Abstract
Male carriers with balanced reciprocal translocations can produce a variable proportion of unbalanced gametes resulting in reproductive failures. The presence of a structural rearrangement may induce an interchromosomal effect. This is characterized by abnormal bivalents not involved in the reorganization thereby yielding non-disjunction, which would present as aneuploid spermatozoa for these chromosomes. In the present case report segregation analysis of the sperm and investigation of interchromosomal effect were carried out using cytogenetic and fluorescence in situ hybridization (FISH) analysis on blood lymphocytes. The karyotype of the patient was 46,XY,t(3;6)(q21;q23). During sperm segregation analysis a total of 2,002 sperms were evaluated, of which 46.8% showed normal/balanced (alternate segregation mode) and 53.2% of sperm showed an abnormal signal pattern. A significant difference in the frequency of the estimated number of chromosome anomalies was observed in the translocation carrier when compared to the normozoospermic group (P < 0.0001) and the oligozoospermic group (P < 0.0001). Meiotic segregation analysis of sperm together with aneuploidy assessment for X, Y, and 17 chromosomes using FISH allows for the determination of a reproductive prognosis in male balanced translocation carriers and can be used for appropriate genetic counseling.
Introduction
Balanced reciprocal translocations, the exchange of chromosomal regions between two heterologous chromosomes, are the most common cytogenetic aberrations found in 1 of every 1,175 newborns [De Braekeleer and Dao Citation1991]. Although the carriers with these balanced translocations do not show any clinical symptoms, they can generate a variable ratio of unbalanced gametes causing abnormal genetic material in the offspring. This may manifest as infertility, habitual spontaneous abortions, or congenital malformations. The frequencies of unbalanced spermatozoa of man carrying a balanced reciprocal translocation vary from 19% to 80% [Morel et al. Citation2004].
During meiosis I in male carriers of a balanced reciprocal translocation, a quadrivalent is formed between the translocated chromosomes and their normal homologues in pachytene spermatocytes, leading to five modes of segregation. The alternate segregation mode is the only one that enables the production of chromosomally normal or balanced gametes. The other four segregation modes (adjacent I, adjacent II, 3:1, and 4:0) produce chromosomally unbalanced gametes [Morel et al. Citation2004].
The study of meiotic segregation was made possible in the late 1980s, owing to the heterospecific fecundation of golden hamster oocytes with human spermatozoa. Since 1990, fluorescence in situ hybridization (FISH) has been introduced to study the chromosomal content of spermatozoa [Morel et al. Citation2004]. The presence of a structural rearrangement may induce an interchromosomal effect (ICE), characterized by abnormal partition of bivalents not involved in the reciprocal translocation resulting in non-disjunction and aneuploid spermatozoa [Lejuene Citation1963].
To our knowledge, the case reported here is the first segregation study of the specific translocation t(3;6) carrier male presenting with infertility. The sperm sample was analyzed by FISH to evaluate the segregation patterns of chromosomes 3 and 6. The possible interchromosomal effects of the balanced translocation was studied by examining the aneuploidy frequencies of chromosomes X, Y, and 17 of which the latter is frequently observed in early abortions causing infertility.
Results
Cytogenetic and FISH analysis
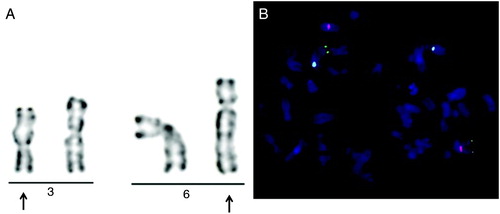
The cytogenetic analysis revealed a balanced translocation between the long arms of chromosomes 3 and 6. The karyotype of the patient was 46,XY,t(3;6)(q21;q23). The translocation was confirmed by FISH (). Metaphase FISH analysis from cultured blood lymphocytes using locus specific probes for 3qter and centomer region specific probes for chromosomes 3 and 6 confirmed the reciprocal translocation between chromosome 3 and chromosome 6. The parents of the patient were not investigated as the father of the patient was deceased and the mother of the patient declined testing. The karyotype of the patient's wife proved to be normal.
Figure 1. Cytogenetic and FISH results of the carrier with balanced reciprocal translocation. A) Partial GTG-banding karyogram showing the t(3;6)(q21;q23) rearrangement. Arrows indicate the abnormal chromosomes. B) FISH analysis on methaphase chromosomes with three color DNA probes for centromeric region of chromosome 3 (CEP3) (red), chromosome 6 (CEP6) (blue), and 3qtel region (green). One green signal (3qter) and one blue signal (CEP6) on the same chromosome confirm the translocation between chromosome 3 and 6.
Segregation analysis of the sperm
The result of segregation analysis of the patient is summerized in the . The overall hybridization efficiency was 99.5%. A total of 2,002 sperms were evaluated, 46.8% showed normal/balanced (alternate) segregation mode while 53.2% of the sperm showed an abnormal signal pattern caused by the unbalanced segregation. Alternate segregation was the most common form of segregation seen in the patient, followed by adjacent I, adjacent II and 3:1 segregations. Adjacent I segregation was observed in 26.07% of the spermatozoa, adjacent II in 13.63%, and 3:1 in 8.49% of sperm. The accessory products were produced in 1.5:1 ratio for adjacent I segregation, and near in 1:1 ratio for adjacent II segregation, with the exception of a higher frequency of cells with red-red-green (RRG) (P < 0.0001) or green-blue-blue (GBB) (P < 0.0001) signal patterns compared to any of the other patterns in this type of segregation. The RRG and GBB phenotypes arose when interstitial recombination does not occur [Vozdova et al. Citation2008]. There were significant differences among the proportions of most accessory products in 3:1 segregation, generally with hypohaploid sperm (R, GB, B, and RG) being more frequent than hyperhaploid (RGGBB, RRGB, RRGGB, and RGBB) (P < 0.0001). Diploidy and 4:0 segregation mode occurred in 0.45% of sperm, however, their individual frequencies could not be distinguished due to the similar signal patterns of the FISH probes. Other signal patterns were observed in 4.54% of sperm heads.
Table 1. Results of meiotic segregation analysis.
Interchromosomal effect
A significant difference in the prevalence of the estimated numerical chromosome anomalies was seen between the translocation carrier and the normozoospermic group (P < 0.0001), and between the translocation carrier and the oligozoospermic group (P < 0.0001). The ICE numerical chromosome anomalies was estimated according to Downie et al. [1997] and Egozcue et al. [1997]. The results are summarized in . The existence of ICE with chromosomes X, Y, and 17 was tested by comparison of the FISH signal patterns in the translocation carrier with results in a control group of five young healthy normospermic men and with results in a group of five oligozoospermic men. The X/Y ratio in the patient and in the two other control groups were close to 1:1. We have found a significantly higher frequency of sex chromosome disomy in the translocation carrier compared to the normozoospermic (P < 0.05) and the oligozoospermic group (P = 0.05). Although higher frequency of disomy 17 was observed in the translocation carrier compared to the normozoospermic controls and the oligozoospermic men, these differences were not significant (P = 0.11 and P = 0.30). The total diploidy frequency of the translocation carrier was significantly higher compared to the two control groups (P < 0.0001, normozoospermic controls; P = 0.006, oligozoospermic men).
Table 2. Frequencies of 17 and sex chromosomes disomy, diploidy and estimated chromosome anomalies detected by FISH with probes for chromosomes X, Y, and 17 in the patient and mean values in controls and in oligozoospermic men.
Discussion
Men carrying a balanced reciprocal translocation frequently generate unbalanced gametes a consequence of the segregation of translocated chromosomes. The frequencies of unbalanced spermatozoa in men carrying a balanced reciprocal translocation vary from 19% to 80% [Morel et al. Citation2004]. These variations confirm that the risk of meiotic imbalance varies according to the feature of the chromosomes involved in the realignments, as well as the size of the translocated regions, centromere, and breakpoint positions. These frequencies are higher than the 10% chromosomally unbalanced children or the 11.5 % chromosomally abnormal fetuses discovered during prenatal diagnosis in couples in whom a partner carries a balanced reciprocal translocation. This observation supports the concept that implantation is inhibited or the unbalanced conceptus is eliminated very early during embryonic development because of significant post-zygotic selection [Boué and Gallano Citation1984; Morel et al. Citation2004].
To our knowledge this is the first report of sperm FISH from an infertile carrier with the balanced reciprocal translocation t(3;6)(q21;q23). Our results showed a prevalence of alternate segregation (46.8%) followed by adjacent I (26.07%), adjacent II (13.63%), 3:1 (8.49%), and other segregants (4.99%) in the patient studied. Our data are in accordance with the results of others where the translocation affects chromosome 3 or 6, but different breakpoints. On one hand, similar meiotic segregation profiles have been observed in sperm of translocation carriers [Rousseaux et al. Citation1995; Martini et al. Citation1998; Lim et al. Citation2003; Perrin et al. Citation2010]. On the other hand, different segregation profiles with some differences have also been observed [Honda et al. Citation1999; Escudero et al. Citation2003].
Investigation of the segregation in carriers with structural chromosome abnormalities and sperm FISH studies provide evidence in support of the existence of aneuploidies for chromosomes not contained in the rearrangement. The interchromosomal effect has been presumed to be a possible attendant of meiotic deffects caused by the rearrangements [Lejuene Citation1963]. Meiotic errors, influencing synapsis through prophase I or meiotic recombination [Egozcue et al. Citation2000], can be considered as hotspots for the production of chromosomally unbalanced spermatozoa. While this phenomenon affecting the chromosomes 3 or 6 has been suggested [Rousseaux et al. Citation1995; Blanco et al. Citation2000; Oliver-Bonet et al. Citation2002], others report no evidence of this mechanism [Estop et al. Citation2000]. Independent of the origin of disomy and diploidy in sperm, carriers of balanced translocations have a greater chance of generating aneuploid gametes in general. Some authors provide evidence that the interchromosomal effects in balanced translocations largely contribute to the formation of aneuploid embryos [Gianaroli et al. Citation2002].
In the present study, the frequencies of aneuploidies for chromosomes X, Y, and 17 were assessed in a translocation carrier. We also examined chromosome 17 aneuploidy frequencies, because it was frequently observed in early abortions causing infertility. Aneuploidies of chromosomes 13, 18, and 21 may result in live births and not solely abortions. In the translocation carrier higher frequencies of disomies of sex chromosomes and chromosome 17 as well as of diploid sperm cells were observed when compared to normozoospermic control donors, and the group of oligozoospermic men. Rearrangement during meiotic segregation is evidenced by the abnormal chromosomal composition of spermatozoa. The high frequency of chromosome 17 disomy and diploidy could be considered as a consequence of an interchromosomal effect.
The analysis of spermatozoa from translocation carriers is a valuable tool in estimating individual risk of the occurence of unbalanced spermatozoal genome. Antenatal diagnosis has to be recommended to those couples in whom the male partner (as well as the female) carries a balanced translocation. Pre-implantation genetic diagnosis (PGD), a method allowing the identification of genetic abnormalities of embryos obtained by in vitro fertilization before in utero transfer, can also be discussed with these couples [Escudero et al. Citation2003]. The percentage of chromosomally abnormal spermatozoa estimated by FISH may have a predictive value for translocation and thus the outcome of PGD [Morel et al. Citation2004]. Meiotic segregation analysis of sperm together with aneuploidy study for X, Y, and 17 chromosomes using FISH allows the determination a reproductive prognosis in male balanced translocation carriers and should provide for appropriate genetic counseling.
Material and Methods
All protocols involving human subjects were approved by the Institutional Review Board for human subjects. Prior to the study, all patients were informed about the investigation and their consents were obtained.
Patient
The blood sample of a 30-year-old man and his 27-year-old wife was directed to the cytogenetic laboratory for constitutional karyotype analysis after four spontaneous abortions during four previous years. The gestational age of the abortions were less than 9 w. The first three abortions were completed as delayed menses. The last one was incomplete and needed curretage, the abortum was hystologically normal, karyotype analysis was not carried out. The couple were clinically healthy.
The patient showed sperm concentration of 81 million/ml with 60% motility. Semen specimen was collected after a requested abstinence of four d. Sperm analysis was carried out according to WHO guidelines and morphology was examined using strict criteria [WHO Citation2010].
Control groups
One control group consisted of five young and healthy normospermic men with proven fertility (age range 23 - 27). The sperm concentration of the normozoospermic control group was above 40 million/ml (range 41-160) with above 60% motility (range 60-95). The other control group consisted of five oligozoospermic men (age range 30 - 41) with normal karyotype. Semen concentration of the oligozoospermic men was under 15 million/ml (range 3 - 15) with above 60% motility (range 60-85). The patient and the two control groups had above 30% morphologically normal spermatozoa.
Cytogenetic and FISH analysis on blood lymphocytes
Peripheral blood lymphocytes were cultured with phytohaemagglutinin-M for 72 h and GTG-banded chromosome analysis was performed according to standard cytogenetic protocol. The result was described according to International System for Human Cytogenetic Nomenclature (ISCN) [Shaffer and Tommerup Citation2009]. FISH was performed on fixed cell suspension in Carnoy fixative using alfa-satellite DNA probes for chromosome 3 (CEP3, Red; Cytocell, Cambridge, United Kingdom) and for chromosome 6 (CEP6, Spectrum Aqua; Kreatech Diagnostics, Amsterdam, The Netherlands) and a subtelomeric DNA probe for the distal portion of the long arm of chromosome 3 (3qter, Spectrum Green; Cytocell) according to the manufacturer's protocols. At least 50 interphase nuclei and 30 metaphase cells were evaluated. Analysis was performed on a Zeiss Axioplan2 (Carl Zeiss, Jena, Germany) fluorescence microscope, the images were captured and analysed by ISIS software (Metasystems, Althussheim, Germany).
FISH analysis on sperm
Smears of semen samples (10 µL) were fixed with methanol: acetic acid (3:1) for 10 min, dehydrated in 70, 85, and 100 % ethanol for 2 min each, and stored at – 20 °C until FISH was performed. For decondensation, the slides with the sperm cells were treated with 10 mmol/L dithiothreitol (DTT; Sigma, St. Louis, Missouri, USA) in 0.1 mol/L Tris-HCl, pH 8.0, at room temperature for 30 min, then with 10 mmol/L lithium diidosalicylate (LIS; Sigma) in Tris-HCl for 1-3 h. After decondensation, the sperm slides were dehydrated in 70, 85, and 100 % ethanol. The meiotic segregation assay was performed using the same FISH probes as used on cell suspension from cultured blood lymphocytes. The interchromosomal effect was studied using three FISH probes: alpha-satellite sequence specific centromeric probes for chromosome 17 (D17Z1, SpectrumAqua), X (DXZ1, SpectrumOrange), and satellite III sequence specific centromeric probes for chromosome Y (DYZ1, SpectrumGreen) (Abbott/Vysis, Des Plains, Illinois, USA).
The slide and the probe codenaturation was carried out at 76°C for 3 min. The hybridization was carried out at 37°C in a moist chamber for 16- 18 h. Posthybridization washes were performed with 50% formamide/2X SSC at 42°C for 15 min. The slides were washed with 2X SSC at room temperature for 10 min and 2X SSC/0.1% NP-40 for 5 min. The nuclei were then stained with 4'- 6' diamidino-2 - phenylindole (DAPI) (Abbott/Vysis).
Scoring criteria
The meiotic segregation study from the patient semen sample was performed by scoring 2,000 sperm heads. The investigation of ICE was carried out by scoring 5,000 sperm heads. Nuclei which were overlapped or displayed no signal due to hybridization failure were eliminated from the scoring.
Statistical analysis
Statistical analysis was performed using chi-square analysis by the Statistica software package (StatSoft, Tulsa, OK, USA). A value of P < 0.05 was considered significant.
Declaration of interest: The authors report no declarations of interest.
Abbreviations
| FISH: | = | fluorescence in situ hybridization |
| ICE: | = | interchromosomal effect |
| PGD: | = | pre-implantation genetic diagnosis |
References
- Blanco, J., Egozcue, J. and Vidal, F. (2000) Interchromosomal effects for chromosome 21 in carriers of structural chromosome reorganizations determined by fluorescence in situ hybridization on sperm nuclei. Hum Genet 106(5):500–505.
- Boué, A. and Gallano, P. (1984) A collaborative study of the segregation of inherited chromosome structural rearrangements in 1356 prenatal diagnoses. Prenat Diagn 4:45–67.
- De Braekeleer, M. and Dao, T.N. (1991) Cytogenetic studies in human spontaneous abortions. In Assisted Human Reproductive Technology ed.Hafez, E.S.E. Hemispere Publication Company, New York City, New York, USA, pp.41–45.
- Downie, S.E.,Flaherty, S.P. and Matthews, C.D. (1997) Detection of chromosomes and estimation of aneuploidy in human spermatozoa using fluorescence in-situ hybridization. Mol Hum Reprod 3(7):585–598.
- Egozcue, J., Blanco, J. and Vidal F. (1997) Chromosome studies in human sperm nuclei using fluorescence in-situ hybridization (FISH). Hum Reprod Update 3(5):441–452.
- Egozcue, S., Blanco, J., Vendrell, J.M., García, F., Veiga, A., Aran, B., (2000) Human male infertility: chromosome anomalies, meiotic disorders, abnormal spermatozoa and recurrent abortion. Hum Reprod Update 6(1):93–105.
- Escudero, T., Abdelhadi, I., Sandalinas, M. and Munné, S. (2003) Predictive value of sperm fluorescence in situ hybridization analysis on the outcome of preimplantation genetic diagnosis for translocations. Fertil Steril 79 Suppl 3: 1528–1534.
- Estop, A.M., Cieply, K., Munne, S., Surti, U., Wakim, A. and Feingold, E. (2000) Is there an interchromosomal effect in reciprocal translocation carriers? Sperm FISH studies. Hum Genet 106(5):517–524.
- Gianaroli, L., Magli, M.C., Ferraretti, A.P., Munné, S., Balicchia, B., Escudero, T. (2002) Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod 17(12):3201–3207.
- Honda, H., Miharu, N., Ohashi, Y., Honda, N., Hara, T. and Ohama, K. (1999) Analysis of segregation and aneuploidy in two reciprocal translocation carriers, t(3;9)(q26.2;q32) and t(3;9)(p25;q32), by triple-color fluorescence in situ hybridization. Hum Genet 105(5):428–436.
- Lejuene, J. (1963) Autosomal disorders. Pediatrics 32:326–337.
- Lim, A.S., Lim, T.H., Kee, S.K., Chieng, R. and Tay, S.K. (2003) Sperm segregation patterns by fluorescence in situ hybridization studies of a 46,XY,t(2;6) heterozygote giving rise to a rare triploid product of conception with a 9,XXY,t(2;6)(p12;q24)der(6)t(2;6)(p12;q24)pat karyotype. Am J Med Genet A 117A(2):172–176.
- Martini, E., von Bergh, A.R., Coonen, E., de Die-Smulders, C.E., Hopman, A.H. and Ramaekers, F.C., (1998) Detection of structural abnormalities in spermatozoa of a translocation carrier t(3;11)(q27.3;q24.3) by triple FISH. Hum Genet 102(2):157–165.
- Morel, F., Douet-Guilbert, N., Le Bris, M.J., Herry, A., Amice, V., Amice, J., (2004) Meiotic segregation of translocations during male gametogenesis. Int J Androl 27 (4):200–212.
- Oliver-Bonet, M., Navarro, J., Carrera, M., Egozcue, J. and Benet, J. (2002) Aneuploid and unbalanced sperm in two translocation carriers: evaluation of the genetic risk. Mol Hum Reprod 8(10):958–963.
- Perrin, A., Morel, F., Douet-Guilbert, N., Le Bris, M.J., Amice, J., Amice, V., (2010) A study of meiotic segregation of chromosomes in spermatozoa of translocation carriers using fluorescent in situ hybridisation. Andrologia 42(1):27–34.
- Rousseaux, S., Chevret, E., Monteil, M., Cozzi, J., Pelletier, R., Devillard, F., (1995) Meiotic segregation in males heterozygote for reciprocal translocations: analysis of sperm nuclei by two and three colour fluorescence in situ hybridization. Cytogenet Cell Genet 71(2):126–130.
- Shaffer, L.G. and Tommerup, N. (2009) ISCN 2009: An International System for Human Cytogenetic Nomenclature. S. Karger, Basel, Switzerland.
- Vozdova, M., Oracova, E., Horinova, V. and Rubes, J. (2008) Sperm fluorescence in situ hybridization study of meiotic segregation and an interchromosomal effect in carriers of t(11;18). Hum Reprod 23(3):581–588.
- WHO (2010) WHO laboratory manual for the examination and processing of human semen. 5:271.