei, K., Clark, A.B., Wong, E., Kane, M.F., Mazur, D.J., Parris, T., (2003) Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev 17:603–614.
- Wohlfahrt-Veje, C., Main, K.M., Skakkebaek, N.E. (2009) Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 71:459–465.
- Wong, E.C., Ferguson, K.A., Chow, V., Ma, S. (2008) Sperm aneuploidy and meiotic sex chromosome configurations in an infertile XYY male. Hum Reprod 23:374–378.
- Writzl, K., Zorn, B., Peterlin, B. (2005) Copy number of DAZ genes in infertile men. Fertil Steril 84:1522–1525.
- Xu, K., Lu, T., Zhou, H., Bai, L., Xiang, Y. (2010) The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of male infertility with azoospermia or severe oligozoospermia. Clin Chim Acta 411:49–52.
- Yang, Y., Ma, M., Li, L., Su, D., Chen, P., Ma, Y., (2010) Differential effect of specific gr/gr deletion subtypes on spermatogenesis in the Chinese Han population. Int J Androl 33:745–754.
- Yatsenko, A.N., Iwamori, N., Iwamori, T., Matzuk, M.M. (2010) The power of mouse genetics to study spermatogenesis. J Androl 31:34–44.
- Yong, E.L., Loy, C.J., Sim, K.S. (2003) Androgen receptor gene and male infertility. Hum Reprod Update 9:1–7.
- Zhou, D., Xia, Y., Li, Y., Song, L., Hu, F., Lu, C., (2011) Higher proportion of haploid round spermatids and spermatogenic disomy rate in relation to idiopathic male infertility. Urology 77:77–82.