Abstract
Adrenomedullin (ADM) is found in male accessory sex glands and is part of the seminal secretion. It plays an important role in protecting the sperm in the female reproductive tract. In this study, we investigated the roles of ADM in inflammation and oxidative stress in the endometrium and in leukocyte and macrophage infiltration in the endometrial stroma. The expression of the ADM gene in the ventral prostate, coagulating gland, and seminal vesicle was determined by real time PCR. The peptide levels in the tissue and secretion were measured using an EIA Kit. The highest ADM mRNA and peptide levels were found in the ventral prostate. Most of the ADM in the seminal vesicle was stored in the tissue while little was secreted. The expression of the IL-1β gene and the secretion of TNFα and IL-6 in uterine tissue decreased significantly after treatment with ADM for 4 hours. Using an immunostaining method, the levels of leukocyte and macrophage infiltration were found to be lower at 24 hours post coitus than 1.5 hours post coitus. The infusion of ADM receptor antagonist reduced the infiltration of leukocyte and macrophages in the endometrial stroma at 24 hours post coitus. As to the anti-oxidative effect of ADM in the female tract, the reactive oxygen species (ROS) level in isolated endometrial epithelial cells was significantly decreased after treatment with ADM or seminal fluid. Our findings demonstrated that ADM in the seminal secretion may modify the inflammatory responses, play an anti-oxidative role, and increase leukocyte and macrophage infiltration in the uterus.
Introduction
Adrenomedullin (ADM) is classified as one of the members in the calcitonin/calcitonin-gene-related peptide (CGRP)/amylin family [Kitamura et al. Citation1993]. ADM mediates its biological response by binding to the calcitonin receptor-like receptor (CALCRL) and receptor activity modifying proteins (Ramp-1, -2, and -3) [Hay et al. Citation2004]. We have shown that ADM inhibits steroidogenesis [Li et al. Citation2008; Chan et al. Citation2008a] but stimulates inhibin production in the testis [Chan et al. Citation2008b] and stimulates epididymal anion transport and fluid secretion [Hwang et al. Citation2003]. In the female, it stimulates steroidogenesis in the rat ovary [Li et al. Citation2008], increases ciliary beat frequency in both human [Chiu et al. Citation2010] and rat [Li et al. Citation2010] oviducts, and inhibits oviductal contraction [Liao et al. Citation2011].
The secretion of the male accessory sex glands nourishes the sperm and serves as the medium for the sperm transportation [Robertson Citation2007]. ADM is secreted by the prostate and its level in the seminal fluid correlates positively with sperm motility [Marinoni et al. Citation2005]. After ejaculation accessory sex gland secretion enters the uterus with the sperm. As a source of invasive antigens, the seminal secretion was reported to cause inflammation and oxidative stress in the epithelial cells of the endometrium [Marinoni et al. Citation2007]. To offset these, components of accessory sex glands secretion protect the integrity of the sperm DNA [Chen et al. Citation2002; Chen et al. Citation2003]. ADM produced by the ventral prostate, seminal vesicle, and coagulating glands [Li et al. Citation2007] may be one of them. Whether ADM in the seminal secretion has any action in the female reproductive tracts to facilitate successful fertilization and pregnancy outcome is not known. In this study, we have measured ADM levels both inside the gland and in the secretion of the ventral prostate, coagulating gland, and the seminal vesicle of the rat and looked at the effects of ADM against inflammation and oxidative stress in the uterine tissue.
Results
ADM gene expression in the accessory sex glands
The highest Adm mRNA level in the tissue (A) and peptide level (C) in the secretion were found in the ventral prostate. Adm mRNA and peptide levels in the coagulating gland were relatively low (P < 0.01, Kruskal Wallis test), while most of the ADM was secreted into the seminal fluid (Fig. 1B, C). Seminal vesicle Adm mRNA was also low (P < 0.01, Kruskal Wallis test). Most of the ADM peptide was inside the seminal vesicle tissue and only a small amount was secreted out into the medium (P < 0.01, Kruskal Wallis test) (Fig. 1B, C). On a per mg protein basis, the secretion from the ventral prostate has the highest level of ADM. However, on a per mg fluid basis, the highest level was found in the secretion of the coagulating gland. The values for the ventral prostate, the coagulating gland, and the seminal vesicle were 4.71 ± 1.37, 50.0 ± 7.2, and 0.36 ± 0.03 fg/mg, respectively.
Figure 1. ADM gene expression and peptide level in male accessory sex glands. Gene expression level of ADM (A) and ADM peptide levels in the secretion (C) were highest in ventral prostate. Tissue, while little was secreted out ADM peptide was highest in seminal vesicle (B) among all the glands, while little was secreted out (C). ADM: adrenomedullin; *P < 0.01, vs. ventral prostate, #P < 0.01, vs. coagulating glands, Kruskal Wallis test; data presented as Mean ±SEM; N = 8 for tissue, N = 5 for the secretion
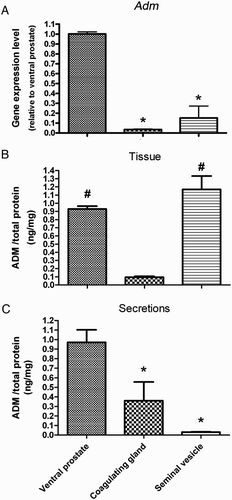
Figure 2. Cytokine mRNA expression levels in uterus tissue treated with different dosages of ADM for 4 hours in vitro. TNFα (A) and IL-6 (C) gene expression remained unchanged after treatment with ADM while IL-1β gene expression decreased significantly (B). ADM: adrenomedullin; TNF: tissue necrosis factor; IL: interleukin; P < 0.05, Newman-Keuls Multiple post test; data presented as Mean ±SEM; N = 5.
ADM decreased cytokine expression or secretion in the uterus tissue
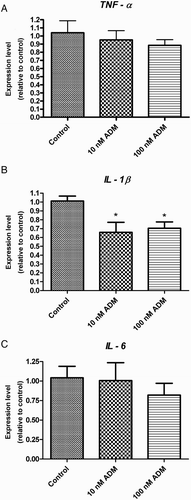
After incubation with ADM for 4 hours, there was a significant decrease in the mRNA level of IL-1β in the uterus tissue (B) and in the secretion of TNFα and IL-6 (A and C) (P < 0.05, Newman-Keuls Mutiple Comparison test).
Figure 3. Percentage changes of cytokines in the culture medium after treatment with ADM for 4 hours in vitro. TNF α (A) and IL-6 (C) secretion was significantly decreased after treatment with ADM (*P < 0.05 vs. control, #P < 0.01 vs. control, +P < 0.01 vs. 10 nM ADM, Newman-Keuls Multiple post test). There was no difference in the IL-1β secretion (B) after ADM treatment. ADM: adrenomedullin; TNF: tissue necrosis factor; IL: interleukin; data were presented as Mean ±SEM; N = 9.
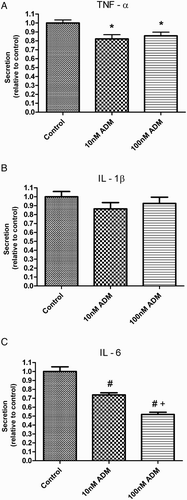
ADM receptor antagonists decreased the leukocyte and macrophage infiltration in the post coitus uterine tissue
Both the CD 45 positive leukocyte and CD 68 positive macrophage were found in the endometrial stroma, especially underneath the epithelium. The number of leukocytes (A) and macrophages (B) were lower at 24 hpc (hours post coitus) than at 1.5 hpc in all treatments (P < 0.01; post-hoc LSD test). There was no significant difference among the different treatment groups at 1.5 hpc However, the number of leukocytes and macrophages decreased significantly in the group treated with the ADM receptor antagonist (hADM22-52) at 24 hpc (P < 0.05, LSD post-hoc test).
Figure 4. Number of leukocytes and macrophages in post coital (pc) uterus segment at different treatment groups. The number of leukocytes (A) and macrophages (B) were lower at 24 hpc than that at 1.5 hpc (*P < 0.01, LSD test).Infusion with the ADM receptor blocker decreased the number of the leukocytesand macrophages at 24 hpc (#P < 0.05, LSD test). ADM: adrenomedull in; data were presented as Mean ±SEM; N = 10.
ADM decreased oxidative stress in isolated endometrial epithelial cells
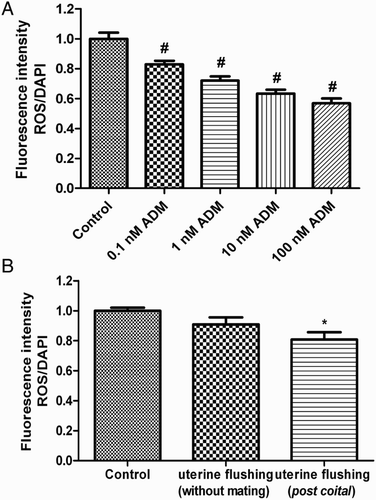
The levels of reactive oxygen species (ROS) in the endometrial epithelial cells after hydrogen peroxide treatment were significantly decreased by incubation with ADM (0.1-100 nM) or diluted post coital uterine flushing () (P < 0.05, Newman-Keuls Multiple Comparison test).
Figure 5. ROS/DAPI fluorescence intensity ratios in isolated uterine epithelial cells treated for 2 hours in vitro. ADM significantly decreased the ROS level in the endometrial epithelial cells (A) (#P < 0.01 vs. control, one way ANOVA with Newman-Keuls multiple post test). ROS level in the epithelial cells decreased after post coital uterine fluid treatment (B). ROS: reactive oxygen species; ADM: adrenomedullin; (*P < 0.05 vs. control, one way ANOVA with Newman-Keuls multiple post test); N = 7.
Discussion
The highest level of Adm mRNA was found in the ventral prostate. This is in line with the previous finding of a much higher level of Adm mRNA in the ventral prostate than in the seminal vesicles [Pewitt et al. l999]. Marinoni et al. [2007] have suggested that the prostate is the major source of seminal fluid ADM in the human. ADM in the prostate may increase blood flow [Shibata et al. Citation2006], and decrease the smooth muscle contraction [Ventura et al. Citation2000], leading to an accumulation of fluid inside the gland. Our results show that much of the ADM in the coagulating gland is secreted while relatively little is secreted from the seminal vesicle. The high level of ADM in the seminal vesicle may be important for the inhibition of contraction as we have found such an effect on NE-induced contraction (Liao, O, and Tang, in press).
Seminal fluid ADM may play an anti-oxidative role in the female reproductive tract. Hydrogen peroxide is a well-documented agent for the generation of intracellular ROS [Barbieri et al. Citation2003] and as shown above in cultured endometrial cells, our result suggests that ADM may modify the level of ROS in the female reproductive tract. Thus ADM may protect the sperm from oxidative damage caused by ROS generated by leukocytes and dead spermatozoa in the ejaculate [Hammadeh et al. Citation2009] or by the uterus [Jain et al. Citation2000]. Post-coital uterine flushing was also found to decrease ROS, in agreement with our previous finding of a protective role of accessory gland secretion against oxidative stress in the sperm [Chen et al. Citation2002].
ADM significantly decreased the level of IL-1β mRNA and the secretion of TNFα and IL-6 in uterine fragments at 4 hour post treatment. A decrease in secretion together with a lack of change in mRNA may suggest an increase in TNFα and IL-6 in the tissue. TNFα and IL-1β are pro-inflammatory while IL-6 is anti-inflammatory [Tilg et al. Citation1997], as IL-6 may inhibit TNFα and IL-1β production [Di Santo E et al. 1997]. The secretion of TNFα decreased in spite of a decrease in IL-6, resulting in an overall anti-inflammatory effect. This may play a significant role in limiting the extent of inflammation brought about by the sperm and the seminal fluid.
Contrary to expectation, blocking ADM action at 1.5 hpc did not increase leukocyte and macrophage infiltrations in the uterus, which are signs of inflammation in response to semen [Marinoni et al. Citation2007] or semen proteins [Sanford et al. Citation1992; Robertson et al. Citation1996]. ADM is known to have an early inflammatory effect by increasing lipopolysaccharide (LPS)-induced macrophage migration inhibitory factor (MIF) in macrophages [Wong et al. Citation2005]. Blocking ADM at 24 hpc, however, did lead to a decrease in leukocyte and macrophage infiltration, with the implication that ADM may be inflammatory. As noted above, the seminal fluid is a source of ADM. Whether there is any increase in ADM production by the endometrium after copulation is not known. The increase in lymphocyte and macrophage infiltration between 1.5 and 24 hpc may be due to other unkown factors.
In conclusion, our findings suggest that ADM may have two different actions: an early action which is anti-inflammatory (as shown by a decrease in TNFα secretion at 4 hours after ADM treatment) to limit the extent of inflammation brought about by the sperm and the seminal fluid, and a late inflammatory action (as evidenced by an increase in leukocytic and macrophagic infiltration with hADM22-52 treatment at 24 hpc). The significance of this remains to be elucidated.
Materials and Methods
Animals
Adult Sprague-Dawley rats (male, 8-10 w; female, 7-9 w) were obtained from the Laboratory Animal Unit, Faculty of Medicine, of the University of Hong Kong. All procedures had been approved by the Committee on the Use of Live Animals for Teaching and Research, the University of Hong Kong, and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Science).
Determination of Adrenomedullin mRNA and peptide level in the accessory sex gland secretion by real time RT-PCR and EIA
The rats were sacrificed by an intraperitoneal injection of sodium pentobarbitone at 200 mg/kg body weight and the ventral prostate, coagulating glands, and seminal vesicles were dissected out. The total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystem, Foster City, CA, USA). Two μg total RNA in a total volume of 20 µl of master mix was incubated for 10 min at 25°C, 2 h at 37°C and 5 sec at 85°C. The concentration of MultiScribeTM Reverse Transcriptase was 2.5 U/µl. The primer sequences, target lengths, and accession numbers of Adm, cytokines, and β-actin were shown in . Real time PCR was conducted using iQ TMSYBR Green supermix under the following condition: 95°C for 10 min for denaturing, followed by 40 cycles of 95°C for 30 sec, 57°C for 30 sec, and 72°C for 30 sec, and extension for 10min. The MyiQTM Real time PCR Detection system was used to perform real-time PCR (Bio-Rad Laboratories, Hercules, CA, USA). Standard curves for each primer pair were prepared using serial dilutions of cDNA to determine the PCR efficiency. The relative quantities of Adm and cytokine mRNAs to β-actin RNA in each sample were calculated using the 2 (-Delta Delta Cq) method where Cq is the threshold cycle and were expressed as fold changes relative to the ventral prostate or to the control group (cytokine study). Melt curve analysis for each primer set revealed only one peak for each product. The qPCR results were analyzed using the Bio-Rad iQ5-standard edition, version 2.1.94.617, (Bio-Rad laboratories).
Table 1. Primers for amplification of genes using real time PCR.
The secretions of ventral prostate, coagulating gland, and seminal vesicle were aspirated with a pasteur pipette. The tissues and secretions were stored at -70°C for future peptide extraction. The frozen samples were homogenized in 2N acetic acid on ice, followed by boiling for 10 min. A 50 µl was taken out for protein assay and the remaining homogenate was centrifuged at 17,000 x g for 20 min at 4°C. The supernatant was lyophilized overnight and stored at -20°C until assay. The fluids inside the glands were diluted in 0.1N trifluoracetic acid and centrifuged at 17,000 x g at 4°C for 1 h and the supernatants were purified by Sep-pak C-18 (Waters Corporation, Milford, MA, USA). The ADM level was measured using an ADM EIA assay Kit (Phoenix Pharmaceuticals Inc, Burlingame, CA, USA). The amount of protein in the sample was measured using a protein assay reagent from Bio-Rad Laboratories (Hercules, CA, USA) and a spectrophotometer (Analytical Instruments, LLC, Golden Valley, MN, USA). ADM levels in the tissues were expressed as ng/mg protein and in the secretion as ng/mg protein or as per mg fluid.
Effect of ADM on TNFα, IL-6 and IL-1β cytokines level in the uterine tissue
Female SD rats aged 7-9 w were injected with PMSG (150 IU/kg), followed by hCG (75 IU/kg) 48 h later. The uterus was dissected out 24 h after hCG injection and cut longitudinally into 2-3 mm pieces and weighed. To each well of a 4-well culture dish (Nunc, Roskilde, Denmark) 4-5 fragments were distributed, followed by incubation at 37°C for 1 h in DMEM/F12 (GIBCO, Invitrogen Corporation) supplement with 10% FBS (GIBCO, Invitrogen Corporation). ADM (0, 10, 100 nM) was added into the new medium for 4 h at 37°C. The medium was collected for cytokine measurement using TNFα, IL-6 and IL-1β ELISA kit (R&D Systems, Inc. Minneapolis, MN, USA). The uterine fragments were collected for real time RT-PCR of these cytokines as previously described.
Effect of ADM on leukocyte and macrophage infiltration in post coital uterus
Female SD rats were primed with PMSG (150 IU/kg) and hCG (75 IU/kg) as described above. On the day of hCG injection, an alzet osmotic pump (model 1003D, Alzet, Durect Corporation, Cupertino, California, USA) was implanted underneath the skin in the dorsal neck region under anesthesia to deliver 250 µg/rat/d of a ADM receptor antagonist or CGRP receptor antagonist or vehicle (sterile normal saline, 0.9% NaCl) at a rate of 1 µl per h. The adult male rat and female rat were allowed to mate for 1 h on the morning after hCG injection and the female rat was sacrificed at 1.5 h or 24 hpc. The uterus sample obtained was fixed in glutaldehyde and paraffin-embedded. Sections of 5 µm thickness were cut on a microtome and dewaxed, rehydrated, and treated with 3% H2O2 in methanol for 30 min. The sections were pretreated in 1% BSA, 0.3% SDS, 2% Triton X-100 in 0.01 M PBS at 37°C for 30 min and blocked with a blocking reagent for 1 h at room temperature. Mouse primary antibody against the macrophage marker (anti-rat CD68) or the leukocyte marker (anti-rat CD45) was added in PBS (at 1:500 dilution) overnight at 4°C. Both anti-sera were from AbD Serotec, Oxford, UK. After washing, the slides were incubated with biotinylated secondary antibody (1:200 goat anti-mouse ABC staining system) at room temperature for 30 min. The slides were washed with PBS for 15 min and incubated with ABC reagent for 30 min at room temperature. After washing with PBS for 15 min, the DAB solution was added to develop a brown color for 5-10 min. The reaction was stopped with PBS and followed by washing with distilled water. The slides were counterstained in Eosin for 1 min and mounted in permount. The images of uterine section were taken using a Zeiss Axioplan Microscope equipped with a Spot RT digital camera system at a magnification of 20x (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). Positive cells in each of the sections were counted and normalized with tissue area.
ADM and uterine flushing decreased the ROS in endometrial epithelial cells
Seven to nine w old female rats were primed with the PMSG and hCG as described above. The uterus were dissected out and cut open longitudinally. The uterus were digested with 10 ml DMEM/F12 medium containing 5 mg trypsin, 25 mg Pancreatin, 0.16 mg DNase I and supplemented with 100 IU/ml penicillin G and 100 µg/ml streptomycin, 2.5 ml/L Fungizone, at 4°C with continuous shaking for 30 min. After continued digestion for 15 min at room temperature, the digestion was stopped by adding an equal volume of DMEM/F12 with 10% fetal bovine serum (FBS) for 10 sec. The medium containing the endothelial epithelial cells were passed through a 100 µM cell strainer (BD Falcon, BD, Franklin Lakes, NJ, USA) to remove cell debris. The cells were washed twice and seeded at a density of 1x 105 per well in a collagen pre-coated 96 well culture plate (IWAKI, Asahi Glass Co., Ltd, Tokyo, Japan).
After overnight incubation with DMEM/F12 without phenol red, the cells were washed and incubated for 30 min with ADM (0, 10, 100 nM) or uterine flushing from the mated female rat. Then oxidative stress was induced by incubation with 1 µM H2O2 for 2 h at 37 °C. The ROS in cells were stained by incubating them with 5 µM H2O2 sensitive fluorophore (CM-H2DCFDA) for 30 min at 37°C. After washing with a fresh medium, the increase in fluorescent intensity was measured by a microplate reader (VICTOR 3TM Multilabel Counter, PerkinElmer, Waltham, MA) with an excitation filter (485 nm) and an emission filter (535 nm) within 20 min. The fluorescent intensity was normalized with cell numbers as determined by DAPI nuclear staining.
Statistics
Data were presented as Mean ± SEM. Expression of the ADM gene in the ventral prostate, coagulating gland, and seminal vesicle was compared by Kruskal Wallis test, Dunns multiple post hoc test. The gene expression and cytokine secretion of IL-1β, TNFα, and IL-6 in the uterus and the ROS levels in the endometrial epithelial cells were subjected to 1-way ANOVA, then Newman Keuls post hoc test. Statistical analyses were performed using Graphpad Prism 4.0 (Graphpad Software Inc., La Jolla, CA, USA). A comparison of the leukocyte and macrophage infiltration between groups at 24 h was done by using 2-way ANOVA, then LSD post hoc test, SPSS.
Declaration of Interest: This study was substantially supported by a grant from the Research Grant Council of the Hong Kong Special Administrative Region, China (HKU 775908). The authors have no declarations of interest.
Authors contribution
Conceived and designed the experiment: Wai Sum O. Performed the experiments: Lowell HM Kong, Su Bin Liao. Contributed reagents/materials/analysis tools: Wai Sum O, Fai Tang. Wrote and revised the manuscript: Su Bin Liao, Fai Tang, Wai Sum O.
Abbreviations
| ADM: | = | adrenomedullin |
| hCG: | = | human chorionic gonadotropin |
| IL: | = | interleukin |
| PMSG: | = | pregnant mare serum gonadotropin |
| ROS: | = | reactive oxygen species |
| TNF: | = | tissue necrosis factor. |
References
- Barbieri, S.S., Eligini, S., Brambilla, M., Tremoli, E. and Colli, S. (2003) Reactive oxygen species mediate cyclooxygenase-2 induction during monocyte to macrophage differentiation: critical role of NADPH oxidase. Cardiovas Res 60:187–197.
- Chan, Y.F., O, W.S. and Tang, F. (2008a) Adrenomedullin in the rat testis I: its production, actions on inhibin secretion, regulation by follicle-stimulating hormone, and its interaction with endothelin 1 in the Leydig cell. Biol Reprod 78:773–779.
- Chan, Y.F., Tang, F. and O, W.S. (2008b) Adrenomedullin in the rat testis II: its production, actions on inhibin secretion, regulation by follicle-stimulating hormone, and its interaction with endothelin 1 in the Sertoli cell. Biol Reprod 78:780–785.
- Chen, H., Chow, P.H., Cheng, S.K., Cheung, A.L., Cheng, L.Y. and O, W.S. (2003) Male genital tract antioxidant enzymes: their source, function in the female, and ability to preserve sperm DNA integrity in the golden hamster. J Androl 24:704–711.
- Chen, H., Cheung, M.P., Chow, P.H., Cheung, A.L., Liu, W. and O, W.S. (2002) Protection of sperm DNA against oxidative stress in vivo by accessory sex gland secretions in male hamsters. Reproduction 124:491–499.
- Chiu, P.C., Liao, S., Tam, W.W., Tang, F., Ho, J.C., Ho, P.C., (2010) Adrenomedullin regulates sperm motility and oviductal ciliary beat via cyclic adenosine 5'-monophosphate/protein kinase A and nitric oxide. Endocrinology 151:3336–3347.
- Di Santo, E., Alonzi, T., Poli, V., Fattori, E., Toniatti, C., Sironi, M., (1997) differential effects of IL-6 on systemic and central production of TNF: a study with IL-6 deficient mice. Cytokine 9:300–306.
- Hammadeh, M.E., Fillippos, A. and Hamad, M.F. (2009) Reactive oxygen species and antioxidant in seminal plasma and their impact on male fertility. Int J Fertil Steril 3:87–110.
- Hay, D.L., Conner, A.C., Howitt, S.G., Smith, D.M. and Poyner, D.R. (2004) The pharmacology of adrenomedullin receptors and their relationship to CGRP receptors. J Mol Neurosci 22:105–113.
- Hwang, I.S., Autleitano, D.J., Wong, P.Y., Leung, G.P. and Tang, F. (2003) Coexpression of adrenomedullin and adrenomedullin receptors in rat epididymis: distinct physiological actions on anion transport. Biol Reprod 68:2005–2012.
- Jain, S., Saxena, D., Kumar, G.P. and Laloraya, M. (2000) NADPH dependent superoxide generation in the ovary and uterus of mice during estrous cycle and early pregnancy. Life Sci 66:1139–1146.
- Kitamura, K., Kangawa, K., Kawamoto, M., Ichiki, Y., Nakamura, S., Matsuo, H., (1993) Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun192:553–560.
- Li, H.W., Liao, S.B., Chiu, P.C., Tam, W.W., Ho, J.C., Ng, E.H., (2010) Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium. J Clin Endocrinol Metab 95:E18–25.
- Li, Y.Y., Li, L., Hwang, I.S., Tang, F. and O, W.S. (2008) Coexpression of adrenomedullin and its receptors in the reproductive system of the rat: effects on steroid secretion in rat ovary. Biol Reprod 79:200–208.
- Li, Y.Y., O, W.S. and Tang, F. (2007) Effect of aging on the expression of adrenomedullin and its receptor component proteins in the male reproductive system of the rat. J Gerontol A Biol Sci Med Sci 62(12):1346–1351.
- Liao, S.B., Ho, J.C., Tang, F. and O, W.S. (2011) Adrenomedullin increases ciliary beat frequency and decreases muscular contraction in the rat oviduct. Reproduction 141:367–372.
- Liao, S.B., O, W.S. and Tang, F. (2012) Adrenomedullin inhibits norepinephrine-induced contraction of rat seminal vesicles. Urology (In Press)
- Marinoni, E., Di Iorio, R., Villaccio, B., Vellucci, O., Di Netta, T., Sessa, M., (2005) Adrenomedullin in human male reproductive system. Eur J Obstet Gynecol Reprod Biol 122:195–198.
- Marinoni, E., Vellucci, O., Letizia, C., Sessa, M., Moscarini, M. and Di Iorio, R. (2007) The level of adrenomedullin immunoreactivity in seminal fluid is higher in oligozoospermic subjects and correlated with semen biochemical parameters. Eur J Obstet Gynecol Reprod Biol 131:169–175.
- Pewitt, E.B., Haleem, R. and Wang, Z. (1999) Adrenomedullin gene is abundantly expressed and directly regulated by androgen in the rat ventral prostate. Endocrinology 140:2382–2386.
- Robertson, S.A. (2007) Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci 85:E36–44.
- Robertson, S.A., Mau, V.J., Tremellen, K.P. and Seamark, R.F. (1996) Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil 107:265–277.
- Sanford, T.R., De, M. and Wood, G.W. (1992) Expression of colony-stimulating factors and inflammatory cytokines in the uterus of CD1 mice during days 1 to 3 of pregnancy. J Reprod Fertil 94:213–220.
- Shibata, Y., Kashiwagi, B., Arai, S., Magarai, T., Suzuki, K. and Honma, S. (2006) Participation of adrenomedullin and its relation with vascular endothelial growth factor in androgen regulation of prostatic blood flow in vivo. Urology 68:1127–1131.
- Tilg, H., Dinarello, C.A. and Mier, J.W. (1997) IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today 18:428–432.
- Ventura, S., Lau, W.A., Buljubasich, S. and Pennefather, J.N. (2000) Calcitonin gene-related peptide (CGRP) inhibits contractions of the prostatic stroma of the rat but not the guinea-pig. Regul Pept. 28:63–73.
- Wong, L.Y., Cheung, B.M., Li, Y.Y. and Tang, F. (2005) Adrenomedullin is both pro-inflammatory and anti-inflammatory: its effects on gene expression and secretion of cytokines and macrophage migration inhibitory factor in NR8383 macrophage cell line. Endocrinology 146:1321–1327.