Abstract
Several studies have reported improved IVF by shortening the time of sperm-oocyte coincubation from 16–18 hours to 1–4 hours. The objective of this study was to examine the advantages and disadvantages of a shortened sperm-oocyte coincubation time in order to assess the effects of this insemination method for clinical IVF practice. Two insemination methods, the shortened method (4 hours) and the standard method (16–18 hours) of coincubation of sperm-oocytes for two groups of patients based on the quality of sperm were compared. Group I, was composed of couples without male factor; Group II, involved couples with mild male factor. Fertilization, good quality embryos, clinical pregnancy, and implantation rates were compared by two different insemination methods. In Group I, fertilization, clinical pregnancy, and implantation rates were not different between the two insemination methods. However, the polyspermy rate was significantly higher (P < 0.05) in the shortened (7.3%) than in the standard (4.1%) insemination method. In Group II, the fertilization rate was significantly lower (P < 0.05) using the shortened insemination method (62.6%) compared to the standard insemination method (68.7%). When fertilization failed with the shortened insemination method, the clinical pregnancy and implantation rates were 34.7% and 24.1%, respectively, from the rescue intracytoplasmic sperm injection (ICSI). The live birth rate from the rescue ICSI was 32.0% with normal infants. The duration of sperm-oocyte coincubation does not affect fertilization, embryo quality, clinical pregnancy, and implantation rates. However, fertilization rates will decrease with the shortened insemination method when the sperm parameters are poor. From the results of the present study we suggest that the combination of the shortened sperm-oocyte coincubation and rescue ICSI method may be an efficient method for IVF treatment in order to prevent fertilization failure when sperm parameters were poor as mild male factor.
Introduction
The traditional in vitro fertilization (IVF) technique co-incubates sperm-oocytes for 16-18 hours in varying volumes of culture medium with or without a paraffin (or mineral oil) overlay. This method has been challenged for improvement, because it has been indicated that reactive oxygen species (ROS) released by the cellular metabolism affects membrane fluidity [Aitken and Clarkson Citation1987; Aitken Citation1994] and DNA fragmentation [Twigg et al. Citation1998] as well as early embryonic development [Gianaroli et al. Citation1996a; Dimfeld et al. 1999; Bedaiwy et al. Citation2004]. Several studies reported that one of the improvement options for IVF technique is shortening the time of sperm-oocyte coincubation from 16-18 hours to 1-4 hours [Gianaroli et al. Citation1996a; Gianaroli et al. 1996; Quinn et al. Citation1998; Katterra and Chen 2003; Bungum et al. Citation2006]. Nevertheless, some reports indicated that equivalent fertilization, embryo quality, and pregnancy rates are obtained from both shortened and standard insemination methods [Coskun et al. Citation1998; Lin et al. Citation2000; Swenson et al. Citation2000] suggesting that there are no beneficial effects of a shortened sperm-oocyte coincubation time in terms of embryo quality and pregnancy rates.
Interestingly Lundqvist et al. [2001] reported a decreased fertilization rate in the shortened sperm-oocyte coincubation compared to the standard 18 hours of sperm-oocyte coincubation, which is supported by recent reports from Barraud-Lange et al. [2008], indicating that a shortened insemination method does not improve embryo quality and pregnancy rates. Therefore, it is unclear whether IVF techniques should adopt the shortened coincubation of sperm-oocytes to improve the fertilization, embryo quality, and pregnancy rates. The objective of this study was to assess the advantages and disadvantages of the shortened coincubation time of sperm-oocytes in order to discern the beneficial effects of the shortened insemination method for clinical IVF practice.
Results
As shown in for the Group I couples (without male factor), fertilization, production of a good quality embryo, clinical pregnancy, and implantation rates were no different between the two insemination methods. However, the rate of polyspermy was significantly higher (P < 0.01) in the shortened coincubation of sperm-oocytes (7.3%) than in the standard coincubation of sperm-oocytes (4.1%). As shown in for Group II couples (mild male factor) the yield of good quality embryos, clinical pregnancy, and implantation rates were not different between the shortened and the standard insemination methods, the fertilization rate was significantly lower (P < 0.05) in the shortened insemination method (62.6%) compared to the standard insemination method (68.7%).
Table 1. Comparison of fertilization, embryo quality, pregnancy and implantation rates in the different durations of sperm-oocyte co-incubation with Group I patients.
Table 2. Comparison of fertilization, embryo quality, pregnancy and implantation rates in the different durations of sperm-oocyte coincubation in Group II patients.
Of 1,699 couples with 1,738 IVF treatment cycles with the shortened coincubation of sperm-oocytes, 77 patients (4.5%) have had rescue ICSI performed and 75 patients completed the treatment cycles (). The fertilization rate was 71.3% and the polyspermy rate was 3.2% following rescue ICSI. Following embryo transfer, the clinical pregnancy and implantation rates were 34.7% and 24.1%, respectively. The live birth rate was 32.0% with a rate of 11.5% miscarriage.
Table 3. Pregnancy and implantation outcome in patients from rescue ICSI.
A total of 31 infants were born with an average of 37.5 wk ± 1.1 gestation period (). From 24 live births with 31 infants, an average of 3,103 ± 466 g birth weight was observed with no birth defects. Karyotype analysis showed all babies to be normal.
Table 4. Obstetric and perinatal outcome of the patients who had live births from rescue ICSI.
Discussion
The results from the present study indicate that there were no differences in terms of fertilization, embryo quality, clinical pregnancy and implantation rates between the two insemination methods when sperm parameters were normal (). It has been reported that more than 80% of oocytes were penetrated by a sperm after a 1 hour exposure to a suitable concentration of spermatozoa in vitro [Plachot et al. Citation1986; Wassarman Citation1987] and completed within 2 to 4 hours [Nagy et al. Citation1994; Payne et al. Citation1997; Plachot et al. Citation1988]. Therefore, it seems that a prolonged sperm-oocyte coincubation is not necessary for IVF. Also the coincubation time can be shortened to 30 seconds which could reduce the rate of polyspermy when the proper sperm concentration was used for the insemination [Bungum et al. Citation2006]. Interestingly, in the results of the present study, the polyspermy rate was higher in the shortened sperm-oocyte coincubation than in the standard sperm-oocyte coincubation (). We do not know exactly why it occurred, but it may be due to the manipulation of the fine denuding pipette to remove the cumulus cells from the oocytes. The procedure of repeated passage of the oocyte through the fine diameter pipette might enhance non-specific attachment of sperm penetrating the oocyte.
Although some reports indicated that short exposure of oocytes to sperm produce better quality embryos [Gianaroli et al. Citation1996a; Gianaroli et al. Citation1996b; Quinn et al. Citation1998; Katterra and Chen 2003; Bungum et al. Citation2006], the results from the present study did not find differences in the embryos obtained between these two insemination methods regardless of sperm quality (Tables and ). It has been reported that the similar fertilization, embryo quality, and pregnancy rates were achieved from both shortened and standard sperm-oocyte coincubation [Coskun et al.,Citation1998; Lin et al. Citation2000; Swenson et al. Citation2000] indicating that there is no extra beneficial effect of a shortened insemination method. However, the results of the present study indicate that the fertilization rate significantly decreased when sperm quality was relatively poor (), which supports previous reports [Lundqvist et al. Citation2001; Barraud-Lange et al. Citation2008].
Cohen et al. [1992] reported that maternal age has a strong correlation with embryo quality and zona pellucida (ZP) thickness. Interestingly, Dirnfeld et al. [2003] reported that ZP is significantly thinner after ICSI than after standard IVF, suggesting that there is a positive effect of a short exposure of the oocytes to sperm on embryo quality and ZP thickness. However, the results of the present study, did not find differences in embryo quality after fertilization between those two insemination methods. Taken together, it seems that the fertilization outcome is related directly to sperm quality and concentrations used, because the fertilization rate decreased when the poor sperm parameters were used in the shortened coincubation of sperm-oocytes ().
The optimal sperm concentration for IVF varies. It has been considered to be from 2 x 104 to 1 x 106 motile sperm/mL depending on insemination method. It is well known that the higher concentration of sperm with the standard insemination method results in increased polyspermy rate in animals and humans [Chian et al. Citation1992; Dumoulin et al. Citation1992; Kattera and Chen Citation2003]. In the present study, we used a concentration of 12–15 x 104 motile sperm/mL in 0.6 mL of fertilization medium with 2 cumulus oocyte complexes (COCs) in a 4-well dish. We believe that this is an optimal sperm concentration for the standard sperm-oocyte coincubation, because the polyspermy rates were 4.1% and 4.4%, respectively, in two groups of patients (Tables and ). Interestingly, the polyspermy rate was similar between the two insemination methods in Group II, although the final fertilization rate was significantly lower in the shorted sperm-oocyte coincubation than in the standard sperm-oocyte coincubation ().
After the first trial year, in the second year, all IVF treatment cycles were performed with the shorted sperm-oocyte coincubation in order to prevent fertilization failure. We observed that fertilization of individual oocyte can be identified 4 hours after sperm-oocyte coincubation based on the presence of 2 polar bodies (PB) in the perivitelline space (PVS) (). Therefore, early rescue ICSI is an option for the treatment cycles in which none of oocytes inspected 6 hours after insemination presented the second polar body. The results from the present study indicate that this is an efficient method to prevent fertilization failure for IVF treatment, although the proportion (4.5%) of the fertilization failure may not be large (). It was surprising to observe 3.2% polyspermy in the oocytes where rescue ICSI was performed. This is also higher than in vitro matured oocytes [Yang et al. Citation2012]. It is unclear why this was observed with the rescued oocytes. It might be possible that some oocytes were penetrated by a sperm but did not extrude 2PB yet after 4 hours of coincubation at the time of rescue ICSI. However, the exact reason why polyspermy was observed requires confirmation. The results from clinical pregnancy and implantation rates as well as the obstetric and perinatal outcome indicate that the combination of the shorten sperm-oocyte coincubation and rescue ICSI may be an efficient method for insemination with IVF treatment cycles (), which can prevent fertilization failure when the sperm parameters were poor.
Figure 1. Human oocyte with 2 polar bodies extruded into perivitelline space, observed at 4 hours after oocyte-sperm coincubation. Scale bar indicates 40 µm.
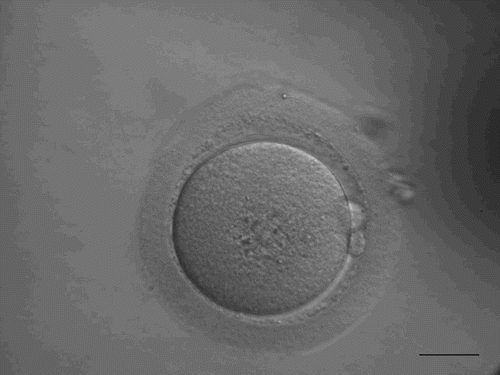
In conclusion, the present study demonstrates that the time of sperm-oocyte coincubation does not affect fertilization, embryo quality, clinical pregnancy and implantation rates when the sperm quality is good. However, the rate of fertilization will decrease using a shortened sperm-oocyte coincubation method when the sperm parameters are poor. Therefore, it is important to employ the combination of the shortened sperm-oocyte coincubation and rescue ICSI method in order to prevent fertilization failure with patients with mild male factor.
Materials and Methods
Patients and study design
A total of 2,130 couples who underwent 2,169 IVF treatment cycles from January 2009 to December 2010 were recruited in this study. All couples signed the informed consent form, and the hospital of Institutional Review Board approved the study. The couples with severe male factor have been excluded from this study.
In the first trial year, 431 couples with 431 treatment cycles were divided into two groups based on quality of sperm as the following criteria: Group I, female patients under 35 years of age, with less than 5 years of infertility duration, and without male factor [WHO Citation1999], and Group II, female patients under 35 years of age, less than 5 years of infertility period, and with mild male factor but suitable for IVF without ICSI. Mild male factor was defined when sperm concentration ranged from 10 x 106 sperm/mL to 20 x 106 sperm/mL and the sperm motility ranged from 10% to 25% with a normal morphology range from 15% to 30% [WHO Citation1999]. In each group, the retrieved COCs were inseminated randomly by two different insemination methods: i) shortened coincubation of sperm-oocyte of 4 h, and ii) standard coincubation of sperm-oocytes of 16–18 h.
Based on the results of the first trial year, we found that it is possible to identify whether the oocytes were fertilized or not 4 h after sperm-oocytes coincubation. Therefore, in the second trial year, 1,699 couples with 1,738 cycles of IVF treatment were inseminated with the shortened coincubation of sperm-oocytes for 4 h. If the oocytes did not have the second PB extrusion 4 h after sperm-oocyte coincubation, the oocytes were rechecked again 2 h later to confirm whether or not there was the 2PB extrusion (Fig.1). At this point, if 2PB was not observed in any oocytes, rescue ICSI was performed for those oocytes. The fertilization check was performed 10-12 h after rescue ICSI was performed.
Controlled ovarian stimulation and egg retrieval
All patients received gonadotropin-releasing hormone agonist (Decapeptyl, Ipsen, Paris, France) for pituitary down-regulation in the middle of the luteal phase of the last menstrual cycle. Recombinant FSH (Serono Laboratories, Switzerland) or HMG (Ferring GmbH, Germany) were administered in a fixed dosage for the first couple days and then modified to each patient's response, which was monitored by daily hormone assessment and ultrasound scanning. When at least one leading follicle reached 18 mm or two reached 17 mm in diameter, 10,000 IU of human chorionic gonadotropin (HCG, Serono Laboratories, Switzerland) was administered. Oocyte retrieval was performed with trans-vaginal ultrasound guided aspiration needle post 34-36 h of HCG injection.
Sperm preparation
All semen samples were collected, in sterile containers, by masturbation. After liquefaction at room temperature, samples were assessed for sperm density, motility, and morphology. Gradient separation (Coopersurgical/SAGE, USA) were used with a centrifuge for 15 min at 300 × g. The sperm pellets were washed twice by Sperm Washing Medium (Coopersurgical/SAGE, USA) and centrifuged for 5 min at 250 × g. The sperm pellets were resuspended in 2 mL Fertilization Medium (Coopersurgical/SAGE, USA) and incubated at 37°C until insemination.
Insemination
A final concentration of 12–15 x 104 motile sperm/mL in the 4-well Falcon (Becton Dickinson and Company, USA) dishes containing 0.6 mL of Fertilization Medium (Coopersurgical/SAGE) with two COCs in each well was used for insemination at 37°C, 6% CO2 incubator. In the shortened coincubation, post 4 h of sperm-oocyte coincubation, COCs were denuded delicately from the cumulus cells and sperm using a fine diameter pipette, and the denuded oocytes were transferred to 50 µL of Cleavage Medium (Coopersurgical/SAGE) droplets under paraffin oil. In standard coincubation, 2 COCs were coincubated for 16-18 h, and then the COCs were denuded gently from the cumulus cells and sperm using fine diameter pipette. The denuded oocytes were transferred to 50 µL of Cleavage Medium.
Fertilization and embryonic development culture
Fertilization was checked in both insemination methods under invert microscope. In the shortened insemination method, the oocytes were checked 4 h after sperm-oocyte coincubation for a confirmation of the extrusion of the second PB, and another confirmation was done in the next morning for the presence of two pronuclei (2PN). For the standard insemination method, the fertilization was checked by the presence of 2PN and 2PB at 16-18 h after sperm-oocyte coincubation.
The fertilized zygotes were cultured further in Cleavage Medium for 48-50 h after insemination. The developed embryos were graded according to the following criteria: Grade 1, even-sized and symmetrical blastomeres with no obvious fragmentation, Grade 2, uneven sized blastomeres, or a total cytoplasmic mass containing <10% fragmentation, Grade 3, 10%-50% cytoplasm fragmentation, and Grade 4, >50% cytoplasmic fragmentation. Grade 1 and grade 2 embryos were considered good quality embryos.
Embryo transfer and luteal support
All embryo transfers were conducted under ultrasound guidance with flexible catheter (Wallace, Smith Medical International Inc., U.K.). Luteal phase support was supplemented with 60mg/d of pure progesterone in oil from the day of oocyte retrieval. Oral progesterone (Dydrogesterone, Solvay Pharmaceuticals, Holland) were also added starting on day 3 of post-oocyte retrieval. Clinical pregnancy was confirmed by the observation of gestational sac using trans-vaginal ultrasonography after 5 w of embryo transfer.
Statistical analysis
All data were analyzed using SPSS 13.0 software /PC Statistics package (SPSS Inc., Chicago, IL, USA). Data expressed as mean ± SD were analyzed by Independent-Samples T Test. Data expressed as counts and percentages were analyzed by the exact χ2 or Fisher's exact test. A probability value < 0.05 was considered to be statistically significant.
Declaration of interest: The authors, Shan-Jun Dai, Yu-Huan Qiao, Hai-Xia Jin, Zhi-Min Xin, Ying-Chun Su, Ying-Pu Sun, Ri-Cheng Chian have no declarations of interest.
Author contributions: Performed primarily the experiments, S-JD; Performed ICSI, Y-HQ, H-XJ; Performed embryo transfer, Z-MX and Y-CS; Designed and supervised the experiments, interpreted the results, and wrote the manuscript, Y-PS and R-CC.
Abbreviations:
| ICSI: | = | intracytoplasmic sperm injection |
| IVF: | = | in vitro fertilization |
| ROS: | = | reactive oxygen species |
| ZP: | = | zona pellucida |
| COCs: | = | cumulus-oocyte complexes |
| PB: | = | polar body |
| PVS: | = | perivitelline space |
| HCG: | = | human chorionic gonadotropin |
| 2PN: | = | two pronuclei. |
References
- Aitken, R.J. (1994) A free radical theory of male infertility. Reprod Fertil Dev 6:19–23; discussion 23–14.
- Aitken, R.J. and Clarkson, J.S. (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 81:459–469.
- Barraud-Lange, V., Sifer, C., Pocate, K., Ziyyat, A., Martin-Pont, B., Porcher, R., (2008) Short gamete co-incubation during in vitro fertilization decreases the fertilization rate and does not improve embryo quality: a prospective auto controlled study. J Assist Reprod Genet 25:305–310.
- Bedaiwy, M.A., Falcone, T., Mohamed, M.S., Aleem, A.A., Sharma, R.K., Worley, S.E., (2004) Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril 82:593–600.
- Bungum, M., Bungum, L. and Humaidan, P. (2006) A prospective study, using sibling oocytes, examining the effect of 30 seconds versus 90 minutes gamete co-incubation in IVF. Hum Reprod 21:518–523.
- Chian, R.C., Niwa, K. and Nakahara, H. (1992) Effect of sperm penetration in vitro on completion of first meiosis by bovine oocytes arrested at various stages in culture. J Reprod Fertil 96:73–78.
- Cohen, J., Alikani, M., Reing, A.M., Ferrara, T.A., Trowbridge, J. and Tucker, M. (1992) Selective assisted hatching of human embryos. Ann Acad Med Singapore 21:565–570.
- Coskun, S., Roca, G.L., Elnour, A.M., al Mayman, H., Hollanders, J.M. and Jaroudi, K.A. (1998) Effects of reducing insemination time in human in vitro fertilization and embryo development by using sibling oocytes. J Assist Reprod Genet 15:605–608.
- Dirnfeld, M., Bider, D., Koifman, M., Calderon, I. and Abramovici, H. (1999) Shortened exposure of oocytes to spermatozoa improves in-vitro fertilization outcome: a prospective, randomized, controlled study. Hum Reprod 14:2562–2564.
- Dirnfeld, M., Shiloh, H., Bider, D., Harari, E., Koifman, M., Lahav-Baratz, S., (2003) A prospective randomized controlled study of the effect of short coincubation of gametes during insemination on zona pellucida thickness. Gynecol Endocrinol 17:397–403.
- Dumoulin, J.C., Bras, M., Land, J.A., Pieters, M.H., Enginsu, M.E., Geraedts, J.P., (1992) Effect of the number of inseminated spermatozoa on subsequent human and mouse embryonic development in vitro. Hum Reprod 7:1010–1013.
- Gianaroli, L., Cristina Magli, M., Ferraretti, A.P., Fiorentino, A., Tosti, E., Panzella, S., (1996a) Reducing the time of sperm-oocyte interaction in human in-vitro fertilization improves the implantation rate. Hum Reprod 11:166–171.
- Gianaroli, L., Fiorentino, A., Magli, M.C., Ferraretti, A.P. and Montanaro, N. (1996b) Prolonged sperm-oocyte exposure and high sperm concentration affect human embryo viability and pregnancy rate. Hum Reprod 11:2507–2511.
- Kattera, S. and Chen, C. (2003) Short coincubation of gametes in in vitro fertilization improves implantation and pregnancy rates: a prospective, randomized, controlled study. Fertil Steril 80:1017–1021.
- Lin, S.P., Lee, R.K., Su, J.T., Lin, M.H. and Hwu, Y.M. (2000) The effects of brief gamete co-incubation in human in vitro fertilization. J Assist Reprod Genet 17:344–348.
- Lundqvist, M., Johansson, U., Lundkvist, O., Milton, K., Westin, C. and Simberg, N. (2001) Reducing the time of coincubation of gametes in human in-vitro fertilization has no beneficial effects. Reprod Biomed Online 3:21–24.
- Nagy, Z.P., Liu, J., Joris, H., Devroey, P. and Van Steirteghem, A. (1994) Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod 9:1743–1748.
- Payne, D., Flaherty, S.P., Barry, M.F. and Matthews, C.D. (1997) Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Reprod 12:532–541.
- Plachot, M., Junca, A.M., Mandelbaum, J., Cohen, J., Salat-Baroux, J. and Da Lage, C. (1986) Timing of in-vitro fertilization of cumulus-free and cumulus-enclosed human oocytes. Hum Reprod 1:237–242.
- Plachot, M., Veiga, A., Montagut, J., de Grouchy, J., Calderon, G., Lepretre, S., (1988) Are clinical and biological IVF parameters correlated with chromosomal disorders in early life: a multicentric study. Hum Reprod 3:627–635.
- Quinn, P., Lydic, M.L., Ho, M., Bastuba, M., Hendee, F. and Brody, S.A. (1998) Confirmation of the beneficial effects of brief coincubation of gametes in human in vitro fertilization. Fertil Steril 69:399–402.
- Swenson, K., Check, J.H., Summers-Chase, D., Choe, J.K. and Check, M.L. (2000) A randomized study comparing the effect of standard versus short incubation of sperm and oocyte on subsequent pregnancy and implantation rates following in vitro fertilization embryo transfer. Arch Androl 45:73–76.
- Twigg, J., Fulton, N., Gomez, E., Irvine, D.S. and Aitken, R.J. (1998) Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod 13:1429–1436.
- Wassarman, P.M. (1987) The biology and chemistry of fertilization. Science 235:553–560.
- WHO (1999) WHO laboratory manual for examination of human semen and semen-cervical mucus interaction, Cambridge University Press, Cambridge, UK.
- Yang, S.H., Patrizio, P., Yoon, S.H., Lim, J.H. and Chian, R.C. (2012) Comparison of pregnancy outcomes in natural cycle IVF/M treatment with or without mature oocytes retrieved at time of egg collection. Syst Biol Reprod Med 58:154–159.