Abstract
The aim of this study was to determine if a relationship exists between the levels of sperm DNA fragmentation and necrospermia in infertile men. Semen samples obtained from 70 men consulting for infertility evaluation were analyzed according to World Health Organization (WHO) guidelines. Patients were subdivided into three groups according to the percentage of necrotic spermatozoa: normozoospermia (<30%; n = 20), moderate necrozoospermia (50-80%; n = 30), and severe necrozoospermia (>80%; n = 20). DNA fragmentation was detected by the terminal desoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL) assay. The sperm DNA fragmentation index (DFI) was 9.28 ± 2.98% in patients with a normal level of necrotic spermatozoa, 20.25 ± 3.21% in patients with moderate necrozoospermia, and 35.31 ± 5.25% in patients with severe necrozoospermia. There was a statistically significant increase of DNA fragmentation in the necrozoospermic group (P < 0.01). A strong correlation was found between the degree of necrozoospermia and sperm DNA fragmentation. We concluded that patients with necrozoospermia showed a high level of DNA fragmentation compared to normozoospermic men. Severe necrozoospermia (>80%) is a predictive factor for increased sperm DNA damage.
Introduction
Male related factors continue to be among the common causes of infertility. They are solely responsible for about 20% of cases of infertility [Thonneau et al. Citation1991]. Assessment of male infertility has traditionally been based on semen analysis classified according to WHO [1999] standards. Necrospermia is defined as sperm degeneration and death that occur when sperm mixes with seminal plasma at ejaculation [Wilton et al. Citation1988]. Necrospermia is still a poorly documented cause of male infertility, with a reported incidence in the literature of 0.2% to 0.4%, and relates to a large variety of etiologies, including infections [Nduwayo et al. Citation1995], chronic medical conditions [Fang and Baker Citation2003; Mallidis et al. Citation2000], and exposure to toxic substances [Lerda and Rizzi Citation1991]. Unexplained necrospermia has been termed epididymal necrospermia.
Several hypotheses have been put forth to explain the relationship between necrospermia and DNA fragmentation realizing that DNA fragmentation would be the final step before the death of spermatozoa [Irvine et al. Citation1994]. In contrast to the somatic cells, the sperm DNA is highly condensed because of the replacement of histones by protamines and increased disulfide bond formation [Philpott and Leno Citation1992]. Normal sperm DNA is highly resistant to physical or chemical denaturation and features a doughnut-shape configuration that is resistant to DNA damage during sperm transport [Bianchi et al. Citation1996]. DNA strand breaks in human spermatozoa are indicators of deteriorating function. Sperm DNA damage may occur by at least one of three important mechanisms. First, defective chromatin condensation during spermiogenesis may be able to induce DNA damage and exert a negative impact on male fertility [Sakkas Citation1999]. Second, apoptosis may be initiated during spermatogenesis or transport of sperm through the male genital tract [Shen and Ong Citation2000]. In addition, such apoptosis may lead to functional elimination of possibly defective germ cells from the genetic pool. Third, oxidative stress mainly resulting from reactive oxygen species arises internally or externally [Agarwal and Saleh Citation2002; O'Brien and Zini Citation2005]. Several studies showed that the process of DNA fragmentation in spermatozoa continues even after ejaculation [Muratori et al. Citation2003; Gosálvez et al. Citation2009a]. Indeed, Muratori et al. [2003] demonstrated that in vitro incubation of swim up selected human spermatozoa in human tubal fluid (HTF) medium without the addition of external factors results in a progressive increase in the percentage of DNA fragmentation sperm. Similar results were observed in mammalian species after several hours of sperm incubation at 37°C, where the basal values of sperm DNA fragmentation tend to increase [López-Fernández et al. Citation2008; Cortés-Gutiérrez et al. Citation2008]. Moreover, sperm viability was shown to decrease rapidly after ejaculation. Cortés-Gutiérrez at al. [2008] showed that sperm viability decreased dramatically after 4 hours at 37°C, and viability was completely lost after 24 hours . For these reasons and in order to obtain more accurate results, sperm DNA fragmentation and sperm viability were assessed within one hour after ejaculation. In the present study we examine the relationship between sperm DNA fragmentation measured by TUNEL assay and necrospermia.
Results
Conventional semen analysis
The characteristics of sperm examination of the different groups of patients are reported in . No differences were observed in age, ejaculate volume, and sperm concentration and morphology between the different groups (p > 0.05). According to WHO [1999] guidelines, these parameters were shown to be normal in all groups. Only motility was shown to be lower in the second and third group with a statistically significant difference compared to the control group (p < 0.05). As expected, the percentage of necrotic spermatozoa was higher in the second and the third group compared to the first group (p < 0.01). The mean value of necrospermia was 65.67 ± 8.85% in the second group (range 50 to 77%) and 83.72 ± 2.79% in the third group (range 80 to 90%). However this value did not exceed 16.67 ± 4.40 % in the first group (range 10 to 24 %).
Table 1. Means of age and conventional semen parameters of patients with moderate necrozoospermia, severe necrozoospermia, and controls.
Analysis of sperm DNA fragmentation
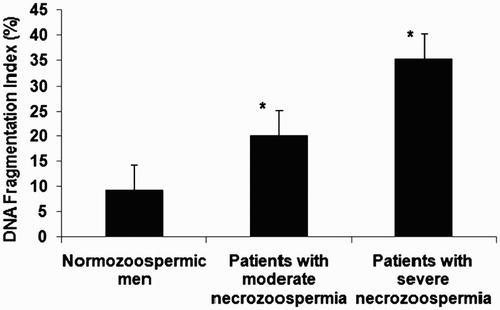
As shown in , the sperm DNA fragmentation index (DFI) was 9.28 ± 2.98% in patients with a normal level of necrotic spermatozoa, 20.25 ± 3.21% in patients with moderate necrozoospermia, and 35.31 ± 5.25% in patients with severe necrozoospermia. Sperm DNA fragmentation was significantly higher in patients with necrozoospermia compared to normozoospermic men with the highest value in patients with necrozoospermia exceeding 80% (p < 0.05).
Figure 1. Results of DNA fragmentation by TUNEL assay in spermatozoa from patients with normal levels of necrozoospermia, moderate necrozoospermia, and severe necrozoospermia (* p value < 0.05 compared to normozoospermic men). Patients with necrozoospermia had a high level of sperm DNA fragmentation compared to normozoospermic men with a statistically significant difference (p value < 0.05). The highest value was found in patients with severe necrozoospermia.
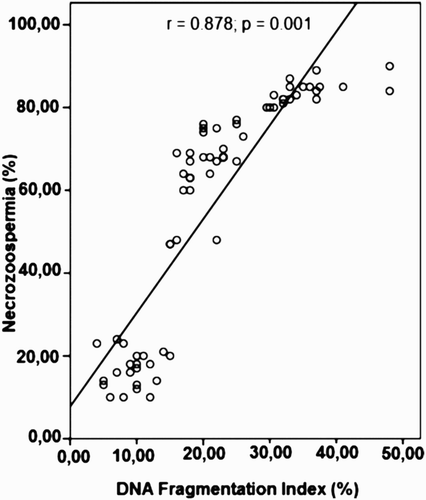
Using Pearson's correlation test, a strong correlation was found between the degree of necrozoospermia and sperm DNA fragmentation (r = 0.878; p = 0.001) (). In addition statistically significant negative correlations were found between the level of DNA fragmentation and sperm motility (r = - 0.646; p = 0.001), and abnormal sperm morphology (r = 0.434; p = 0.001). However no evident correlation was found between the percentage of sperm DNA fragmentation and sperm count, and paternal age (p > 0.05).
Discussion
Necrozoospermia is a problem primarily related with membrane stability. In the present study, we examined the relationship between DNA fragmentation and necrozoospermia. As far as we know, this is the first study examining DNA fragmentation in patients presenting with necrozoospermia, normal sperm concentration and morphology according to WHO [1999] guidelines. The majority of previous studies have been carried out in a heterogenic study group with more than one affected semen parameters. In addition few data are available concerning the relationship between DNA damage and necrozoospermia. Using a TUNEL assay, our results show that patients with necrozoospermia had a high level of sperm DNA damage compared to the normozoospermic men. In addition the highest value of DNA fragmentation was observed in patients with necrozoospermia exceeding 80%. A statistically significant correlation was observed between levels of sperm DNA fragmentation and necrozoospermia. Our data are concordant with the reports of several recent studies which showed an inverse relationship between DNA damage and vitality [Guérin et al Citation2005; Cohen-Bacrie et al Citation2008; Shen et al. Citation2002]. Indeed, in a previous study performed on swim up -selected sperm samples, and after incubation for 0, 4, and 24 hours at 37°C in HTF medium, Muratori et al. [2003] found a strong correlation between TUNEL-labeled sperm at each incubation time point and the percentages of necrotic sperm . In addition, a good or partial negative correlation was observed in the sperm samples of Equus asinus when sperm DNA fragmentation was compared with sperm viability. The loss of membrane integrity can be considered as a pathological process because it can result in the uncontrolled release of active enzymes. This can lead to autodestructive necrotic processes or alternatively to the activation of apoptotic pathways, including oxidative stress that results in fragmented DNA [Cortés-Gutiérrez et al. Citation2008]. However, in a recent study, Gosálvez et al. [2009b] showed that the loss of viability does not directly cause sperm DNA fragmentation.
When semen parameters were correlated with sperm DNA fragmentation, statistically significant correlations were found between the DNA fragmentation level and abnormal sperm morphology, and sperm motility. However no evident correlation was found between the percentage of sperm DNA fragmentation and sperm count, and paternal age. Regarding the results of the literature, correlations of sperm DNA fragmentation with other sperm quality parameters have been extremely variable. DNA fragmentation has been found to be negatively correlated with sperm concentration in certain instances [Sun et al. Citation1997; Irvine et al. Citation2000] but not in others [Lopes et al. Citation1998]. Negative correlations with the percentage of normal sperm morphology have also been found [Irvine et al. Citation2000; Lopes et al. Citation1998; Sills et al. Citation2003; Brahem et al. Citation2011]. The correlation most frequently reported for DNA fragmentation is a high negative relationship with sperm motility [Lopes et al. Citation1998; Sills et al. Citation2003; Giwercman et al. Citation2003], as shown in our study.
Many attempts have been made in several laboratories to use ejaculated, immotile (frequently dead) human spermatozoa for intracytoplasmic sperm injection (ICSI) for treatment of infertility due to severe astheno- or necrozoospermia [Dozortsev et al. Citation1995; Nagy et al. Citation1995]. Fertilization using initially immotile and presumably dead spermatozoa is reported to be very low [Dozortsev et al. Citation1995; Nagy et al. Citation1995]. This poor fertilization rate could be due to the inability of spermatozoa to trigger the activation process of the oocytes. In 1999, Ahmadi and Ng showed that a similar fertilization rate as that using live intact spermatozoon can be achieved following activation [Ahmadi and Ng Citation1999]. However, the development of the resultant embryos to blastocyst and to term is very much related to the integrity of the DNA [Ahmadi and Ng Citation1999]. A much higher degree of negative association between the degree of DNA damage with various indices of fertility such as embryo cleavage rate, implantation rate, pregnancy rate, and live birth rate, have been observed [Lewis and Aitken Citation2005]. Using a TUNEL assay, a normal quality sample would present a DFI lower than 15%. Levels between 16% and 29% would be considered from regular to good quality. A percentage higher than 30% would correspond to individuals with low fertility potential [Sergerie et al. Citation2005]. Our study indicates that the DFI value of 30% may be expected in patients with severe necrozoospermia. Regarding our results and those in the literature, we can predict that the injection of these compromised spermatozoa may affect the outcome of assisted reproduction in multiple ways, including effects on the fertilization rates, embryo cleavage, and pregnancy rates. In addition, our data show that severe necrozoospermia (>80%) which is a critical semen parameter associated with infertility, is a predictive factor for an increased risk of sperm DNA damage. Therefore we recommended the evaluation of sperm DNA status and extent of damage in patients with a high level of necrozoospermia before any attempt of assisted reproduction.
Materials and Methods
Semen collection and analysis
Semen samples were obtained from 70 men consulting for infertility evaluation at our laboratory of Cytogenetics and Reproductive Biology, Farhat Hached University Teaching Hospital, Sousse (Tunisia). Patients were subdivided into three groups according to the percentage of dead spermatozoa. The first group included 20 patients with normal level of dead spermatozoa as recommended by WHO [1999] guidelines (<30%). The second group was composed of 30 patients with a percentage of sperm death between 50 and 80% (moderate necrozoospermia). The third group consisted of 20 patients with necrozoospermia more than 80% (severe necrozoospermia). For all the patients, no history of orchitis, toxic exposure, trauma, radiotherapy, chemotherapy, chronic illness, or medication was found. For each patient, sperm viability and sperm DNA fragmentation were assessed within 1 h after ejaculation because both parameters were shown to be increased rapidly after ejaculation. This protocol was approved by the local ethics committee and all patients had previously given informed consent for the study.
Semen analysis
Samples were produced by masturbation after 3 d of sexual abstinence. After liquefaction of semen, at room temperature, standard semen parameters were obtained according to WHO [1999] guidelines. Sperm viability was assessed 30 min after ejaculation. The eosin-nigrosin viability test was performed by dissolving 1g eosin with 1g of fresh sperm and then 3g nigrosin. The percentage of viable sperm (sperm head unstained; living spermatozoa) and non viable (sperm head stained; dead spermatozoa) was assessed by counting a minimum of 100 spermatozoa.
Semen preparation
Approximately 1 ml of fresh semen was washed twice in 8 ml of Phosphate Buffered Saline (PBS, pH 7.4) (Sigma: St Louis, MO, USA) by centrifugation at 400 x g for 5 min. Spermatozoa were fixed with 5 ml of acetic acid/methanol mixture (MERCK, Darmstadt, Allemande, Germany) for at least 30 min at 4°C, then centrifuged at 400 x g for 5 min. Aliquots (40-50 µl) of the resulting suspension of nuclei were smeared on slides.
Measurements of DNA fragmentation by TUNEL assay
The presence of DNA strand breaks in spermatozoa was evaluated by the TUNEL assay, using the ApopTag® Apoptosis Detection Kit (QBiogene, Paris, France). The assay was performed according to the manufacturer's instructions and as published elsewhere [Brahem et al. Citation2011]. For each assay, a negative control without the addition of TdT and a positive control with DNase I treatment were always included to ensure the reproducibility of the assay. A total of 500 sperm cells of each sample were analyzed. Each sperm cell was considered as having intact DNA (blue-gray colored nuclei) or as having DNA fragmentation (brown colored nuclei).
Statistical evaluation
Data were analyzed using SPSS.15 (SPSS, Chicago, IL, USA). Group comparisons were made using student's t-test. Pearson's correlation was performed to examine the relationship between the percentage of sperm with DNA fragmentation and standard semen parameters. All hypothesis testing were two sided with a probability value of 0.05 deemed as significant.
Declaration of interest: The authors report no conflicts of interest.
Author contributions: Contributed to the study design, analyzed and interpreted the data, and drafted and revised the manuscript: SB, SJ; Participated in the semen analysis: SI; Revised the manuscript and final approval: AS, MM. Read and approved the final manuscript: all authors.
Abbreviations
| WHO: | = | World Health Organization |
| TUNEL: | = | terminal desoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labelling |
| DFI: | = | DNA fragmentation index |
| HTF: | = | human tubal fluid |
| ICSI: | = | intracytoplasmic sperm injection. |
References
- Agarwal, A. and Saleh, R.A. (2002) Utility of oxidative stress test in the male infertility clinic. Zhonghua Nan Ke Xue 8:1–9.
- Ahmadi, A. and Ng, S.C. (1999) Developmental capacity of damaged spermatozoa. Hum Reprod 14:2279–2285.
- Bianchi, P.G., Manicardi, G.C., Urner, F., Campana, A. and Sakkas, D. (1996) Chromatin packaging and morphology in ejaculated human spermatozoa: evidence of hidden anomalies in normal spermatozoa. Mol Hum Reprod 2:139–144.
- Brahem, S., Mehdi, M., Elghezal, H. and Saad, A. (2011) Detection of DNA fragmentation and meiotic segregation in human with isolated teratozoospermia. J Assist Reprod Genet 28:41–48.
- Cohen-Bacrie, P., Belloc, S., Ménézo, Y.J., Clement, P., Hamidi, J. and Benkhalifa, M. (2008) Correlation between DNA damage and sperm parameters: a prospective study of 1,633 patients. Fertil Steril 91:1801–1805.
- Cortés-Gutiérrez, E., Crespo, F., Gosálvez, J., Dávila-Rodriguez, M., López-Fernández, C. and Gosálvez, J. (2008) DNA fragmentation in frozen sperm of Equus asinus: Zamorano-Leonés, a breed at risk of extinction. Theryogenology 8:1022–1032.
- Dozortsev, D., Rybouchkin, A., Dsutter, P., Qian, C. and Dhont, M. (1995) Human oocytes activation following intracytoplasmic injection: the role of the sperm cell. Hum Reprod 10:403–407.
- Fang, S. and Baker, H.W. (2003) Male infertility and adult polycystic kidney disease are associated with necrospermia. Fertil Steril 79:643–644.
- Giwercman, A., Richthoff, J., Hjøllund, H., Bonde, J.P., Jepson, K., Frohm, B., (2003) Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril 80:1404–1412.
- Gosálvez, J., Cortés-Gutierez, E., López-Fernández, C., Fernández, J.L., Caballero, P. and Nuñez, R. (2009a) Sperm DNA fragmentation dynamics in fertile donors. Fertil Steril 92:170–173.
- Gosálvez, J., Cortés-Gutiérrez, E., Rocio Nuñez, R., Fernández, J.L., Caballero, P., López-Fernández, C., (2009b) A dynamic assessment of sperm DNA fragmentation versus sperm viability in proven fertile human donors. Fertil Steril 92:1915–1919.
- Guérin, P., Matillon, C., Bleau, G., Lévy, R. and Ménézo, Y. (2005) Impact of sperm DNA fragmentation on ART outcome. Gynécol Obstet Fertil 33:665–668.
- Irvine, D.S., Macleod, I.C., Templeton, A.A., Masterton, A. and Taylor, A. (1994) A prospective clinical study of the relationship between the computer-assisted assessment of human semen quality and the achievement of pregnancy in vivo. Hum Reprod 9:2324–2334.
- Irvine, D.S., Twigg, J.P., Gordon, E.L., Fulton, N., Milne, P.A. and Aitken, R.J. (2000) DNA integrity in human spermatozoa: relationships with semen quality. J Androl 21:33–44.
- Lerda, D. and Rizzi, R. (1991) Study of retrospective function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid(2,4-D). Mutat Res 262:47–50.
- Lewis, S.E. and Aitken, R.J. (2005) DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res 322:33–41.
- Lopes, S., Sun, J.G., Juriscova, A., Meriano, J. and Casper, R.F. (1998) Sperm deoxyribonucleic acid fragmentation is increased in poor quality sperm samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril 69:528–532.
- López-Fernández, C., Fernández, J.L., Gosálbez, A., Arroyo, F., Vázquez, J.M. and Gosálvez, J. (2008) Dynamics of sperm DNA fragmentation in domestic animals III. Ram. Therigenology 70:898–908.
- Mallidis, C., Lim, T.C., Hill, S.T., Skinner, D.J., Brown, D.J., Johnston, W.I., (2000) Necrospermia and chronic spinal cord injury. Fertil Steril 74:221–227.
- Muratori, M., Maggi, M., Spinelli, S., Filimberti, E., Forti, G. and Baldi, E. (2003) Spontaneous DNA fragmentation in swim-up selected human spermatozoa during long term incubation. J Androl 24:253–262.
- Nagy, Z.P., Liu, J., Joris, H., Verheyen, G., Tournaye, H., Camus, M., (1995) The results of intracytoplasmic injection sperm is not related to any of the three basic sperm parameters. Hum Reprod 10:1123–1129.
- Nduwayo, L., Barthelemy, C., Lansac, J., Tharanne, M.J. and Lecomte, P. (1995) Management of necrospermia. Contracept Fertil Sex 23:682–685.
- O'Brien, J. and Zini, A. (2005) Sperm DNA integrity and male infertility. Urology 65:16–22.
- Philpott, A. and Leno, G.H. (1992) Nucleoplasmin remodels sperm chromatin in xenopus egg extracts. Cell 69:759–767.
- Sakkas, D. (1999) The use of blastocyst culture to ovoid inheritance of an abnormal paternal genome after ICSI. Hum Reprod 14:4–5.
- Sergerie, M., Laforest, G., Bujan, L., Bissonnette, F. and Bleau, G. (2005) Sperm DNA fragmentation: threshold value in male fertility. Hum Reprod 20:3446–3451.
- Shen, H. and Ong, C. (2000) Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med 28:529–536.
- Shen, H.M., Dai, J., Chai, S.E., Lim, A. and Ong, C.N. (2002) Detection of apoptotic alterations in sperm in subfertile patients and their correlation with sperm quality. Hum Reprod 17:1266–1273.
- Sills, E.S., Frydman, J.T., Perloe, M., Michels, K.B. and Tucker, M.J. (2003) Chromatin fluorescence characteristics and standard semen analysis parameters: correlations observed in andrology testing among 136 males referred for infertility evaluation. J Obstet Gynaecol 24:74–77.
- Sun, J.G., Jurisicova, A. and Casper, R.F. (1997) Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 56:602–607.
- Thonneau, P., Marchand, S., Tallec, A., Ferial, M.L., Ducot, B., Lansac, J., (1991) Incidence and main causes of infertility in a resident population(1,850,000) of three French regions(1988-1989). Hum Reprod 6:811–816.
- Wilton, L.J., Temple-Smith, P.D., Baker, H.W. and de Krester, D.M. (1988) Human male infertility caused by degeneration and death of sperm in the epididymis. Fertil Steril 49:1052–1058.
- WHO (1999) World Health Organization (WHO) Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th edn. Cambridge University Press, New York.