Abstract
The objective of the present study was to evaluate the effects of ghrelin on the concentrations of estrogen (E2) and progesterone (P4) in serum and the mRNA expression of estrogen receptor beta (ERβ) and progesterone receptor (PRA+B) in ovary in rats during estrous cycle. Adult female Sprague Dawley rats were intracerebroventricularly (i.c.v.) injected with 3 nmol ghrelin during the estrous cycle, and sacrificed 15 min later. Blood samples and ovaries were collected. The concentrations of serum E2 and P4 were measured by radioimmunoassay, while the amount of ERβ and PRA+B mRNA was assessed by real-time quantitative PCR. Our studies showed that ghrelin could significantly reduce the serum concentration of E2 throughout the estrous cycle (P < 0.05), the serum level of P4 (P < 0.05), and the amount of ERβ mRNA during metestrus (P < 0.05). Meanwhile, the amount of PRA+B mRNA was only reduced during diestrus (P < 0.05). Overall, our present findings provide the first evidence that i.c.v. injection of ghrelin could reduce the serum concentration of E2 and P4 and the level of ERβ and PRA+B mRNA expression, supporting the role of ghrelin in reproduction.
Keywords:
Introduction
Ghrelin, a 28-amino-acid peptide requires serine 3 n-octanoylation. It is a pleiotropic regulator involved in a wide array of endocrine and non-endocrine functions [Wren et al. Citation2000; Horvath et al. Citation2001]. Moreover, a growing number of studies suggest that ghrelin may play a role in the regulation of reproduction. For example, the concentration of estrogen (E2) and progesterone (P4) in cultured granulosal-luteal cells of women is significantly reduced by ghrelin in a dose-dependent manner [Viani et al. Citation2008]. The treatment of cultured granulosal cells of rabbits with ghrelin has recently been found to increase P4 secretion at 1 ng/mL and decrease it at 10 ng/mL [Sirotkin et al. Citation2009]. Nevertheless, it has been shown that 250 or 500 pg/mL doses of ghrelin stimulates E2 secretion of prepubertal porcine follicular cells in vitro [Rak and Gregoraszczuk Citation2008]. Increasing evidence indicates that ghrelin could also be involved in controlling gonadal function [Wang et al. 2011].
E2 and P4 play important roles in the regulation of female reproduction, and their actions are mediated through their receptors. It has been shown that most female reproductive organs express both ERα and ERβ [Mowa and Iwanaga Citation2000]. However, estrogen receptor subtypes differ in both concentration and ratio in various tissues [Jefferson et al. Citation2000; Igarashi et al. Citation2001]. It has been suggested that ERβ is the dominant estrogen receptor (ER) subtype in the ovary [Byers et al. Citation1997]. The granulosal and thecal cells of the preovulatory follicles express both progesterone receptor A and B (PRA+B) [Gava et al. Citation2004]. The relative levels of expression of these receptors may play a major role in the ovary. To gain further insight, the serum level of E2 and P4 and the expression of ERβ and PRA+B mRNA in the ovary were assessed in rat following i.c.v. injection of ghrelin.
Results
E2 and P4 concentration changes after i.c.v. injection of ghrelin
The highest E2 levels were observed proestrus. The i.c.v injection of ghrelin in cyclic rats induced a significant decrease in serum levels of E2 throughout the estrous cycle compared with the control (P < 0.05) ().
Table 1. Serum level of E2 (mean ± SD) after i.c.v. injection of ghrelin in rats during the estrus cycle.
The results in show that the level of P4 was the highest in diestrus but lowest in estrus. The serum levels of P4 were inhibited during metestrus compared with the control (P < 0.05) following ghrelin i.c.v. injection to cycling rats.
Table 2. Serum level of P4 (mean ± SD) after i.c.v. injection of ghrelin in rats during the estrus cycle.
ERβ mRNA analysis in ovary
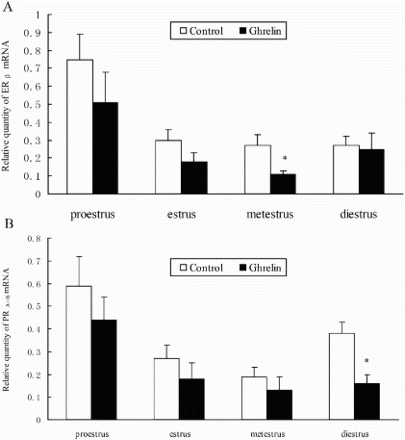
The results showed that the level of ERβ mRNA was highest proestrus. In comparison, the ERβ mRNA in ghrelin injected rats, was significantly inhibited during metestrus (P < 0.05). As summarized in A, no significant change was observed in ERβ mRNA expression during the other stages of the estrous cycle (P > 0.05).
Figure 1. A) Levels of ERβ mRNA in the ovary after i.c.v. injection of ghrelin during the estrus cycle. The RNA levels during proestrus, estrus, metestrus, and diestrus are compared to the saline control following ghrelin administration. B) Expression of PRA+B mRNA in the ovary after i.c.v. injection of ghrelin in rats during the estrus cycle. *indicates significant difference between control and ghrelin injected group (P < 0.05)
PRA+B mRNA level in ovary
The PCR primers used in this study along with the corresponding products are described in . The amount of PRA+B mRNA was highest in proestrus, and lowest in metestrus (B) in both the control and ghrelin groups. The level of PRA+B mRNA was significantly reduced during diestrus compared with the control (P < 0.05) following i.c.v. injection of ghrelin. However, there was no difference in PRA+B mRNA expression at other stages (P > 0.05) (A).
Table 3. Real-time PCR primer and size of the amplification products of target genes (ERβ,PRA+B) and housekeeping (β- actin).
Discussion
Emerging evidence suggests that ghrelin can regulate E2 and P4 secretion in vitro. The data presented in this communication demonstrated that ghrelin could induce differential inhibition of E2 and P4 secretion as well as ERβ and PRA+B mRNA expression. I.c.v. injection of ghrelin, inhibited the release of E2 throughout the estrous cycle. As such, the level of E2 detected in serum decreased. This may reflect the action of luteinizing hormone (LH), which could stimulate the ovaries to produce E2. It has been observed that ghrelin can inhibit the release of LH at all stages of the estrous cycle [Fernández-Fernández et al. Citation2005]. Recently, Rak and Gregoraszczuk [2008] showed that ghrelin directly inhibits aromatase activity thereby decreasing E2 secretion. However, Messini et al. [2009] showed that the serum concentration of E2 remained constant in women with a normal menstrual cycle who were subject to i.v. injection of ghrelin (1 mg/kg). This discrepancy may result from the delivery methods and different dosages of ghrelin.
Tropea et al. [2007] have shown that 10−13-10−7M ghrelin could significantly inhibit basal and human chorionic gonadotropin-stimulated progesterone release in women. As shown here, i.c.v injection of ghrelin evoked a significant decrease of P4 during metestrus. This is likely due to the increase of GHS-R1a in the hypothalamus during metestrus [Fernández-Fernández et al. Citation2005]. In addition, ghrelin could inhibit P4 secretion through mitogen-activated protein kinase (MAPK) and protein kinase A (PKA)-dependent intracellular mechanisms, at the post-receptor level [Sirotkin and Grossmann Citation2007; Citation2008]. In contrast, the concentration of serum P4 was not altered in women with a normal menstrual cycle who received 1 mg/kg ghrelin [Messini et al. Citation2009]. Results obtained from rats and women support the view that ghrelin may regulated P4 secretion in a species or delivery specific manner. If correct this can be only one of the mechanisms regualting the secretion of P4 since it is also influenced by platelet-activating factor, epidermal growth factor, vascular endothelial growth factor, prostaglandin, and their interactions [Rabinovici and Angle Citation1991; Liu et al. Citation1998; Dickson et al. Citation2001; Arosh et al. Citation2004].
The results of the present study show that ERβ mRNA was present during the estrous cycle with the level being maximum during proestrus and then decreasing during estrus. This is similar to what has been observed by others [Byers et al. Citation1997; Misao et al. Citation1999]. However, the opposite pattern of ER expression has been reported for the rhesus monkey [Duffy et al. Citation2000]. I.c.v. injection of ghrelin evoked a significant decrease of ERβ mRNA during metestrus. This may reflect the action of follicle stimulating hormone (FSH) since the level of ERβ mRNA decreases significantly in FSH-antiserum-treated ovaries [Yang et al. Citation2004]. However, FSH secretion was inhibited by ghrelin during metestrus [Fernandez-Fernandez et al. 2005]. This suggests that ghrelin plays a physiological role in ERβ mRNA expression in rats during the estrous cycle. However, ERβ mRNA expression is also subjected to a range of other factors, including histone deacetylase inhibitors, and estrogen [Read et al. Citation1989; Duong et al. Citation2006].
Although the PRA+B protein has been observed in the ovary [Gava et al. Citation2004], information regarding the control of gene expression at the mRNA level is still lacking. As shown above, the amount of PRA+B mRNA decreased in proestrus and increased in diestrus, the patterns of which were similar to the PRA+B mRNA expression in the anterior pituitary [Szabo et al. Citation2000]. Others have shown that the PRA+B mRNA level was differentially regulated in follicles and corpus luteum during the estrous cycle [Berisha et al. Citation2002]. This discrepancy suggests that PRA+B mRNA expression was regulated in a tissue-specific manner. However, PRA+B mRNA expression in the anterior pituitary and ovary showed approximately the same changes. The reasons for this apparent dichotomy are unclear. The amount of PRA+B mRNA was significantly inhibited during diestrus when the rats were treated with ghrelin. It may be associated with an increased plasma P4 level in diestrus, because PRA+B mRNA expression was down-regulated by P4 [Kraus and Katzenellenbogen Citation1993]. During metestrus, P4 secretion was significantly inhibited by ghrelin. While the administration of ghrelin by i.c.v has suggested a possible role in the control of the gonadal hormone secretion and the expression in ERβ mRNA and PRA+B mRNA, the mechanisms of such actions remain obscure. Further research is needed to identify the precise role of ghrelin.
Materials and Methods
Animals and experimental design
Sprague Dawley rats (180-240g) were purchased from the Experimental Animal Center of Anhui Medical University. The study was approved by the Animal Care and Use Committee of Anhui Agricultural University. The rats were kept (6 per cage) under controlled conditions of light (12 h light, 12 h darkness, light at 07:00 h) and temperature (22oC), with free access to pelleted food and tap water, and relative humidity of 50%-60%. Vaginal smears were examined daily for identifying the stage of estrus cycle. Only those animals exhibiting at least two consecutive 4-day estrous cycles were used. Animals were divided into four groups (proestrus, estrus, metestrus, and diestrus) by vaginal cell morphology [Zhang et al. Citation2006], each experimental group and control group consisted of eight animals. Ghrelin was obtained from Sigma (USA) and dissolved in saline solution immediately before use. The experimental group of rats were intracerebroventricularly (i.c.v.) injected with 2 µL ghrelin solution (2 nmol ghrelin), while the control animals received 2 µL of saline. The ghrelin or saline injection time was either in the afternoon of proestrus (17:30 h), the morning of estrus (07: 30 h), metestrus (10:00 h), or diestrus (9:00 h ) [Fernández-Fernández et al. Citation2005]. All animals were decapitated 15 min after injection with ghrelin (plasma ghrelin levels rapidly decreased with a half-life of 11 min). Approximately 5mL of blood samples were collected into centrifuge tubes without heparin and centrifuged (1,600 g at 4oC for 20 min) immediately, serum was collected and stored at −20oC until use. In parallel, the ovaries were rapidly dissected out and then stored at −80oC until analysis.
E2 and P4 determination
The concentrations of E2 and P4 in serum and culture media were measured using a commercial RIA system according to manufacturer's instructions (Furui Bioengineering Corporation, Beijing, China). The intra- and inter-assay coefficients of variation were less than 7.3% and 10.3% for E2 and 8.8% and 12.1% for P4, respectively. The sensitivity was 0.5 pg/mL for E2 and 0.002 ng/mL for P4. All samples were assayed in duplicate.
Total RNA isolation and reverse transcription
Total RNA was extracted from the ovary, granulosa cells, and luteal cells using a Trizol reagent (TransGen, Beijing, China) according to the manufacturer's instructions. The quality of total RNA was assessed by formaldehyde gel electrophoresis. Samples that showed good RNA quality (i.e., low degradation and presence of 2 ribosomal peaks) were selected for further reverse transcription. Reverse transcription was performed by TransScript First-Strand cDNA Synthesis SuperMix (TransGen, Beijing, China) according to the manufacturer's instructions. A total of 4 μg of total RNA in 30 μL was used for reverse transcription.
Real-time PCR
The ERβ and PRA+B target genes, and β-actin as the housekeeping internal control gene primers are listed in . Real-time PCR was performed using SYBR Premix Ex TaqTM Kits (TaKaRa, Dalian, China). Each reaction well in a 96-well plate contains 25 pmol/μL forward and reverse primers, 1× Hotstart Fluo-PCR mix and 1 µL cDNA. The final reaction volume was 30 µL and all samples were run in triplicate. The following PCR conditions were used: preliminary denaturation at 95°C for 30 s, followed by 40 cycles with temperature profiles of 5 s at 95°C, 20 s at 55°C, and 15 s at 72°C. Melting curves were determined. Quantitative analysis was performed using a relative standard curve method. The data were analyzed and shown as a ratio of analyzed gene expression to β-actin.
Statistical analyses
Results presented as mean ± SD. Differences in the concentrations of E2 and P4, the ERβ and PRA+B relative mRNAs were determined by one-way repeated-measures ANOVA, where P < 0.05 was considered statistically significant.
Declaration of Interest: Supported by Natural Science Foundation of Anhui Education Department (Grant KJ2011A117) (Anhui, P. R. China). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Abbreviations
| GHS-R1a | = | growth hormone secretagogue receptors |
| i.c.v | = | intracerebroventricularly |
| GHS-R | = | growth hormone secretagogue receptor |
| LH | = | luteinizing hormone |
| FSH | = | follicle-stimulating hormone |
| E2 | = | estrogen |
| P4 | = | progesterone |
| ER | = | estrogen receptor |
| ERβ | = | estrogen receptor beta |
| MAPK | = | mitogen-activated protein kinase |
| PKA | = | protein kinase A |
| PRA+B | = | progesterone receptor A and B. |
References
- Arosh, J.A., Banu, S.K., Chapdelaine, P., Madore, E., Sirois, J. and Fortier, M.A. (2004) Prostaglandin biosynthesis, transport and signaling in corpus luteum: a basis for autoregulation of luteal function. Endocrinology 145:2551–2560.
- Berisha, B., Pfaffl, M.W. and Schams, D. (2002) Expression of estrogen and progesterone receptors in the bovine ovary during estrous cycle. Endocrine 17:207–214.
- Byers, M., Kuiper, G.G, Gustafsson, J.A. and Park-Sarge, O.K. (1997) Estrogen Receptor-ß mRNA Expression in Rat Ovary: Down-Regulation by Gonadotropins. Mol Endocrinol 11:172–182.
- Dickson, S.E., Bicknell, R. and Fraser, H.M. (2001) Mid-luteal angiogenesis and function in the primate is dependent on vascular endothelial growth factor. J Endocrinol 168:409–416.
- Duffy, D.M., Chaffin, C.L. and Stouffer, R.L. (2000) Expression of estrogen receptor alpha and beta in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology 141:1711–1717.
- Duong, V., Licznar, A., Margueron, R., Boulle, N., Busson, M., Lacroix, M., (2006) ERalpha and ERbeta expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene 25:1799–1806.
- Fernández-Fernández, R., Tena-semepere, M., Navarro, V.M., Barreiro, M.L., Castellano, J.M., Aguilar, E. (2005) Effects of Ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats; in vivo and in vitro studies. Neuroendocrinology 82:245–255.
- Gava, N., Clarke, C.L., Byth, K., Arnett-Mansfield, R.L. and deFazio, A. (2004) Expression of Progesterone Receptors A and B in the Mouse Ovary during the Estrous Cycle. Endocrinology 7:3487–3494.
- Horvath, T.L., Diano, S., Sotonyi, P., Heiman, M. and Tschöp, M. (2001) Ghrelin and the regulation of energy balance-A hypothalamic perspective. Endocrinology 142:4163–4169.
- Igarashi, H., Kouro, T., Yokota, T., Comp, P.C. and Kincade, P.W. (2001) Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proceedings of the National Academy of Sciences 98:15131–15136.
- Jefferson, W.N., Couse, J.F., Banks, E.P., Korach, K.S. and Newbold, R.R. (2000) Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod 62:310–317.
- Kraus, W.L. and Katzenellenbogen, B.S. (1993) Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology 132:2371–2379.
- Liu, J.H., Zhang, G.H., Peng, R.X., Wang, Q.F. and Cheng, Z.P. (1998) Effect of EGF on hCG-induced progesterone production of luteal cells in vitro. Basic & Clinical Medicine 18: 27–32 (In Chinese).
- Messini, C.I., Dafopoulos, K., Chalvatzas, N., Georgoulias, P. and Messinis, I.E. (2009) Effect of ghrelin on gonadotrophin secretion in women during the menstrual cycle. Hum Reprod 24:976–981.
- Misao, R., Nakanishi, Y., Sun, W.S., Fujimoto, J., Iwagaki, S., Hirose, R., (1999) Expression of estrogen receptor alpha and beta mRNA in corpus luteum of human subjects. Mol Hum Reprod 5:17–21.
- Mowa, C.N. and Iwanaga, T. (2000) Differential distribution of oestrogen receptor- and - mRNAs in the female reproductive organ of rats as revealed by in situ hybridization. J Endocrinol 165:59–66.
- Rabinovici, J. and Angle, M.J. (1991) Platelet-activating factor induces progesterone secretion and changes in morphological appearance in luteinizing granulosa cells in vitro. Fertil Steril 55:1106–1111.
- Rak, A. and Gregoraszczuk, E.L. (2008) Modulatory effects of Ghrelin in prepubertal porcine ovarian follicles. J Physiol Pharmacol 59:781–793.
- Read, L.D., Greene, G.L. and Katzenellenbogen, B.S. (1989) Regulation of estrogen receptor messenger ribonucleic acid and protein levels in human breast cancer cell lines by sex steroid hormones, their antagonists, and growth factors. Mol Endocrinol 3:295–304.
- Sirotkin, A.V. and Grossmann, R. (2007) The role of Ghrelin and some intracellular mechanisms in controlling the secretory activity of chicken ovarian cells. Comp Biochem Physiol A Mol Integr Physiol 147:239–246.
- Sirotkin, A.V. and Grossmann, R. (2008) Effects of Ghrelin and its analogues on chicken ovarian granulosa cells. Domestic Animal Endocrinology 34:125–134.
- Sirotkin, A.V., Rafav, J., Kotwica, J., Darlak, K. and Valenzuela, F. (2009) Role of Ghrelin in regulating rabbit ovarian function and the response to LH and IGF-I. Domest Anim Endocrinol 36:162–172.
- Szabo, M., Kilen, S.M., Nho, S.J. and Schwartz, N.B. (2000) Progesterone receptor A and B messenger ribonucleic acid levels in the anterior pituitary of rats are regulated by estrogen. Biol Reprod 62:95–102.
- Tropea, F., Tiberi, F., Minici, F., Orlando, M., Gangale, M.F., Romani, F., (2007) Ghrelin affects the release of lutolytic and luteotropic factors in human luteal cells. J Clin Endocrinol Metab 92:3239–3245.
- Viani, I., Vottero, A., Tassi, F., Cremonini, G., Sartori, C., Bernasconi, S., (2008) Ghrelin inhibits steroid biosynthesis by cultured granulosa–lutein cells. J Clin Endocrinol Metab 93:1476–1481.
- Wang, L., Fang, F., Li, Y., Zhang, Y., Pu, Y. and Zhang, X. (2011) Role of ghrelin on testosterone secretion and the mRNA expression of androgen receptors in adult rat testis. Syst Biol Reprod Med 57:119–123
- Wren, A.M., Small, C.J., Ward, H.L., Murphy, K.G.., Dakin, C.L., Taheri, S., (2000) The novel hypothalamic peptide Ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328.
- Yang, P., Wang, J., Shen, Y. and Roy, S.K. (2004) Developmental expression of estrogen receptor (ER) α and ERß in the hamster ovary: regulation by follicle-stimulating hormone. Endocrinology 145:5757–5766.
- Zhang, S., Wang, X, Wei S.B. and Tan J.H. (2006) Vaginal cell morphology of rat during estrus cycle. Veterinary Medicine 27:69–72 (In Chinese).