Abstract
We explored the relationship between sperm chromatin integrity, hormone levels, seminal plasma total antioxidant capacity (TAC), and routine sperm parameters in men with male factor (MF, n = 81) and non-male factor (NMF, n = 52) infertility. Semen and blood were collected and examined from men undergoing evaluation for infertility in the Avicenna Infertility Clinic. We have examined each patient for serum hormones (LH, FSH, E2, DHEA), sperm chromatin damage, level of protamination and seminal plasma TAC. Levels of FSH, LH, sperm chromatin damage, and abnormal protamination were significantly higher in MF vs. NMF groups (p < 0.001). Sperm chromatin damage was correlated with percentage of CMA3- positive sperm (r = 0.64, p < 0.001) and with sperm concentration (r = −0.36, p < 0.001), motility (r = −0.21, p < 0.05), and morphologically normal spermatozoa (r = −0.29, p < 0.001). Linear regression showed sperm chromatin damage was related to percentage of CMA3- positive sperm (p < 0.001) in ungrouped patients. It was related to both percentage of CMA3- positive sperm and serum DHEA in the MF group (p < 0.001 and p < 0.05, respectively). Sperm chromatin maturity assessed by CMA3 test was inversely related to sperm chromatin damage assessed by the toludine blue assay. Male factor infertility associated with sperm chromatin damage may be related to sperm protamination and to serum DHEA.
Introduction
Intact sperm chromatin and sperm maturity are important for the reproductive potential of men [Meeker et al. Citation2008]. Sperm chromatin damage negatively affects fertility potential and it may result in poor embryo quality with low pregnancy rates [Agarwal et al. 2004; Lewis and Aitken Citation2005; Morris et al. Citation2002]. Higher levels of DNA damage observed in spermatozoa compared to somatic cells may be due to sperm susceptibility to damage at a number of stages of spermatogenesis and spermiogenesis [Lewis et al. Citation2008].
Sperm chromatin damage may take place within the testis, epididymis, or ejaculate. The sperm plasma membrane is rich in polyunsaturated fatty acids (PUFAs) which predispose sperm to oxidative damage [Jones et al. Citation1979]. De Iuliis et al. [2009] indicated that defects in chromatin remodeling during spermiogenesis followed by a free radical attack likely induces sperm DNA damage. Sperm chromatin damage may resolve as: i) oxidative stress (OS), ii) poor chromatin packaging or abnormal packing due to defective protamination, iii) abortive apoptosis, and iv) impairment in recombination during spermatogenesis resulting in cell apoptosis [Agarwal and Said Citation2003; Erenpreiss et al. Citation2006; Sakkas et al. Citation1999]. The most widely reported causes are OS, sperm nuclear protamination, and serum hormones [Appasamy et al. Citation2007; Meeker et al. Citation2008].
OS is an imbalance between the available total antioxidant capacity (TAC) and reactive oxygen species (ROS) generation in spermatozoa, either through overproduction of ROS and/ or decreased protective TAC levels which cannot scavenge abnormal free radicals [Lewis and Aitken Citation2005; Sharma and Agarwal Citation1996]. Immature spermatozoa and semen leukocytes are the major sources of ROS generation in semen [Aitken and West Citation1990; Gil-Guzman et al. Citation2001].
ROS overproduction oxidizes lipids in sperm plasma membrane and intracellular proteins leading to sperm damage and male infertility [Sikka Citation1996]. In addition, OS creates several forms of DNA and chromatin damage, e.g., chromatin cross-linking, DNA strand breaks and oxidized nucleotide bases [Aitken et al. Citation2003).
Impairment of DNA protamination during late spermatogenesis is associated with chromatin damage in human spermatozoa [De Iuliis et al. Citation2009]. Serum gonadotropins and androgens are critical for healthy spermatogenesis and male reproductive function [Lo and Lamb Citation2004). However, there are plenty of mechanisms involved that have not been completely elucidated. Recently, reports showed that estradiol and thyroid hormones may play an important role in spermatogenesis and germ cell survival [Akingbemi Citation2005; Hess et al. Citation1997; Jannini et al. Citation1995; Krassas and Pontikides Citation2004; O'Donnell et al. Citation2001; Trokoudes et al. Citation2006]. Dehydroepiandrosterone (DHEA) is a derivative of C19 steroid, mainly secreted by the adrenal cortex [Parker Citation1999]. DHEA may play a role in the level of antioxidants and in cellular pathways. Exogenous DHEA can exert an antioxidant or prooxidant effect, depending on dose, schedule, and target tissue type [Jacob et al. Citation2008). There is a lack of understanding of the levels of DHEA and antioxidants in male infertility. This study aimed to evaluate the relationships between sperm chromatin integrity, serum hormonal levels, seminal plasma TAC, and routine sperm parameters in infertile men.
Results
There were no significant differences between serum hormone levels and semen parameters in men when the MF vs. NMF groups were compared or between group-A (sperm chromatin damage ≥ 30%) vs. group-B (). Representative patient samples subjected to TB staining and CMA3 are shown in and , respectively.
Figure 1. Toluidine blue (TB) staining. Light blue sperm cell heads shown with white arrows were scored as normal integrity and those with purple (deep violet) heads shown with black arrows were scored as having damaged chromatin.
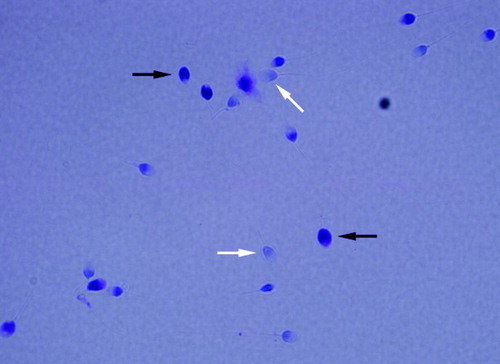
Figure 2. Chromomycin A3 (CMA3) staining. Dull sperm cell heads shown with hollow arrows were scored as CMA3 negative cell and bright yellow sperm cell heads shown with bold arrows were scored as CMA3 positive cell.
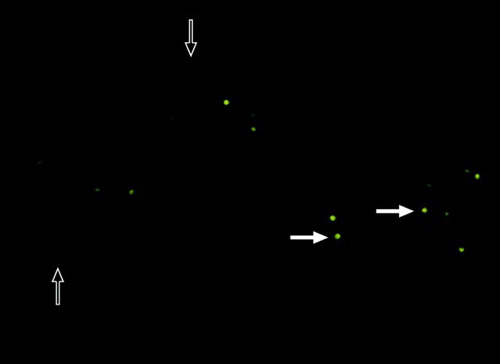
Table 1. Semen parameters, male reproductive hormones, seminal plasma total antioxidant capacity (TAC), sperm chromatin damage, and percentage of CMA3-positive sperm in MF infertility (n = 81) and NMF infertility (n = 52) also in group with sperm chromatin damage ≥ 30% (n = 49) and in group with sperm chromatin damage < 30% (n = 81).
There were no significant differences observed in terms of the age of infertile men when group 1 was compared to group 2, as well as between group-A vs. group-B. However, patients with NMF generally exhibited higher sperm motility (%), sperm concentration (Mil/ml), and normal morphologically spermatozoa (%) (p < 0.001 for all) ().
In the NMF group, 43 patients (82.69%) showed low sperm chromatin damage (< 30% stained blue with TB test) while 38 patients (46.91%) in the MF group had sperm chromatin damage (≥ 30% stained violet with TB test). The MF group exhibited a higher incidence of CMA3-positive sperm (46.5 ± 16.2) compared to the NMF group (31.4 ± 12.7) (p < 0.001). While group A showed a significantly higher CMA3-positive sperm (52 ± 15.8) compared to group B (34.1 ± 13.3; p < 0.001).
MF patients showed significantly higher levels of serum LH compared with the NMF patients group (9.7 ± 4.8 mIU/ml vs 6.5 ± 3.5 mIU/ml; p < 0.001). However patients with high chromatin damage (group A) did not have a higher level of LH compared with group B (9.5 ± 4.7 mIU/ml vs 8 ± 4.5 mIU/ml; p ≥ 0.05). Serum FSH was significantly higher in MF patients compared with NMF patient groups (9.4 ± 6.3 mIU/ml vs 5.7 ± 4.2 mIU/ml; p < 0.001). However, group A, patients with high chromatin damage, showed significantly higher levels of FSH compared to group B, men with low chromatin damage, (9.2 ± 6.1 mIU/ml vs 7.4 ± 5.8 mIU/ml; p < 0.05). There were no significant differences in serum testosterone, DHEA, or E2 between MF vs. NMF groups as well as between group A or group B.
Correlation studies in all patients
Patient age was negatively correlated with serum testosterone (r = −0.19, p = 0.05) and DHEA (r = −0.45, p < 0.001). Serum LH was negatively correlated with sperm concentration (r = −0.38, p < 0.001), sperm motility (%) (r = −0.3, p <0.01), and with morphologically normal spermatozoa (%) (r = −0.37, p < 0.001). As expected, a positive correlation was observed between serum LH and serum testosterone (r = 0.31, p < 0.01) and between serum LH and serum DHEA (r = 0.2, p < 0.05). Interestingly, serum FSH was negatively correlated with sperm concentration (r = −0.41, p < 0.001), sperm motility (r = −0.25, p < 0.01), and morphologically normal spermatozoa (r = −0.35, p < 0.001). There was also a significant positive correlation between serum FSH and LH (r = 0.5, p < 0.001) and between serum FSH and testosterone (r = 0.27, p < 0.01). Surprisingly, a significant positive correlation was observed between serum DHEA and serum E2 (r = 0.41, p < 0.001) in all patients.
Sperm chromatin damage showed significant correlations with serum LH (r = 0.23, p < 0.05) and serum FSH (r = 0.23, p < 0.05) and non significant correlations with serum levels of DHEA, E2, and testosterone. Sperm chromatin damage showed significant negative correlations with sperm concentration (r = −0.36, p < 0.001), sperm motility (r = −0.21, p < 0.05), and with morphologically normal spermatozoa (r = −0.29, p < 0.01).
The percentage of CMA3-positive sperm showed significantly negative correlations with sperm count (r = −0.45, p < 0.001), sperm motility (r = −0.3, p < 0.001), and morphologically normal spermatozoa (r = −0.36, p < 0.001). CMA3-positive sperm showed significant positive correlations with sperm chromatin damage (r = 0.64, p < 0.001), serum LH (r = 0.27, p < 0.01), serum FSH (r = 0.27, p < 0.01),and serum DHEA (r = 0.19, p < 0.05).
Correlation studies within stratified patients' group
Only sperm chromatin damage was positively correlated with the percentage of CMA3- positive sperm in the MF group (r = 0.62, p < 0.001) and in the NMF group (r = 0.38, p < 0.01). On one hand, in infertile men with low sperm chromatin damage, group B, sperm chromatin damage was negatively correlated with sperm count (r = −0.39, p <0.001) and morphologically normal spermatozoa (r = −0.23, p < 0.05), while it was positively correlated with percentage of CMA3- positive sperm (r = 0.3, p < 0.01). On the other hand, sperm chromatin damage was positively correlated with serum LH (r = 0.37, p < 0.05) and the percentage of CMA3- positive sperm (r = 0.59, p < 0.001) in group-A, men with high chromatin damage.
Multivariate linear regression in all and in stratified patients
Sperm chromatin damage was evaluated as a function of routine sperm parameters, hormonal levels, percentage of CMA3- positive sperm, and seminal plasma TAC in each and all patient groups. In the absence or presence of confounding variables such as sperm concentration, sperm motility, and sperm normal morphology. Regression models, suggested that only percentage of CMA3- positive sperm was related to sperm chromatin damage (p <0.001, 95% CI: 0.45, 0.78) when all patients were considered (). In group 1, with and without entering confounding parameters, the percentage of CMA3- positive sperm (p <0.001, 95% CI: 0.39, 0.78) and serum DHEA (p <0.05, 95% CI: - 0.05, - 0.002) were related to sperm chromatin damage (), but in group 2, without entering confounding variables no significant relationship was found between the variables and sperm chromatin damage (). However, the percentage of CMA3- positive sperm was closely but not significantly related to sperm chromatin damage (p = 0.055, 95% CI: - 0.007, 0.61). The confounding variables of sperm concentration (p <0.05, 95% CI: 0, 0) and sperm normal morphology (p <0.05, 95% CI: 0.23, 1.6) had a significant relationship with sperm chromatin damage ().
Table 2. Adjusted regression coefficients for relationship of sperm chromatin damage with semen parameters, hormones, percentage of CMA3-positive sperm, and seminal plasma TAC in all patients (MF & NMF groups).
Table 3. Adjusted regression coefficients for relationship of sperm chromatin damage with semen parameters, hormones, percentage of CMA3- positive sperm, and seminal plasma TAC in MF (group 1).
Table 4. Adjusted regression coefficients for relationship of sperm chromatin damage with semen parameters, hormones, percentage of CMA3- positive sperm, and seminal plasma TAC in NMF infertility (group 2).
Multivariate linear regression for evaluating the association of percentage of CMA3- positive sperm and sperm chromatin damage with hormone levels, seminal plasma TAC, and semen parameters
Without considering confounding variables in the models, only seminal TAC was correlated with the percentage of CMA3- positive sperm (p <0.05, 95% CI: -17.76, -0.12), but when confounding variables were incorporated, sperm concentration (p < 0.05, 95% CI: 0, 0) and sperm motility (p <0.05, 95% CI: - 0.39, - 0.02) were correlated with the percentage of CMA3- positive sperm (). On one hand when confounding variables were not considered, seminal TAC was correlated with sperm chromatin damage (p <0.05, 95% CI: - 18.06, -1.03). On the other hand, when confounding variables were considered, sperm concentration was correlated with sperm chromatin damage (p < 0.001, 95% CI: 0, 0) ().
Table 5. Adjusted regression coefficients for relationship of percentage of CMA3- positive sperm with semen parameters, hormones and seminal plasma TAC.
Table 6. Adjusted regression coefficients for relationship of sperm chromatin damage with semen parameters, hormones and seminal plasma TAC.
Odds ratio (OR) evaluation in group A and group B
In general, the patients presenting with a high percentage of CMA3-positive sperm, were more likely to have high sperm chromatin damage (≥ 30%) compared to patients with low sperm chromatin damage (< 30%) (OR: 1.08, 95% CI: 1.04-1.12). The same trend of having high percentage of CMA3-positive sperm in patients with high sperm chromatin damage (≥ 30%) to that of patients with low sperm chromatin damage (< 30%) (OR: 1.08, 95% CI: 1.03-1.13) was observed when the samples were grouped. Interestingly, the MF patient was more likely to have a high level of sperm chromatin damage (≥30%) compared to the NMF patient (OR: 9.3, 95% CI: 1.79-48.8).
Discussion
Spermatogenesis is controlled through the integration of local and systemic factors. Healthy mature spermatozoa reflect appropriate testicular tissue function in response to androgens [Aoki et al. Citation2006; Steger et al. Citation2000]. Sperm chromatin structure plays a major role in the fertility potential of a male. Its status is reflective of both maturation and integrity and susceptibility to modification throughout spermatogenesis. Oxidative stress and serum hormones may affect final sperm chromatin status [Agarwal et al. Citation2009; Barratt et al. Citation2010].
In this study we examined the relationship of sperm chromatin status with routine sperm parameters, hormonal levels, and seminal plasma TAC in male partners of infertile couples due to either MF or NMF. This study shows that sperm chromatin damage is inversely related to sperm motility, concentration as well as the number of morphologically normal spermatozoa in infertile men. This confirms that sperm chromatin reflects the status of spermatogenesis in infertile men and is complementary to routine sperm parameters [Appasamy et al. Citation2007; Saleh et al. Citation2003]. However, this association is lost in NMF infertility, as previously reported [Varghese et al. Citation2009].
The study presented above is in agreement with Appasamy et al. [2007] and disagreement with reports that showed low TAC in infertile men compared to fertile men [Pasqualotto et al. Citation2000; Shi et al. Citation2005]. Use of the ROS/TAC score may be better suited for patients who may have border line seminal plasma TAC values [Saleh et al. Citation2003].
Tarozzi et al. [2009], reported a significant negative relationship between protamine deficiency and seminal TAC. This agrees with our study, using multivariate linear regression when hormones and TAC were considered. Seminal TAC was significantly related with CMA3-positive sperm. However, this relationship was lost with the addition of sperm concentration, sperm motility, and normal morphologically sperm into the model [Tarozzi et al. Citation2009].
Recent reports showed that sperm DNA damage is related to protamine deficiency [Aoki et al. Citation2006; Aoki et al. Citation2005; Nili et al. Citation2009]. Interestingly the above showed significant positive correlation between sperm under-protamination and sperm chromatin damage. This confirms that protamine deficiency is one of the causes of sperm chromatin damage or abnormal integrity.
The above showed that the percentage of CMA3-positive spermatozoa exhibits a significant positive relationship to FSH, LH, and DHEA in all patients. In addition, sperm chromatin damage has a significant positive relationship to LH, FSH but not DHEA in all infertile men. However, these relationships disappeared when patients were classified based on male factor. The observed correlations may be due to the relationship of classical sperm parameters (count, motility, and morphology) with CMA3-positive spermatozoa and sperm chromatin damage.
In the present study, we, for the first time, showed that sperm chromatin damage is positively correlated with the percentage of CMA3-positive sperm and negatively with serum DHEA levels. These findings may explain the high incidence of chromatin damage in male factor infertility in men who have impaired or low androgen status. DHEA and its sulfate ester (DHEA-S) are the most abundant steroid hormones in humans [Charlton et al. Citation2008]. DHEA exhibits a potent mediator property for ROS scavenger production [Bednarek-Tupikowska et al. Citation2000]). Chronic administration of DHEA was reported to have no determinantal effect on the qualitative or quantitative analysis of spermatogenesis [Gobbi et al. Citation2003]. However, the response of spermatogenesis to external DHEA administration may vary in terms of dosage and timing [Yapanoglu et al. Citation2008]. In our study, sperm chromatin damage was associated with the percentage of CMA3-positive spermatozoa in all patient groups. This highlights the importance of sperm chromatin maturity to protect sperm DNA and chromatin against injurious agents.
The clinical and diagnostic importance of this study includes the further understanding of sperm pathology in relation to various hormones, but also adds an important issue for the infertility clinic. These findings may add novel diagnostic biomarkers to the reproductive laboratory including seminal TAC and serum DHEA in infertile men along with sperm chromatin that is known to be critical for sperm fertility potential [Bungum et al. Citation2011; Zini Citation2011]. Our study limitations include the absence of fertile men as a control group. The use of an appropriately sized group of normozoospermic men with proven fertility would clearly add sperm fertility potential as a comparator.
In conclusion, our study shows that assessment of sperm chromatin by CMA3, as a maturity assay, and toluidine blue, as an integrity assay, may be complementary to each other in reflecting the actual status of sperm chromatin. Multivariate models showed that sperm chromatin damage may be related to sperm protamination in all men while it is also related to levels of protamination and serum DHEA in men with MF infertility. Additional studies are recommended to understand the in vitro or in vivo effects of pharmacologic doses of DHEA, FSH, and LH on sperm chromatin integrity/maturity.
Materials and Methods
Patient characteristics
This study was conducted following the Institutional approval by the ethical committee of Avicenna Research Institute (Tehran, Iran). Informed consent was obtained from each patient before their participation in this study. Blood and semen specimens were collected from enrolled patients who were referred to Avicenna Infertility Clinic (AIC) for infertility treatment. Patients were classified into 2 groups: group 1, includes patients with male factor infertility (MF, n = 81) and group 2 includes male partners of infertile couples with undetectable male factor infertility (NMF, n = 52). The NMF group was used as a control to MF as they were normozoospermic men. While MF showed abnormal semen report such as oligo-, astheno-, and/ or terato-zooeprmia. Furthermore, another classification was used for enrolled men. Subjects were also classified according to their percentage of sperm chromatin damage. Group A includes patients with sperm chromatin damage ≥ 30% (high chromatin damage), as detected by TB staining and group B includes patients with sperm chromatin damage < 30% (low chromatin damage).
Inclusion/exclusion criteria
MF was defined to include all male partners from the consented infertile couples, with no detectable female partner abnormalities, who had an abnormal sperm parameter according to WHO [1999] reference values. MF patients showed one or more of the conventional sperm parameters: sperm count < 20 × 106 /ml, percentage of normal forms < 30%, and total sperm motility < 50% WHO [1999] criteria. The NMF group had normal semen parameters with unexplained infertility or with female factor infertility such as ovulatory/ tubal dysfunction. Patients with leukocytospermia (≥ 1 × 106/ml), varicocele, genital infection, congenital anomalies, a history of smoking and alcohol consumption were excluded.
Specimen selection and preparation
Semen samples were obtained after at least 48 h of abstinence. Routine semen analysis was carried out according to WHO [1999] criteria. Each semen specimen was divided in 2 aliquots; aliquot 1 was freshly used for CMA3 and TB assays while aliquot 2 was centrifuged at 300 x g for 10 min to obtain clear seminal plasma for TAC assay. The supernatant was collected and re-centrifuged at 1500 x g for 10 min at room temperature. Seminal plasma supernatants were stored at −70°C until running time for TAC assay. Serum specimens were obtained by centrifugation of 10 ml whole clotted blood for 10 min at 1,500 x g. Then, aliquots were stored at −20°C until hormone levels were determined.
Measurement of sperm chromatin integrity by toluidine blue
Spermatozoa were pelleted at 100 x g for 10 min then washed twice in phosphate buffered saline (PBS, pH = 7.4). Slide smears were prepared for each specimen, then left for 10 min to air dry. Subsequently, dried smears were fixed in a freshly prepared ethanol (96%): acetone (1:1) at 4°C for 30 min. The slides were allowed to air dry after fixation.
A staining solution of 0.05% TB (Merck, Germany) in staining buffer was applied for 10 min. Staining buffer is comprised of 50% citrate phosphate (McIlvain buffer, pH 3.5 [Elving et al. Citation1956]). Slides were rinsed briefly in distilled water and air dried again. Finally, slides were examined using oil immersion objective lens (×100). Sperm heads were scored based upon their staining characteristics; light blue stained sperm heads were scored as of normal integrity while those stained deep violet were scored as having damaged chromatin. Staining quality was assessed using a positive control of sperm treated with hydrogen peroxide (100 µmol/l) for 10 min, along with patient specimens [Hodjat et al. Citation2008].
Measurement of protamination level in sperm by chromomycin A3
CMA3 staining was performed by treating slides with 100 µl of CMA3 solution (0.25 mg/ml in McIlvaine buffer, pH 7.0, 10 mM MgCI2) for 20 min. After staining, the slides were rinsed in buffer and air dried. Finally, slides were scored using the fluorescence microscope (Carl Zeiss MicroImaging GmbH, Germany) with suitable filters (460-495 nm). Dull sperm heads were examined as CMA3 negatively stained cells. Bright yellow or green sperm heads were scored as CMA3 positively stained cells [Sakkas et al. Citation2000]. The semen specimens were evaluated individually by two expert laboratory staff. Both inter-assay and intra-assay coefficient of variation of semen analysis, CMA3 and TB staining were <10%.
Serum hormones levels
Hormone assays (Estradiol (E2), LH, FSH, testosterone, DHEA) were examined in serum for all patients. Levels of E2, LH, FSH, and testosterone were examined by chemiluminescencence as per manufacturer's instructions (Diasorine, Italy). Analytical sensitivity of each test was ≤0.05 ng/ml, < 0.2 mIU/ml and < 0.25 mIU/ml for testosterone, LH, and FSH, respectively. Intra- and inter-assay variability for testosterone, LH, and FSH were 3.0% and 4.0%, 4.0% and 3.6%, and 3.0% and 3.3%, respectively. DHEA was measured using a commercial radioimmunoassay (RIA) kit (Immunotech, France). Analytical sensitivity of this assay was 6.0 µg/100 ml. Intra- and inter-assay variations were ≤7.4% and ≤10.6%, respectively. E2 was measured using a commercial radioimmunoassay (RIA) kit (Immunotech, France). Analytical sensitivity was < 6 pg/ml. Its intra- and inter-assay variations were ≤12.1% and ≤11.2%, respectively.
Measurement of total antioxidant capacity
TAC examines OS status indirectly. Seminal TAC levels were measured using the colorimetric assay (Randox kit, USA) according to the manufacture's protocol. Briefly, the spectrophotometer (Cecil instruments, United Kingdom) was adjusted to 600 nm with the temperature at 37°C. Trolox (6-hydroxyl-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid) at a concentration of 1.73 mmol/L was used as the standard. TAC level of the specimen was calculated and expressed in terms of Trolox equivalents units, using the following formula: TAC = Concentration of the Standard × (∆A Blank - ∆A Sample)/ (∆A Blank - ∆A Standard). TAC results were represented as micromolar (μM) of Trolox equivalents. For validation of the assay, control serum (Randox, USA) was used [Said et al. Citation2003].
Statistical analysis
Statistical analysis was accomplished using the Statistics Package for Social Sciences (SPSS), version 13 (SPSS Inc, IL, USA) for microsoft windows. The one-sample kolmogorov-smirnov test showed age, normal morphology, CMA3, TB, TAC, LH, testosterone, and DHEA were normally distributed but count, motility, FSH, and E2 were not normally distributed. Variables, showed a normal distribution, were compared in two groups by independent samples t-test while non-parametric tests were used for other comparisons. Mann-Whitney U-test was used for comparison of variables which were not normally distributed in groups. Pearson's correlation coefficient was used for parametric correlations and Spearman's rank correlation was used for non-parametric correlations. Multivariate linear regression was used to explore relationship between sperm chromatin damage and hormone levels, TAC and percentage of CMA3-positive sperm and age. Multivariate linear regression analysis was used to explore the relationship of CMA3-positive sperm with TAC and hormonal levels. Correlation of sperm chromatin damage was examined with hormonal levels and seminal TAC. A regression model was fit with adjustment for confounding variables such as sperm concentration, sperm with normal morphology, and sperm motility. Multivariate logistic regression was used to calculate odds ratio for diverse variables in group A and B. In this study, p-values less than 0.05 (5%) are considered statistically significant [Markel Citation1991].
Abbreviations
| ARI: | = | Avicenna Research Institute |
| MF: | = | male factor |
| NMF: | = | non-male factor |
| PBS: | = | phosphate buffered saline |
| TAC: | = | total antioxidant capacity |
| DNA: | = | deoxyribonucleic Acid |
| CMA3: | = | chromomycin A3 |
| DHEA: | = | dehydroepiandrosterone |
| LH: | = | luteinizing hormone |
| FSH: | = | follicle stimulating hormone |
| PUFAs: | = | polyunsaturated fatty acids |
| OS: | = | oxidative stress |
| ROS: | = | reactive oxygen species |
| TB: | = | toluidine blue |
| WHO: | = | World Health Organization |
| RIA: | = | radioimmunoassay |
| SPSS: | = | statistics package for social sciences. |
Acknowledgments
The authors would like to thank Ms Elham Savadi Shirazi and Ms Lima Asgharpour for their contribution in collecting samples from Avicenna Infertility Clinic (Tehran, Iran).
Declaration of interest: This study was supported by grant No. 840106-22 from the Avicenna Research Institute (ARI, Tehran, Iran). There are no conflicts of interest.
Author Contributions: NL performed the laboratory tests, wrote the manuscript and was involved in the design of the study and interpreting the data. RZM and AA interpreted the results and revised the manuscript. MMA, NAJ and HKH helped to select patients and to design the study. HZ was involved in the statistical analysis and interpreting the results. MRS was involved in the design of the study and reviewed the paper and also supervised the whole study.
References
- Agarwal, A. and Said, T.M. (2003) Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 9:331–345.
- Agarwal, A. and Allamaneni, S.S. (2004) The effect of sperm DNA damage on assisted reproduction outcomes. Minerva Ginecol 56:235–245.
- Agarwal, A., Varghese, A.C. and Sharma, R.K. (2009) Markers of oxidative stress and sperm chromatin integrity. Methods Mol Biol 590:377–402.
- Aitken, R.J., Baker, M.A. and Sawyer, D. (2003) Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online 7:65–70.
- Aitken, R.J. and West, K.M. (1990) Analysis of the relationship between reactive oxygen species production and leucocyte infiltration in fractions of human semen separated on Percoll gradients. Int J Androl 13:433–451.
- Akingbemi, B.T. (2005) Estrogen regulation of testicular function. Reprod Biol Endocrinol 3:51.
- Appasamy, M., Muttukrishna, S., Pizzey, A.R., Ozturk, O., Groome, N.P., Serhal, P. and Jauniaux, E. (2007) Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online 14:59–165.
- Aoki, V.W., Emery, B.R., Liu, L. and Carrell, D.T. (2006) Protamine levels vary between individual sperm cells of infertile human males and correlate with viability and DNA integrity. J Androl 27:890–898.
- Aoki, V.W., Moskovtsev, S.I., Willis, J., Liu, L., Mullen, J.B. and Carrell, D.T. (2005) DNA integrity is compromised in protamine-deficient human sperm. J Androl 26:741–748.
- Barratt, C.L., Aitken, R.J., Björndahl, L., Carrell, D.T., de Boer, P., Kvist, U., Lewis, S.E., Perreault, S.D., Perry, M.J., Ramos, L., Robaire, B., Ward, S. and Zini, A. (2010) Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications--a position report. Hum Reprod 25:824–838.
- Bednarek-Tupikowska, G., Gosk, I., Szuba, A., Bohdanowicz-Pawlak, A., Kosowska, B., Bidzinska, B., (2000) Influence of dehydroepiandrosterone on platelet aggregation, superoxide dismutase activity and serum lipid peroxide concentrations in rabbits with induced hypercholesterolemia. Med Sci Monit 6:40–45.
- Bungum, M., Bungum, L. and Giwercman, A. (2011) Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 13:69–75.
- Charlton, M., Angulo, P., Chalasani, N., Merriman, R., Viker, K., Charatcharoenwitthaya, P., Sanderson, S., Gawrieh, S., Krishnan and A., Lindor, K. (2008) Low circulating levels of dehydroepiandrosterone in histologically advanced nonalcoholic fatty liver disease. Hepatology 47:484–492.
- De Iuliis, G.N., Thomson, L.K., Mitchell, L.A., Finnie, J.M., Koppers, A.J., Hedges, A., (2009) DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2’-deoxyguanosine, a marker of oxidative stress. Biol Reprod 81:517–524.
- Elving, P.J., Markowitz, J.M., Isadore, Rosenthal (1956) Preparation of Buffer Systems of Constant Ionic Strength. Anal Chem 28:1179–1180.
- Erenpreiss, J., Spano, M., Erenpreisa, J., Bungum, M. and Giwercman, A. (2006) Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl 8:11–29.
- Gil-Guzman, E., Ollero, M., Lopez, M.C., Sharma, R.K., Alvarez, J.G., Thomas, A.J., (2001) Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod 16:1922–1930.
- Gobbi, D., Rhoden, E.L., Menti, E., Lulhier, F. and Rhoden, C. (2003) Effects of the chronic use of dehydroepiandrosterone (DHEA) on testicular weight and spermatogenesis: experimental study in rats. Int Urol Nephrol 35:119–122.
- Hess, R.A., Bunick, D., Lee, K.H., Bahr, J., Taylor, J.A., Korach, K. S., (1997) A role for oestrogens in the male reproductive system. Nature 390:509–512.
- Hodjat, M., Akhondi, M.A., Al-Hasani, S., Mobaraki, M. and Sadeghi, M.R. (2008) Increased sperm ubiquitination correlates with abnormal chromatin integrity. Reprod Biomed Online 17:324–330.
- Jacob, M.H., Janner Dda, R., Bello-Klein, A., Llesuy, S.F. and Ribeiro, M.F. (2008) Dehydroepiandrosterone modulates antioxidant enzymes and Akt signaling in healthy Wistar rat hearts. J Steroid Biochem Mol Biol 112:138–144.
- Jannini, E.A., Ulisse, S. and D'Armiento, M. (1995) Thyroid hormone and male gonadal function. Endocrine reviews 16:443–459.
- Jones, R., Mann, T. and Sherins, R. (1979) Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides and protective action of seminal plasma. Fertil Steril 31:531–537.
- Krassas, G.E. and Pontikides, N. (2004) Male reproductive function in relation with thyroid alterations. Best Pract Res Clin Endocrinol Metab 18:183–195.
- Lewis, S.E., Agbaje, I. and Alvarez, J. (2008) Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med 54:111–125.
- Lewis, S.E. and Aitken, R.J. (2005) DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res 322:33–41.
- Lo, K.C and Lamb, D. (2004) The testis and male accessory organs. In Strauss, J.F. and Barbieri, R.L., eds. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 5thed. Elsevier, Inc, Philadelphia, PA pp.367–387.
- Markel, M.D. (1991) The power of a statistical test. What does insignificance mean? Vet Surg 3:209–214.
- Meeker, J.D., Singh, N.P. and Hauser, R. (2008) Serum concentrations of estradiol and free T4 are inversely correlated with sperm DNA damage in men from an infertility clinic. J Androl 29:379–388.
- Morris, I.D., Ilott, S., Dixon, L. and Brison, D.R. (2002) The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod 17:990–998.
- Nili, H.A., Mozdarani, H. and Aleyasin, A. (2009) Correlation of sperm DNA damage with protamine deficiency in Iranian subfertile men. Reprod Biomed Online 18:479–485.
- O'Donnell, L., Robertson, K.M., Jones, M.E. and Simpson, E.R. (2001) Estrogen and spermatogenesis. Endocrine reviews 22:289–318.
- Parker, C.R., Jr. (1999) Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 64:640–647.
- Pasqualotto, F.F., Sharma, R.K., Nelson, D.R., Thomas, A.J. and Agarwal, A. (2000) Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril 73:459–464.
- Sakkas, D., Mariethoz, E., Manicardi, G., Bizzaro, D., Bianchi, P.G. and Bianchi, U. (1999) Origin of DNA damage in ejaculated human spermatozoa. Reviews of reproduction 4:31–37.
- Sakkas, D., Manicardi, G.C., Tomlinson, M., Mandrioli, M., Bizzaro, D., Bianchi, P.G., (2000) The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod 15:1112–1116.
- Said, T.M.Kattal, N., Sharma, R.K., Sikka, S.C., Thomas, A.J. and Mascha, E.Agarwal, A. (2003) Enhanced chemiluminescence assay vs colorimetric assay for measurement of the total antioxidant capacity of human seminal plasma. J Androl 24:676–680.
- Said, T.M., Kattal, N., Sharma, R.K., Sikka, S.C., Thomas, A.J., Mascha, E., (2003) Enhanced chemiluminescence assay vs colorimetric assay for measurement of the total antioxidant capacity of human seminal plasma. J Androl 24:676–680.
- Saleh, R.A., Agarwal, A., Nada, E.A., El-Tonsy, M.H., Sharma, R.K., Meyer, A., (2003) Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 79: 1597–1605.
- Sharma, R.K. and Agarwal, A. (1996) Role of reactive oxygen species in male infertility. Urology 48:835–850.
- Shi, Y.C., Sun, H.M., Shang, X.J., Zhu, P.Y. and Huang, Y.F. (2005) Total antioxidant capacity of seminal plasma in fertile and infertile men. Zhonghua Nan Ke Xue 11:915–917.
- Sikka, S.C. (1996) Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front Biosci 1:e78–86.
- Steger, K., Pauls, K., Klonisch, T., Franke, F.E. and Bergmann, M. (2000) Expression of protamine-1 and -2 mRNA during human spermiogenesis. Mol Hum Reprod 6:219–225.
- Tarozzi, N., Nadalini, M., Stronati, A., Bizzaro, D., Dal Prato, L., Coticchio, G., (2009) Anomalies in sperm chromatin packaging: implications for assisted reproduction techniques. Reprod Biomed Online 18:486–495.
- Trokoudes, K.M., Skordis, N. and Picolos, M.K. (2006) Infertility and thyroid disorders. Curr Opin Obstet Gynecol 18:446–451.
- Varghese, A.C., Bragais, F.M., Mukhopadhyay, D., Kundu, S., Pal, M., Bhattacharyya, A.K., (2009) Human sperm DNA integrity in normal and abnormal semen samples and its correlation with sperm characteristics. Andrologia 41:207–215.
- WHO (1999) World Health Organization laboratory manual for the examination of human semen and sperm-servical mucus interaction, 4th edn.Cambridge University Press, Cambridge.
- Yapanoglu, T., Aksoy, Y., Gursan, N., Ozbey, I., Ziypak, T. and Calik, M. (2008) Antiapoptotic effects of dehydroepiandrosterone on testicular torsion/detorsion in rats. Andrologia 40:38–43.
- Zini, A. (2011) Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med 57:78–85.