Abstract
Sperm function is essential for fertilization and embryogenesis yet semen contain a heterogeneous population of sperm. This study was designed to evaluate two different sperm populations separated by the density gradient method. Semen from 25 idiopathic normozoospermic infertile men was processed by double density gradient centrifugation and evaluated for sperm present in the 50% (upper) layer and the 90% (lower) layer for reactive oxygen species (ROS), sperm chromatin integrity, and morphology. The population of sperm in the 90% layer showed significantly lower ROS levels (22.90 (0.92, 85.32) vs. 382.03 (158.30, 1409.51) and lower DNA fragmentation index (DFI) (24.26 (22.54, 25.50) vs. 29.93 (28.48, 31.25) and higher number of sperm with normal morphology (55 (45.0, 60.0) vs. 32.5 (20, 40) compared to sperm in the 50% layer. However, in the original raw semen, sperm DFI (27.02 (26.19, 27.76)) and percentage high DNA stainability (% HDS) (3.1 (2.40, 3.78)) cells were significantly higher compared to the 90% layer population. Density gradient separation of the sperm subpopulation from the original semen favors the selection of sperm with genome integrity, low levels of ROS, and normal morphology. Therefore presence of pathological sperm in the semen may disrupt the function of normal spermatozoa, and hence the selection of the normal sperm subpopulation may be a better candidate for assisted conception. Further studies are required to evaluate the gradient separated sperm population in assisted reproductive techniques (ART).
Introduction
Infertility is a major reproductive health problem that affects 1 in 7 couples and male factor accounts for about half of the cases [Dada et al. Citation2008; Tuttelmann et al. Citation2011]. In approximately fifty percent of these cases the exact cause is not known and hence they are categorized as idiopathic. Increased levels of reactive oxygen species (ROS) and abnormal sperm chromatin structure/impaired sperm DNA integrity are believed to be major etiological factors in idiopathic infertility [Bungum et al. Citation2011; Gharagozloo and Aitken Citation2011; Venkatesh et al. Citation2011a]. Numerous groups have documented high ROS levels in ejaculate of infertile men with normal and abnormal sperm parameters [Kothari et al. Citation2010; Venkatesh et al. Citation2009]. Supraphysiological levels of ROS impair sperm function by reacting with polyunsaturated fatty-acids and may cause mitochondrial and nuclear DNA damage [Rivlin et al. Citation2004; Venkatesh et al. Citation2011b]. Similarly sperm DNA fragmentation has been reported as one of the major reasons for impaired fertility in idiopathic cases. Moreover, the use of sperm with an increased DNA fragmentation index (DFI) in assisted reproductive technology (ART) may result in the birth of offspring with increased incidence of congenital malformations, cancer, and epigenetic defects [Aitken et al. Citation2003; Lewis et al. Citation2008; Palermo et al. Citation2008]. In a large cohort of cases it was reported that the risk for abnormalities like childhood malignancies was increased in offspring conceived by ART in cases with increased sperm DNA damage [Roman et al. Citation1997]. Sperm DNA damage measured by TUNEL and the acridine orange assay revealed that greater than 12% sperm with DNA damage resulted in no pregnancy by intrauterine insemination (IUI) [Duran et al. Citation2002]. Similarly in another study sperm DNA fragmentation measured by sperm chromatin structure assay (SCSA) showed a significantly lower pregnancy rate if the DFI is >27% [Saleh et al. Citation2003]. Though numerous studies have evaluated the ROS levels and sperm DNA damage in the whole semen, data of levels of ROS and DFI in different sperm populations in the semen remains unclear. Therefore, it is important to distinguish sperm subpopulations which produce normal or high levels of ROS so that segregation of sperm producing supraphysiological levels of free radicals may not induce oxidative DNA damage in sperm subpopulations with normal ROS levels.
Various procedures like swim up, density and percoll gradient centrifugation are used to select good quality spermatozoa for ART [Allamaneni et al. Citation2005a; Ebner et al. Citation2011; Henkel and Schill Citation2003]. However, swim up protocols result in retrieval of motile sperm; whereas density gradient separation is typically not dependent on motile sperm and hence, can also be applied to asthenozoospermic samples. Though the separated better quality sperm look morphologically normal, its DNA quality and ROS levels are not known. Normal ROS levels and sperm DNA integrity are critical determinants of reproductive potential and the birth of healthy offspring. Therefore this study was planned to evaluate sperm parameters, ROS levels, and sperm DNA integrity in two different sperm populations separated by density gradient centrifugation.
Results
The semen parameters, ROS levels, sperm DFI, and percent high DNA stainability (HDS) of the original samples obtained from the infertile men were tabulated (). Among the sperm parameters, sperm morphology was evaluated in the sperm separated by the density gradient method. The population of sperm separated in the lower layer showed significantly better morphology compared to the upper layer and the original semen ().
Table 1. Sperm parameters of raw semen, 50%, and 90% gradient separated semen sample.
ROS
The levels of ROS in the lower gradient layer were found to be significantly (P < 0.05) lower compared to the levels of ROS in the upper gradient layer (). However, the levels of ROS in the two sperm subpopulations were higher compared to the ROS levels of original neat semen (). This may be because neat semen contains both enzymatic and non-enzymatic antioxidants in the seminal plasma. The median ROS levels in the upper layer with abnormal sperm morphology was approximately 16 fold higher than in the lower layer sperm population with normal morphology.
DFI
In the sperm subpopulation separated by gradient centrifugation, the lower layer (90% layer) showed a significantly (P < 0.001) lower DNA fragmentation index (24.26) compared to the upper layer (29.93) (50% layer) and the raw semen (27.02) (). The density gradient centrifugation separated sperm subpopulation have less DNA damage (90% lower layer) than the original semen, which has comparatively more DNA damage (). HDS cells are those which take more DNA staining and are considered to be immature germ cells. The presence of HDS cells were also found to be significantly (p < 0.0001) higher in the upper gradient layer compared to the lower gradient layer and the original semen (). The density gradient separated sperm population showed minimum and maximum DNA damage in the lower and upper gradient layers, respectively ().
Figure 1. Box plot distribution of percent high DNA staining (% HDS) cells from original semen, lower and upper gradient layers. The lower layer showed significantly (p < 0.0001) lower % HDS cells compared to upper layer. The box plot displays the 25th percentile, median, and 75th percentile. The circle represents outlier. The horizontal lines outside the box display minimum and maximum value.
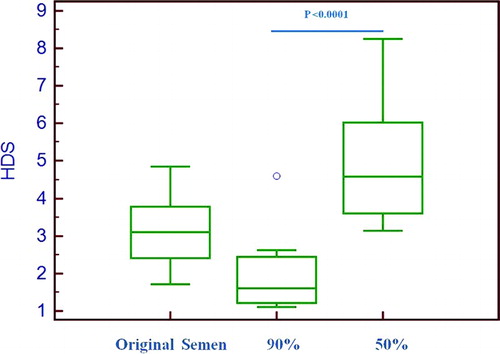
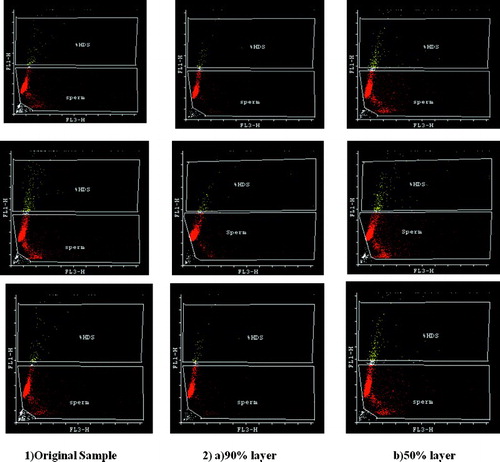
Figure 2. The sperm chromatin structure assay (SCSA) representative flow cytograms gated for sperm and high DNA stainability cells (HDS) from: 1) Original semen showing larger sperm population shifted towards X-axis (fragmented DNA) and 2) Density gradient separated: a) 90% layer showing less number of sperm population towards X-axis and b) 50% layer semen showing large number of sperm shifted towards X-axis.
Discussion
Assisted reproductive techniques have revolutionized the management of male factor infertility. But despite professional expertise and state of the art technology, the percentage of live birth rates in ART is low [Simon et al. Citation2011a]. The majority of men with idiopathic infertility have compromised sperm DNA quality and raised ROS levels [Agarwal et al. Citation1994; Bungum, et al. Citation2011] . As semen contains a heterogeneous mixture of both good and poor quality sperm, it is important to differentiate these classes since increased levels of ROS from one subpopulation can oxidatively damage normal sperm. In ART settings it is always preferred to retrieve good quality spermatozoa [de Lamirande and Gagnon Citation1995]. The major findings in this study highlight the need to segregate the normal sperm fraction from the population of sperm that produce high levels of ROS and may induce oxidative damage to sperm with normal function. This is important in samples being prepared for ART to prevent oxidative damage to sperm with normal function/morphology. Seminal plasma is rich in both enzymatic and non-enzymatic antioxidants which neutralize excess ROS produced by the spermatozoa. During ART, the semen is processed by washing after removing the seminal plasma. The removal of seminal plasma may increase ROS induced injury as sperm are deficient in cytosolic antioxidants. The speed and duration of centrifugation may also artificially aggravate the production of ROS due to heat and mechanical sheering forces generated during centrifugation. Normal sperm produce higher levels of ROS following centrifugation as compared to sperm with impaired motility and abnormal morphology [Agarwal et al. Citation1994].
Numerous methods have been used to separate normal spermatozoa prior to ART, e.g., discontinuous percoll density gradient centrifugation [Chen and Bongso Citation1999], sperm selection through albumin gradient [Soderlund and Lundin Citation2000], glass wool filtration [Grunewald et al. Citation2007], and the swim up technique [Paasch et al. Citation2007]. Most commonly swim up and density gradient separation are employed to separate good quality sperm [Allamaneni et al. Citation2005b; Jackson et al. Citation2010; Li et al. Citation2011]. However swim-up requires motile sperm and yields a lower number of sperm as compared to density gradient where a greater number of sperm are retrieved [Allamaneni et al. Citation2005b]. Though various methods are used to select spermatozoa they are merely based on their characteristic features with respect to separation media, the quality of sperm population separated with respect to sperm DNA integrity and ROS levels in the sperm remain unclear. Therefore we evaluated the quality of different sperm subpopulations separated by density gradient centrifugation for ROS levels and DFI, which are important biomarkers of male infertility [Simon et al. Citation2011b; Venkatesh et al. Citation2009].
The two sperm subpopulations obtained from the gradient separation showed differences in their morphology, ROS, and DFI (). The sperm population in the lower 90% gradient layer was morphologically (microscopic) normal and the absence of other somatic cells and debris was noted. The sperm population in the upper 50% layer contained morphologically abnormal cells including immature germ cells compared to the lower (90%) layer. Immature germ cells were identified by their more intense nuclear staining due to improper chromatin condensation as defined by HDS cells (). They may produce higher levels of ROS [Gil-Guzman et al. Citation2001]. In accord, the 50% layer that contained the abnormal sperm cells produced high levels of ROS and had higher DFI (). However, the DFI levels in the 90% layer were within the normal range of the fertile controls (25.65) as reported in our previous study [Venkatesh et al. Citation2011b]. They showed significantly lower levels of DFI than the original semen. The use of this sperm population may yield a better success rate by ART. This study also highlights that following sperm washing, the two sperm subpopulations may be segregated so that high levels of ROS produced by morphologically abnormal sperm do not induce oxidative DNA damage to the normal sperm population, which is especially important in sperm being processed for ART [Bakos et al. Citation2008; Erenpreiss et al. Citation2008]. Though the levels of ROS in the 90% layer are significantly lower than the 50% layer (16 fold higher), these levels are significantly higher than the ROS levels of the original semen. This may be due to the removal of seminal plasma from the original semen, which contains an array of ROS neutralizing antioxidants. Studies have shown that infertile men have reduced seminal antioxidant capacity compared to controls [Adeel et al. Citation2011]. Therefore supplementation with antioxidants is recommended in the semen sample after washing and centrifugation [Zelen et al. Citation2011] during ART selection. Recent studies have also reported that supplementation of various antioxidants both in vivo and in vitro showed improvement in the semen quality [Armstrong et al. Citation1998; Chi et al. Citation2008; Moslemi and Tavanbakhsh Citation2011; Nadjarzadeh et al. Citation2011].
Though density gradients typically employ centrifugation to separate sperm cells of good quality, centrifugation itself may be harmful to ejaculated spermatozoa and may be a disadvantage of the current method [Mortimer Citation1994]. However, the use of the density gradient technique yields a clean sperm, fraction eliminates leukocytes to a large extent, and eliminates sperm producing high levels of ROS [Henkel and Schill Citation2003]. A possible way to minimize the effect of ROS production during centrifugation is supplementing the separation media with suitable ROS neutralizing antioxidants. Moreover it has also been reported that various methods of separating spermatozoa have different capacities to eliminate apoptotic germ cells [Marti et al. Citation2006]. Apoptotic germ cells may also be present in the semen due to various environmental stresses, and tissue injury which may decrease fertility potential. Also persistence of sperm DNA damage increases mutation load in every cell of the embryo which could result in miscarriage or even lead to cancer in the offspring [Aitken and Krausz Citation2001]. A recent report suggests that sperm samples prepared by density gradient method have more stable DNA than other methods [Zhang et al. Citation2011]. Therefore sperm cells isolated in the lower gradient layer by density gradient centrifugation have better chromatin structure and thus using this sperm subpopulation may improve the success rate of ART.
Materials and Methods
Study population
The study included 25 idiopathic infertile men with normal sperm parameters. They were in the age range of 25 to 35 y. The study was approved by the ethical committee of Institutional Review Board (IEC/NP-127/2010) and detailed informed consent was obtained from each subject. Physical examination was performed to exclude cases with known causes of infertility such as varicocele, cryptorchidism, and hypospadias.
Semen analysis
Semen analysis was carried out as per WHO [1999] guidelines. Semen sample was collected and analyzed for sperm parameters as described previously [Venkatesh et al. Citation2011c]. From each ejaculate one ml of semen was taken for the density-gradient centrifugation separation. Leukocytospermic and highly viscous samples were excluded from the study.
Density gradient centrifugation
ISolate density gradient media (Irvine Scientific, CA, USA) was used in the study as per the manufacturer's protocol. ISolate is a membrane filtered, aseptically processed colloidal suspension of silica particles stabilized with covalently bound hydrophilic silane in HEPES-buffered HTF. Using a sterile pipette, 2ml of the lower layer (90% ISolate gradient) was first placed in the 15 ml conical tube and 2ml of upper layer (50% ISolate gradient) was gently placed on top of the lower layer. One ml of liquefied semen sample was placed on top of the upper layer and centrifuged at 300 × g for 15 min. The resulting cells in the two layers were carefully separated and washed with sperm washing media at 300g for 10 min. Finally the pellet was resuspended in phosphate buffer saline (pH 7.4) and immediately used for further evaluation like sperm concentration, sperm morphology, and ROS. However, an aliquot was stored at -80°C for sperm chromatin structure assay. Chemiluminescence assay was performed immediately after processing sperm samples.
ROS measurement by chemiluminescence assay
Assessment of ROS was performed by chemiluminescence assay as described previously [Athayde et al. 2007; Venkatesh et al. Citation2011c]. ROS levels in the samples were expressed as 104 relative light units (RLU) per 20 x106 sperm.
Sperm chromatin structure (SCSA) assay and DFI calculation
Sperm chromatin structure assay was employed using Flow cytometry to find the DFI levels in each sample as described earlier [Evenson et al. Citation1999; Venkatesh et al. Citation2011b]. The aliquot from each ejaculate stored at -80°C after semen analysis was thawed at 37°C before analysis. The quality control was maintained by performing SCSA analysis of fertile semen samples that was aliquoted and stored at -80°C. For every 10 samples one reference sample was analyzed to ensure the quality control. Post acquisition, the data was analyzed using Cyflogic Flow cytometry software (CyFlo Ltd, Turku, Finland) to find out the DFI levels, where DFI= (red fluorescence/red fluorescence + mean green fluorescence) x 100. The % HDS were also recorded in each sample manually from the graph plot.
Statistical analysis
Sperm parameters, ROS levels, DFI, and % HDS between sperm population from upper and lower density gradient layer and original semen were compared by Kruskal-Wallis test. However, Mann-Whitney test was used to compare ROS levels, DFI, and % HDS between sperm subpopulations from upper and lower density gradient layers. Since most of the data were not distributed normally, a non-parametric statistics was used. The values were expressed as median and interquartile range. P < 0.05 was considered as statistically significant. Statistical analysis was performed using MedCalc trial version for Windows, (MedCalc Software, Mariakerke, Belgium).
Declaration of interest: The authors are grateful to the Department of Biotechnology (DBT), and the Indian Council of Medical Research, (ICMR), India for partial financial support. The authors report no conflicts of interest.
Author Contributions: JT – Performed all research experiments and wrote the manuscript; SV- Performed sperm DNA damage assessment and statistical analysis; RK- Performed clinical evaluation of all subjects; RD- Designed study, gave critical inputs during experimental work and in the manuscript.
Abbreviations
| ROS: | = | Reactive oxygen species |
| DFI: | = | DNA fragmentation index |
| ART: | = | assisted reproductive technology |
| HDS: | = | high DNA stainability |
| IUI: | = | intrauterine insemination |
| ICSI: | = | intracytoplasmic sperm injection |
| WHO: | = | World Health Organization. |
References
- Adeel, A.L., Jahan, S., Subhan, F., Alam, W. and Bibi, R. (2011) Total anti-oxidant status: a biochemical predictor of human male fertility. Andrologia Epub 2011 Aug 2.
- Athayde, K.S., Cocuzza, M., Agarwal, A., Krajcir, N., Lucon, A.M., Srougi, M., (2007) Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl 28:613–620.
- Agarwal, A., Ikemoto, I. and Loughlin, K.R. (1994) Effect of sperm washing on levels of reactive oxygen species in semen. Arch Androl 33:157–162.
- Aitken, R.J., Baker, M.A. and Sawyer, D. (2003) Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online 7:65–70.
- Aitken, R.J. and Krausz, C. (2001) Oxidative stress, DNA damage and the Y chromosome. Reproduction 122:497–506.
- Allamaneni, S.S., Agarwal, A., Nallella, K.P., Sharma, R.K., Thomas, A.J., and Sikka, S.C. (2005a) Characterization of oxidative stress status by evaluation of reactive oxygen species levels in whole semen and isolated spermatozoa. Fertil Steril 83:800–803.
- Allamaneni, S.S., Agarwal, A., Rama, S., Ranganathan, P. and Sharma, R.K. (2005b) Comparative study on density gradients and swim-up preparation techniques utilizing neat and cryopreserved spermatozoa. Asian J Androl 7:86–92.
- Armstrong, J.S., Rajasekaran, M., Hellstrom, W.J. and Sikka, S.C. (1998) Antioxidant potential of human serum albumin: role in the recovery of high quality human spermatozoa for assisted reproductive technology. J Androl 19:412–419.
- Athayde, K.S., Cocuzza, M., Agarwal, A., Krajcir, N., Lucon, A.M., Srougi, M., (2007) Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl 28:613–620.
- Bakos, H.W., Thompson, J.G., Feil, D. and Lane, M. (2008) Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl 31:518–526.
- Bungum, M., Bungum, L. and Giwercman, A. (2011) Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 13:69–75.
- Chen, M.J. and Bongso, A. (1999) Comparative evaluation of two density gradient preparations for sperm separation for medically assisted conception. Hum Reprod 14:759–764.
- Chi, H.J., Kim, J.H., Ryu, C.S., Lee, J.Y., Park, J.S., Chung, D.Y., (2008) Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum Reprod 23:1023–1028.
- Dada, R., Kumar, R., Shamsi, M.B., Tanwar, M., Pathak, D., Venkatesh S, (2008) Genetic screening in couples experiencing recurrent assisted procreation failure. Indian J Biochem Biophys 45:116–120.
- de Lamirande, E. and Gagnon, C. (1995) Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med 18:487–495.
- Duran, E.H., Morshedi, M., Taylor, S. and Oehninger, S. (2002) Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 17:3122–3128.
- Ebner, T., Shebl, O., Moser, M., Mayer, R.B., Arzt, W. and Tews, G. (2011) Easy sperm processing technique allowing exclusive accumulation and later usage of DNA-strandbreak-free spermatozoa. Reprod Biomed Online 22:37–43.
- Erenpreiss, J., Elzanaty, S. and Giwercman, A. (2008) Sperm DNA damage in men from infertile couples. Asian J Androl 10:786–790.
- Evenson, D.P., Jost, L.K., Marshall, D., Zinaman, M.J., Clegg, E., Purvis, K., (1999) Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 14:1039–1049.
- Gharagozloo, P. and Aitken, R.J. (2011) The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 26:1628–1640.
- Gil-Guzman, E., Ollero, M., Lopez, M.C., Sharma, R.K., Alvarez, J.G., Thomas, A.J., (2001) Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod 16:1922–1930.
- Grunewald, S., Miska, W., Miska, G., Rasch, M., Reinhardt, M., Glander, H.J., (2007) Molecular glass wool filtration as a new tool for sperm preparation. Hum Reprod 22:1405–1412.
- Henkel, R.R. and Schill, W.B. (2003) Sperm preparation for ART. Reprod Biol Endocrinol 1:108.
- Jackson, R.E., Bormann, C.L., Hassun, P.A., Rocha, A.M., Motta, E.L., Serafini, P.C., (2010) Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil Steril 94:2626–2630.
- Kothari, S., Thompson, A., Agarwal, A. and du Plessis, S.S. (2010) Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol 48:425–435.
- Lewis, S.E., Agbaje, I. and Alvarez, J. (2008) Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med 54:111–125.
- Li, Z., Zhou, Y., Liu, R., Lin, H., Liu, W., Xiao, W., (2011) Effects of semen processing on the generation of reactive oxygen species and mitochondrial membrane potential of human spermatozoa. Andrologia Epub 2011 Jul 6.
- Marti, E., Perez-Pe, R., Muino-Blanco, T. and Cebrian-Perez, J.A. (2006) Comparative study of four different sperm washing methods using apoptotic markers in ram spermatozoa. J Androl 27:746–753.
- Mortimer, D. (1994) Sperm recovery techniques to maximize fertilizing capacity. Reprod Fertil Dev 6:25–31.
- Moslemi, M.K. and Tavanbakhsh, S. (2011) Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med 4:99–104.
- Nadjarzadeh, A., Sadeghi, M.R., Amirjannati, N., Vafa, M.R., Motevalian, S.A., Gohari, M.R., (2011) Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in Idiopathic Oligoasthenoteratozoospermia: a Randomized Double- blind, Placebo Controlled Trial. J Endocrinol Invest 34(8):e224–228
- Paasch, U., Grunewald, S. and Glander, H.J. (2007) Sperm selection in assisted reproductive techniques. Soc Reprod Fertil Suppl 65:515–525.
- Palermo, G.D., Neri, Q.V., Takeuchi, T., Squires, J., Moy, F. and Rosenwaks, Z. (2008) Genetic and epigenetic characteristics of ICSI children. Reprod Biomed Online 17:820–833.
- Rivlin, J., Mendel, J., Rubinstein, S., Etkovitz, N. and Breitbart, H. (2004) Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod 70:518–522.
- Roman, E., Ansell, P. and Bull, D. (1997) Leukaemia and non-Hodgkin's lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer 76:406–415.
- Saleh, R.A., Agarwal, A., Nada, E.A., El-Tonsy, M.H., Sharma, R.K., Meyer, A., (2003) Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 79 Suppl 3:1597–1605.
- Simon, L., Castillo, J., Oliva, R. and Lewis, S.E. (2011a) Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod Biomed Online 23(6):724–734.
- Simon, L., Lutton, D., McManus, J. and Lewis, S.E. (2011b) Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril 95:652–657.
- Soderlund, B. and Lundin, K. (2000) The use of silane-coated silica particles for density gradient centrifugation in in-vitro fertilization. Hum Reprod 15:857–860.
- Tuttelmann, F., Simoni, M., Kliesch, S., Ledig, S., Dworniczak, B., Wieacker, P., (2011) Copy number variants in patients with severe oligozoospermia and sertoli-cell-only syndrome. PLoS One 6:e19426.
- Venkatesh, S., Riyaz, A.M., Shamsi, M.B., Kumar, R., Gupta, N.P., Mittal, S., (2009) Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia 41:251–256.
- Venkatesh, S., Shamsi, M.B., Dudeja, S., Kumar, R. and Dada, R. (2011a) Reactive oxygen species measurement in neat and washed semen: comparative analysis and its significance in male infertility assessment. Arch Gynecol Obstet 283:121–126.
- Venkatesh, S., Singh, A., Shamsi, M.B., Thilagavathi, J., Kumar, R., Mitra, D.K., (2011b) Clinical Significance of Sperm DNA Damage Threshold Value in the Assessment of Male Infertility. Reprod Sci. 18:1005–1013.
- Venkatesh, S., Shamsi, M.B., Deka, D., Saxena, V., Kumar, R. and Dada, R. (2011c) Clinical implications of oxidative stress & sperm DNA damage in normozoospermic infertile men. Indian J Med Res 134:396–398.
- WHO (1999) WHO laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. World Health Organization, Cambridge University Press.
- Zelen, I., Mitrovic, M., Jurisic-Skevin, A. and Arsenijevic, S. (2011) Activity of superoxide dismutase and catalase and content of malondialdehyde in seminal plasma of infertile patients. Med Pregl 63:624–629.
- Zhang, X.D., Chen, M.Y., Gao, Y., Han, W., Liu, D.Y. and Huang, G.N. (2011) The effects of different sperm preparation methods and incubation time on the sperm DNA fragmentation. Hum Fertil (Camb) 14:187–191.