Abstract
This study was conducted as part of an epidemiological survey of 126 nonsmokers and 178 smokers, showing primary infertility residing around Kolkata region of Eastern India. Their lifestyle history including smoking habits along with semen and blood were collected. The study examined the association of cigarette smoking with the risk of infertility, by determining the semen quality, follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone levels, and androgen receptor (AR)-CAG repeat length in a group of smokers compared with a control group (non smokers). Based on conventional WHO criteria, lower sperm motility (P < 0.001) and increased sperm morphological defects (P < 0.0001) were associated with smoking habits. Binary logistic regression analysis for the effect of smoking status on sperm DNA integrity demonstrated significant positive correlation (p = 0.006). Serum FSH and LH levels were higher for smokers compared with non-smokers while the testosterone level decreased significantly with the increasing smoking habit. The mean length of CAG repeats in AR gene was significantly higher for smokers with low testosterone compared to non-smokers. The study suggested that smoking is associated with altered semen quality, endocrine hormonal status, and number of CAG repeats in the AR gene.
Introduction
Infertility is traditionally defined as the inability of a couple to conceive following one year of unprotected intercourse. It is not an uncommon problem; as the numbers of infertile couples are rising day by day. In those couples who seek evaluation from a reproductive specialist, more than 30% of males have defective sperm quality that significantly contributes to their infertility [Gaur et al. Citation2007]. There are several factors which contribute to the increase in the male factor infertility [Lo et al. Citation2005]. Among these, exposure to several environmental, occupational, and behavioral factors have been reported as risk factors for male infertility. Since cigarette smoke contains mutagens and carcinogens and smoking is very common all over the world, there have been increased concerns of the unfavorable effects of smoking on male reproductive function [Mukhopadhyay et al. Citation2010].
Semen analysis is frequently used to assess male infertility in clinical andrology. Human sperm quality is usually defined by standard WHO semen analysis parameters: count, motility, and morphology [WHO Citation1999]. Recently the relationship between DNA integrity and conventional semen parameters has been established. An increased percentage of DNA damage is associated with a rise in semen abnormality [Trisini et al. Citation2004; Varghese et al. Citation2009].
Environmental factors can affect the male reproductive system either directly, causing decreased or altered sperm production in the testis, and/or indirectly through the endocrine system causing hormonal imbalance. The levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone could be correlated with male infertility [Padron et al. Citation1989]. Androgen is an important steroid hormone responsible for normal spermatogenesis, decreasing the level of this hormone leads to impaired sperm production. All androgens act through a single intracellular androgen receptor (AR), which is encoded by a single-copy gene mapped on the long arm (Xq11-12) of the X-chromosome [Chang et al. Citation1988]. The AR gene has 8 exons, of which exon 1 harbors a CAG repeat motif coding for a polyglutamine stretch. Reduced androgenicity in 20% of infertile men is caused by the expansion of CAG tract [Yong et al. Citation1998].
The present study undertook an analysis of semen parameters such as sperm count, motility, morphology, DNA integrity, endocrine hormones, and CAG repeats in the AR gene among smoker and non-smoker infertile men to assess the association of cigarette smoking with infertility in this population of the eastern region of India.
Results and Discussion
The study population consisted of patients attending our infertility clinic for initial evaluation. Patients of age group 30-60 y were included in the study after a thorough investigation of their past medical history and habits. The body mass index (BMI) for all of the patients was calculated from the information obtained, nonsmokers and light smokers showed almost similar BMI (25.92 ± 4.77 and 25.68 ± 5.92, respectively); in comparison, the mean BMI among heavy smokers were significantly lower being 21.31 ± 4.22 kg/m2 (Table 1). The sperm count among the heavy smokers (50 x 106 ±20) and light smokers (76 x 106 ± 50) was significantly lower compared to non-smokers (130 x 106 ± 50, p = 0.001). Among the nonsmokers, most of the patients were normozoospermic (60.3%), other seminal phenotypes are also present: teratozoospermic (19%), asthenozoospermic (4%), and about 24.1% showed sperm DNA damage. Most of the light smokers presented with normozoospermic (50%), azoospermic (14.3%), and teratozoospermic (16.3%) phenotype, with 42.5% of the patients showing damaged sperm DNA integrity. Heavy smokers produced strikingly different parameters compared to the other two groups. About 85% of the patients showed a defect in seminal phenotype; patients were mostly azoospermic (22.5%), oligozoospermic (20%), and teratozoospermic (30%); about 37.9% presented head defects and 43% sperm DNA damage was prevalent among the heavy smokers ().
Table 2. Characteristics of semen, reproductive hormones for participants according to level of current smoking.
The frequency χ2-test shows significant association between smoking habit and seminal phenotype (χ2 = 59.462, p-value ≈ 0.000). In order to begin to understand the nature of association, logistic regression analysis was carried out as smoking habit and seminal phenotype are categorical variables, as follows: phenotype normozoospermic (0, Reference Event), asthenozoospermic (5), teratozoospermic (4), severe oligozoospermic (3), oligozoospermic (2), and finally azoospermic (1) (). A borderline effect of BMI upon seminal phenotype was noted (OR 0.87 95% CI (0.75-1.00), p = 0.05) in logit 1, with an indication for the effect of BMI upon decreasing phenotype which tends to shift from normo- to aesthenozoopermia. In logit 5 the BMI is significantly lower in the azoospermic group (p < 0.001). Logits 1, 2, 3, and 5 indicate a phenotypical changes from normozoospermia to azo/oligo/severe oligo/asthenozoospermia respectively with increasing smoking habits (p < 0.05) ().
Table 3A. Logistic regression analysis of the confounding effect of age and BMI on seminal phenotype and smoking.
Since the levels in both the smoking habit and motility categories can be ordered, we carried out ordinal logistic regression analysis. The frequency χ2-test shows significant association between the smoking habit and sperm motility (χ2 = 23.188, p ≈ 0.000) (). Further, the negative coefficient for smoking habit and odds ratio less than 1 indicates that higher smoking levels tend to be associated with lower motility levels.
The frequency χ2 test shows significant association between smoking habit and sperm morphology (χ2 = 25.90 p = 0.000, ). Since morphology has two categories, normal and abnormal, binary logistic regression analysis was used to study the nature of association. Further, a positive coefficient for smoking habit and odds ratio greater that 1 indicates that higher smoking levels tend to be associated with abnormal morphology. It was noted that among heavy smokers with abnormal morphology, the percentage having only a head defect is significantly greater than the percentage having only a tail defect (p = 0.037).
Table 3B. Logistic regression analysis of sperm motility, morphology, and DNA integrity with smoking habit.
A significant association was also observed between smoking habit and DNA integrity (χ2 = 9.704, p = 0.008). As DNA integrity has only two categories, normal DNA and damaged DNA (), a binary logistic regression analysis was pursued. Further, a positive coefficient for smoking habit and an odds ratio greater than 1 indicates that a higher level of smoking tends to be associated with sperm DNA damaged.
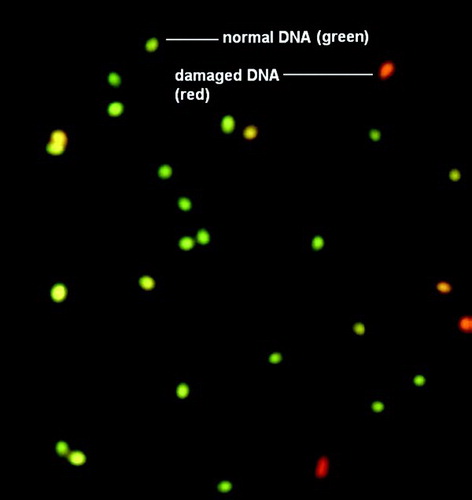
Figure 1. Assessment of human sperm DNA integrity by acridine orange staining (40X). DNA integrity was assessed using acridine orange staining following sperm fixation. The percentage of green (normal DNA integrity) and orange red (abnormal DNA integrity) spermatozoa per 200 spermatozoa in each sample was calculated. An abnormal integrity of sperm DNA was considered as more that 30% denaturation (orange-red).
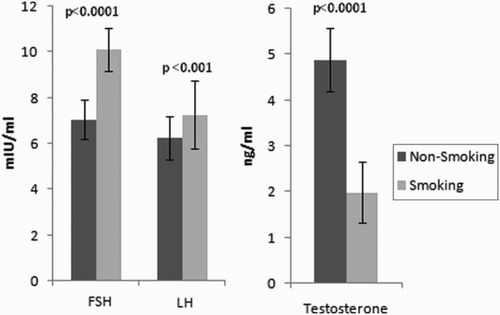
A significant difference in the FSH, LH, and testosterone levels was observed between non-smokers and heavy smokers (). Testosterone levels were found to be significantly lower among heavy smoking-severe oligo/azoospermic patients as compared to nonsmoking-normozoospermic controls (P < 0.0001, Student's paired t test), while FSH and LH levels were significantly higher among heavy smoking-severe oligo/azoospermic patients ().
Figure 2. FSH, LH, and testosterone levels among non-smokers and smokers. Follicle stimulating hormone (FSH), leutanising hormone (LH), and testosterone concentrations were determined in the serum of smokers (severeoligo/azoospermic) and nonsmokers (normozoospermic). Values are mean and SD from two independent experiments. mIU, mili International Unit
Among nonsmoking infertile men, four different CAG repeat sizes (alleles) ranging from 14 − 29 were observed (), and among heavy smoking-severe oligo/azoospermic study patients six different CAG repeat sizes were observed ranging from 16 − 33 (). The mean number of CAG repeats in the non-smoking group was 19.1 + 5.04. In the heavy smoking group the repeat size was significantly higher 26 + 5.8 (p = 0.03).
Figure 3. CAG repeat length in the AR genes of subjects. The CAG repeat segment of the AR gene was amplified by PCR with primers and conditions described in Materials and Methods. The representative products analyzed by agarose gel electrophoresis are shown. L1 and L2 represent repeat numbers 33 and 16, respectively. M, 500 base pair DNA size marker; AR: androgen receptor
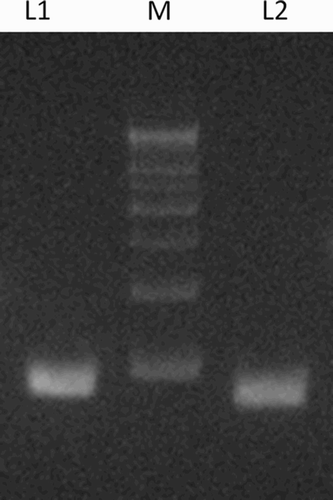
Table 4. Distribution of length of CAG repeats in the androgen receptor gene.
Despite worldwide anti-smoking campaigns, cigarette smoking is very common. The outcome of the present study should be analyzed in the context of the urban lifestyle of the city of Kolkata. However, the results reflect the changing semen quality of smokers attending an andrology laboratory with infertility related problems and should not be linked to the condition of the general fertile population. The subjects in the present study consisted of patients without extreme pathological conditions and had a sperm count > 20 x 106 /ml. The study presented above shows that 58.5% of the smokers were among the group of infertile men. Moreover, the present study includes semen parameters with different seminal phenotypes, motility, morphology, DNA integrity, and level of endocrine hormones such as FSH, LH, and testosterone as well as androgen receptor genes with varied sized CAG repeats. Cigarette smoke is a cell mutagen and carcinogen and certain components in cigarette smoke interact directly or indirectly with the male and female gametes affecting their function [Zenzes Citation2000]. Tobacco consumption involves about 4,000 compounds. The major components such as lead, cadmium, nicotine, and benzopyrene are known to affect semen parameters and sperm function [Zenzes Citation2000] as well as the endocrine status.
It is noted in this study that smoking is associated with decreased sperm concentration and motility (). The effects of tobacco smoking on reproductive function have been performed on a group of infertile patients. A large study conducted by Pasqualotto et al. [ 2006] showed a decline in semen volume with an increased number of cigarettes smoked, although no statistically significant differences were observed between the groups in terms of sperm motility, concentration, or morphology [Zenzes Citation2000]. Another study reported a non-significant change towards higher sperm concentration with more smoking [Oldereid et al. Citation1989], while others showed either a statistically significant dose response relation or a trend towards decreased sperm concentration with increased smoking [Zenzes Citation2000]. In heavy smokers, azoospermic / oligozoospermic / severe oligozoospermic / teratozoospermic /asthenozoospermic phenotypes were found in 85% of cases compared to 39% for non-smokers and 50% of smokers suggesting a trend towards deviation from normozoospermic phenotype with increased smoking. Earlier studies have concluded that toxins in cigarette smoke reach the male reproductive system and interact directly with components of the seminal fluid and the accessory endocrine system leading to increased viscosity, reduced seminal volume, and delayed liquefaction. This ultimately leads to the reduction of forward linear progression of spermatozoa, manifest as asthenozoospermia [Chia et al. Citation1994; Kulikauskas et al. Citation1985; Kunzle et al. Citation2003; Zhang et al. Citation2000]. However, in this population of infertile men, there is a trend towards increased oligozoospermic, teratozoospermic, and azoospermic phenotypes. The presence of the dominant asthenozoospermic phenotype has been observed in both smokers and non-smokers in the study conducted at Dehradun, India [Gaur et al. Citation2007].
Nicotine, present in the smoke, proved to be a potential oxidant which can affect spermatogenesis. Its damage to membrane structure of the spermatozoa is a major cause of the impaired motility [Tejada et al. Citation1984].The mammalian sperm and neuron appear to share many of the ‘neuronal’ receptors. Exocytosis in neurons and sperm is essential to the functions of these cells and is strongly influenced by similar receptors. ‘Neuronal’ receptor types in sperm may also play a role in the control of sperm motility [Arabi Citation2004]. It is highly likely that nicotine disrupts the activity of the nicotinic acetylcholine receptor of spermatozoa and thus weakens the intracellular Ca+2 dynamics resulting in impaired motility.
The study showed poor sperm morphology among the smokers. Cigarettes contain heavy metals, which cause high oxidative stress. Due to the oxidative stress, membrane damage can occur which can lead to morphological defects. Heavy metals also cause DNA damage during spermatogenesis which may result in abnormal development of sperm topography. It was noted that DNA damage is increased with habitual smoking. There have been previous studies highlighting the induction of DNA damage as well as the inhibition of DNA repair mechanisms by heavy metals [Hengstler et al. Citation2003; Meizel Citation2004]. A range of molecular mechanisms was proposed, reflective of their diverse chemical properties. Heavy metals present in cigarette smoke, like lead and cadmium causes an increase in the production of reactive oxygen species (ROS) such as hydroxyl radical (HO.), superoxide radical (O2.−), or hydrogen peroxide (H2O2). Enhanced generation of ROS can overwhelm the cells' intrinsic antioxidant defenses, and result in ‘oxidative stress’ [Meizel Citation2004]. Cells under oxidative stress can cause oxidative DNA damage, strand breaks, and induce mutations [Hengstler et al. Citation2003]. A decrease in repair capacity may increase susceptibility to reactive oxygen species generated by these heavy metals [Hengstler et al. Citation2003]. It has also been shown that repair of 8-hydroxy-2'-deoxyguanosine decreased with increasing cadmium exposure and was inversely correlated with the level of DNA-single strand breaks [Meizel Citation2004]. In this study DNA fragmentation was estimated by acridine orange (AO) staining. In the AO test, the fluorescent dye intercalates between the bases in intact double-stranded DNA producing green fluorescence (530 nm). However, when there is a strand break, the dye forms a weaker bond with the single strand along the back-bone giving red fluorescence (650 nm) (). Since an AO-test can be done much more easily than tests like SCSA, it can serve as a routine screening method to estimate the gross DNA damage in a sperm population [Ercal et al. Citation2001].
The present study showed higher FSH, LH, and lower testosterone levels with increased smoking. This is in contrast to earlier studies which reported higher testosterone and LH levels in addition to higher LH/ free testosterone ratios with increased smoking [Trummer et al. Citation2002], or a positive association between smoking and testosterone [Alexandersen et al. Citation1996]. Nicotine, one of the toxic components in cigarette smoke, had been reported to inhibit androgen biosynthesis and Leydig-cell growth [Bergmann et al. Citation1994; Funabashi et al. Citation2005] which might be responsible for the observed decline in testosterone among the heavy smokers. A high FSH level is usually diagnostic of primary testicular failure, a condition in which the seminiferous tubules in the testes do not produce sperm normally, because they are damaged.
Patients having mutations in AR gene exhibited elevated LH levels [Quigley Citation1998] which might be in response to the impaired feedback signal of testosterone in the pituitary. Leydig cell androgen secretion is mainly regulated via LH receptor mediated events and a receptor down regulation occurs in the presence of elevated LH levels. The mechanisms by which androgens regulate spermatogenesis are still elusive. In human testis, AR is expressed in Leydig as well as in peritubular and sertoli cells and provides sites for regulation of spermatogenesis and sperm outlet. The AR has a dual role in impaired spermatogenesis and reducing the levels of testosterone reflective of its CAG repeat length. The observed effect that CAG repeat length is positively correlated with smoking and inversely correlated with sperm concentration indicating that the variable polyglutamine tract size as deduced from the CAG repeat length in the AR gene, mediates androgen action with varying intensity. This modulation of AR androgenecity might be reflected by the serum testosterone level.
The above two studies [Davis-Dao et al. Citation2007; Mifsud et al. Citation2001] has shown a direct correlation of AR CAG length with free testosterone in fertile men. Although not observed in all populations [Dadze et al. Citation2000], depressed spermatogenesis has been associated with long CAG tracts in men from Singapore [Tut et al. Citation1997], Australia [Dowsing et al. Citation1999], Japan [Yoshida et al. Citation1999], and the USA [Mifsud et al. Citation2001; Patrizio et al. Citation2001]. A previous study by Thangaraj et al. [2002] shows no association of AR-CAG length with infertility when 280 azoospermic men were compared with 201 fertile men from this city. In the present study, we have included 20 infertile men out of which 10 were heavy smokers with low testosterone and 10 non-smokers with a normal level of testosterone and observed the AR-CAG length variation to assess the confounding effect of low testosterone and smoking. This study presents a significantly increased CAG repeat length among heavy smokers with low testosterone. Strikingly contradicting results exist in the literature regarding the association between testosterone and AR-CAG tract in subfertile patients [Mifsud et al. Citation2001]. However most of the studies have compared fertile and infertile men, and in this manner have tried to negate this bias and to observe the individual effect of smoking on CAG repeat length. Only infertile men were considered in the present study. Further studies on the effect of increased CAG repeat and smoking status with a larger sample size are required.
Materials and Methods
Sample population
A total of 304 men of Indian nationals around Kolkata city, who attended the out patient department of the Institute of Reproductive Medicine, Kolkata, India with a history of infertility for at least 1 year, and were able to provide an ejaculate were evaluated. Men meeting the following inclusion criteria were chosen: no vasectomy, no medical history of genital disease, and no abnormality in the reproductive organs. Subjects were excluded from the study if they had pathologies that aggravate erythrocytosis, cardiovascular, and renal disease. Full data was collected through a questionnaire including the number of cigarettes per day. For body mass index (BMI), height, and weight were obtained for each subject and BMI was calculated as kg/m2. Men who had smoked cigarettes for > 6 months and were still smoking were classified as smokers. Smokers were categorized as light (1−20 cigarettes per day) and heavy (> 20 cigarettes per day) smokers. The study was approved by the institutional ethical committee as per guidelines.
Semen analyses
Semen samples were collected by masturbation in a sterile wide-mouthed plastic container after a sexual abstinence of 4-5 d. The sample was allowed to liquefy at 37o C for 20 min before analysis. Sperm concentration and motility were evaluated using a Makler's counting chamber (Sefi Medical Instruments, Haifa, Israel). Total sperm count of a given semen sample was calculated by multiplying the seminal sperm concentration with the volume. Sperm morphology was assessed using Papanicolaou staining method.
Semen analysis was carried out by standard WHO procedure as described earlier and was classified according to the nomenclature of semen variables [WHO Citation1999]. Normozoospermia was diagnosed when sperm concentration, motility, and morphology were within the reference values. The reference values for sperm count is ≥ 20×106 sperm/ml, for motility 50% sperm with forward progression, for morphology ≥ 30% sperm with normal morphology, and for DNA integrity 50% sperm should have intact DNA. Oligozoospermia was determined when the sperm count was less than the reference value and severe-oligoozoospermia when sperm concentration was < 5×106/ml. Likewise, asthenozoospermia and teratozoospermia were diagnosed when motility and morphology were below the reference values respectively. Azoospermia was diagnosed when not a single spermatozoa was found in the ejaculate.
Sperm DNA integrity by acridine orange (AO) fluorescence
A smear was drawn on a clean slide using 20 µl of the semen sample. For assessment of sperm DNA integrity, the smears were air dried for 1 hr and then fixed overnight in freshly prepared Carnoy's solution (1:3 glacial acetic acid: methanol). The slides were washed in distilled water and allowed to dry before staining. Each sample was then stained in acridine orange (Sigma Chemical Co, St. Louis, USA) solution (10ml of 1% AO in distilled water added to a mixture of 40ml of 0.1 M citric acid and 2.5ml of 0.3 M Na2HPO4, 2H2O) for 5 min in dark. The preparations were gently washed in distilled water and examined on the same day using a Fluoroscent microscope (Leica DM 3000, Leica Microsystem Ltd., Switzerland) excitation at 450 to 490 nm. The percentage of green (normal DNA integrity) and orange red (abnormal DNA integrity) spermatozoa per 200 spermatozoa in each sample was calculated by the same single person. An abnormal integrity of sperm DNA was considered as more that 30% denaturation (orange-red).
Hormone assays
Blood (∼ 2 ml) was collected from the antecubital vein in vials without EDTA (clot vials) and serum isolated by centrifugation (1000 x rpm, 10 min). Follicle stimulating hormone (FSH), leutanising hormone (LH), and testosterone concentrations were determined in the serum using standard ELISA kits (Biorad Laboratories, USA) to evaluate the endocrine status of individuals involved in the study group consisting of heavy smoking-severeoligo/azoospermic patients while nonsmoking-normozoospermic group were kept as controls.
DNA isolation
Genomic DNA was extracted from peripheral blood of heavy-smoker (with low testosterone level) and non-smoker subjects using a standard procedure. Briefly, erythrocytes were lysed using erythrocyte lysis buffer (10 mM Tris pH 8.0, 320 mM sucrose, 5 mM MgCl2, 1% Triton X - 100) for 5 min. Leukocytes were pelleted by centrifugation at 500 x g for 5 min, and the pellet dissolved in leukocyte lysis buffer (400mM Tris, 60mM EDTA, 150 mM NaCl, 1% SDS). 5M sodium perchlorate (Sigma Aldrich, USA) was added and mixed thoroughly for 2-3min. DNA was extracted with chloroform and precipitated with isopropanol and finally washed with 70% ethanol and dissolved in TE buffer (10 mM Tris pH 8, 1 mM EDTA).
PCR assay for CAG repeat count
CAG repeat segment in exon 1 of androgen receptor (AR) gene was amplified with the primers: forward 5'AGAGCGTGCGGGAAGCGTTCCAGAACCCG3' and reverse 3'AACGTGGATGGGGCAGCTGAGTCAT5'. PCR amplification of each sample was performed using 5 ng of template DNA 10 pM of each primer, 2.5 mM deoxy Nucleotide triphosphate, 1X PCR buffer containing 1.5mM MgCl2 and 0.5 u of Taq polymerase enzyme (Bangalore Genei, India). Amplification was carried out in ABI 2700 thermal cycler (Applied Biosystems, USA) using the following conditions: 30 cycles of denaturation at 94°C for 30 sec, annealing at 55°C for 45 sec, and extension at 72°C for 60 sec. The final extension was at 72°C. In the first cycle the sample was denatured for 5min.
Automated DNA sequencing
To confirm CAG repeat numbers duplicate samples from each repeat size were sequenced in ABI PRISM 3700 (Perkin Elmer, USA) automated DNA sequencers using the manufacturers protocol.
Statistical analysis
Statistical analysis of the data has been performed using MINITABTM Statistical Software (version: 13.31, Minitab nc. PA, USA). Frequency χ2-test has been used to examine the association between smoking habit and seminal phenotype, sperm motility, morphology, and DNA integrity. Logistic regression analysis was carried out to study the nature of dependence, wherever applicable. A p-value less than 0.05 were considered as statistically significant.
Table 1. Demographic status of the population included in the present study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author Contributions: Amrita Mitra: Literature search, experiments, data-collection and manuscript preparation
Baidyanath Chakraborty: Study designing and clinical evaluation
Dyutiman Mukhopadhay: Data-analysis
Manisha Pal: Statistical analysis
Sanjit Mukherjee: manuscript preparation and experimentation
Samir Banerjee: Study designing and data-analysis
Keya Chaudhuri: Literature search, study design, data analysis, data interpretation, manuscript preparation
References
- Alexandersen, P., Haarbo, J. and Christiansen, C. (1996) The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis 125:1–13.
- Arabi, M. (2004) Nicotinic infertility: assessing DNA and plasma membrane integrity of human spermatozoa. Andrologia 36:305–310.
- Bergmann, M., Behre, H.M. and Nieschlag, E. (1994) Serum FSH and testicular morphology in male infertility. Clin Endocrinol (Oxf) 40:133–136.
- Chang, C.S., Kokontis, J. and Liao, S.T. (1988) Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240:324–326.
- Chia, S.E., Ong, C.N. and Tsakok, F.M. (1994) Effects of cigarette smoking on human semen quality. Arch Androl 33:163–168.
- Dadze, S., Wieland, C., Jakubiczka, S., Funke, K., Schroder, E., Royer-Pokora, B., (2000) The size of the CAG repeat in exon 1 of the androgen receptor gene shows no significant relationship to impaired spermatogenesis in an infertile Caucasoid sample of German origin. Mol Hum Reprod 6:207–214.
- Davis-Dao, C.A., Tuazon, E.D., Sokol, R.Z. and Cortessis, V.K. (2007) Male infertility and variation in CAG repeat length in the androgen receptor gene: a meta-analysis. J Clin Endocrinol Metab 92:4319–4326.
- Dowsing, A.T., Yong, E.L., Clark, M., McLachlan, R.I., de Kretser, D.M. and Trounson, A.O. (1999) Linkage between male infertility and trinucleotide repeat expansion in the androgen-receptor gene. Lancet 354:640–643.
- Ercal, N., Gurer-Orhan, H. and Aykin-Burns, N. (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539.
- Funabashi, T., Sano, A., Mitsushima, D. and Kimura, F. (2005) Nicotine inhibits pulsatile luteinizing hormone secretion in human males but not in human females, and tolerance to this nicotine effect is lost within one week of quitting smoking. J Clin Endocrinol Metab 90:3908–3913.
- Gaur, D.S., Talekar, M. and Pathak, V.P. (2007) Effect of cigarette smoking on semen quality of infertile men. Singapore Med J 48:119–123.
- Hengstler, J.G., Bolm-Audorff, U., Faldum, A., Janssen, K., Reifenrath, M., Gotte, W., (2003) Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 24:63–73.
- Kulikauskas, V., Blaustein, D. and Ablin, R.J. (1985) Cigarette smoking and its possible effects on sperm. Fertil Steril 44:526–528.
- Kunzle, R., Mueller, M.D., Hanggi, W., Birkhauser, M.H., Drescher, H. and Bersinger, N.A. (2003) Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril 79:287–291.
- Lo, K.C., Brugh, V.M., 3rd, Parker, M., and Lamb, D.J. (2005) Isolation and enrichment of murine spermatogonial stem cells using rhodamine 123 mitochondrial dye. Biol Reprod 72:767–771.
- Meizel, S. (2004) The sperm, a neuron with a tail: ‘neuronal’ receptors in mammalian sperm. Biol Rev Camb Philos Soc 79:713–732.
- Mifsud, A., Choon, A.T., Fang, D. and Yong, E.L. (2001) Prostate-specific antigen, testosterone, sex-hormone binding globulin and androgen receptor CAG repeat polymorphisms in subfertile and normal men. Mol Hum Reprod 7:1007–1013.
- Mifsud, A., Sim, C.K., Boettger-Tong, H., Moreira, S., Lamb, D.J., Lipshultz, L.I., (2001) Trinucleotide (CAG) repeat polymorphisms in the androgen receptor gene: molecular markers of risk for male infertility. Fertil Steril 75:275–281.
- Mukhopadhyay, D., Nandi, P., Varghese, A.C., Gutgutia, R., Banerjee, S. and Bhattacharyya, A.K. (2010) The in vitro effect of benzo[a]pyrene on human sperm hyperactivation and acrosome reaction. Fertil Steril 94:595–598.
- Oldereid, N.B., Rui, H., Clausen, O.P. and Purvis, K. (1989) Cigarette smoking and human sperm quality assessed by laser-Doppler spectroscopy and DNA flow cytometry. J Reprod Fertil 86:731–736.
- Padron, R.S., Mas, J., Zamora, R., Riverol, F., Licea, M., Mallea, L., (1989) Lipids and testicular function. Int Urol Nephrol 21:515–519.
- Pasqualotto, F.F., Sobreiro, B.P., Hallak, J., Pasqualotto, E.B. and Lucon, A.M. (2006) Cigarette smoking is related to a decrease in semen volume in a population of fertile men. BJU Int 97:324–326.
- Patrizio, P., Leonard, D.G., Chen, K.L., Hernandez-Ayup, S. and Trounson, A.O. (2001) Larger trinucleotide repeat size in the androgen receptor gene of infertile men with extremely severe oligozoospermia. J Androl 22:444–448.
- Quigley, C.A. (1998) The androgen receptor: physiology and pathophysiology. In: Nieschlag E, Behre HM, eds. Testosterone: action, deficiency, substitution. New York: Springer; 33–106
- Tejada, R.I., Mitchell, J.C., Norman, A., Marik, J.J. and Friedman, S. (1984) A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril 42:87–91.
- Thangaraj, K., Joshi, M.B., Reddy, A.G., Gupta, N.J., Chakravarty, B. and Singh, L. (2002) CAG repeat expansion in the androgen receptor gene is not associated with male infertility in Indian populations. J Androl 23:815–818.
- Trisini, A.T., Singh, N.P., Duty, S.M. and Hauser, R. (2004) Relationship between human semen parameters and deoxyribonucleic acid damage assessed by the neutral comet assay. Fertil Steril 82:1623–1632.
- Trummer, H., Habermann, H., Haas, J. and Pummer, K. (2002) The impact of cigarette smoking on human semen parameters and hormones. Hum Reprod 17:1554–1559.
- Tut, T.G., Ghadessy, F.J., Trifiro, M.A., Pinsky, L. and Yong, E.L. (1997) Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J Clin Endocrinol Metab 82:3777–3782.
- Varghese, A.C., Bragais, F.M., Mukhopadhyay, D., Kundu, S., Pal, M., Bhattacharyya, A.K., (2009) Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia 41:207–215.
- WHO (1999) WHO laboratory manual for the examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th edn, Cambridge University Press, Cambridge, UK.
- Yong, E.L., Ghadessy, F., Wang, Q., Mifsud, A. and Ng, S.C. (1998) Androgen receptor transactivation domain and control of spermatogenesis. Rev Reprod 3:141–144.
- Yoshida, K.I., Yano, M., Chiba, K., Honda, M. and Kitahara, S. (1999) CAG repeat length in the androgen receptor gene is enhanced in patients with idiopathic azoospermia. Urology 54:1078–1081.
- Zenzes, M.T. (2000) Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update 6:122–131.
- Zhang, J.P., Meng, Q.Y., Wang, Q., Zhang, L.J., Mao, Y.L. and Sun, Z.X. (2000) Effect of smoking on semen quality of infertile men in Shandong, China. Asian J Androl Jun 2(2):143–6
