Abstract
The aim of the present study was to compare two different techniques of sperm cell morphology evaluation in teratozoospermic boars: computer assisted semen morphology analysis and conventional assessment of stained semen smears. The semen samples were collected manually from 30 boars with reduced semen quality. In all samples the percentage of morphologically normal spermatozoa was below 70%. Computer assisted semen morphology assessment was performed using the Real Time Morphology (RTM) software (IVOS ver. 12.2, Hamilton Thorne Bioscience). The assessment was made by phase contrast optics, with the magnification of 20 x 3.8 and without staining. Conventional morphology assessment was performed by bright field microscopy with 1,000 x magnification after staining with Giemsa. At least 200 spermatozoa were evaluated per slide in both methods. The Bland-Altman plot indicated a general agreement between both methods of sperm morphology evaluation. The plots revealed the widest limits of agreement (mean ± 1.96 SD) for the percentage of midpiece anomalies (from −16 to 13.2), and the narrowest for the percentage of looped tail (from −1.49 to 1.09). The Bland Altman plot indicates general agreement between RTM and Giemsa staining in the percentage of major and minor defects. However, it was not possible to evaluate acrosomes using RTM. Otherwise, RTM proved to be a valuable tool in sperm morphology assessment, with accuracy equal to typical conventional methods.
Introduction
The assessment of sperm quality has been based on subjective, microscopic evaluation of parameters, such as motility, concentration, morphology abnormalities, and the determination of the percentage of live and dead spermatozoa [Iguer-ouada and Verstegen 2001]. However, it has been generally assumed that the morphological evaluation of spermatozoa is of the most crucial clinical importance [Ombelet et al. Citation1997]. Several studies have shown that the percentage of normal spermatozoa is highly correlated with in vivo fertilizing ability of sperm cells in humans and boars [Hirai et al. Citation2001; Kozdrowski and Dubiel Citation2004; Marnet et al. Citation2000; Ombelet et al. Citation1995]. The ability of spermatozoa to bind to the zona pellucida, initiates the acrosomal reaction and binding to the oolemma that are necessary for a normal fertilization [Marnet et al. Citation2000]. These phenomena occur in a subpopulation of spermatozoa with normal morphology [Menkveld et al. Citation1997]. Moreover, Ron-el et al. [1991] showed that human spermatozoal morphological defects are correlated with reduced male fertility, a lowered fertilization rate, poor embryo morphology, and reduced pregnancy rate.
In general, evaluation of spermatozoal morphology is carried out on stained smears by means of a light microscope. There are many different stains that a laboratory can use to facilitate the study of sperm. The most popular dyes are: Diff-Quik [Coetzee et al. Citation2001], Papanicolaou [Coetzee et al. Citation2001], Giemsa [Niżański Citation2006], Spermac® [Chong et al. Citation1984], Shorr [Menkveld et al. Citation1997], SpermBlue® [van der Horst et al. 2009], and nigrosin-eosin dye [Dott and Foster Citation1972]. Unfortunately, choice may influence the results obtained, e.g., Diff-Quik increases the size of the sperm head [Graves et al. Citation2005]. In smears stained with Giemsa, acrosomal damage is not always visible, and Papanicolaou and Shorr methods of staining are time-consuming [Graves et al. Citation2005]. Staining using eosin dye is also not optimal. For many years various protocols were recommended which differ considerably with regard to the type and concentration of eosin, duration of exposure to eosin, degree of semen dilution, and osmotic conditions [Björndahl et al. Citation2004]. Furthermore, the results obtained differ considerably among the many methods currently available [Niżański Citation2006]. There are also differences in results obtained among laboratories and evaluators of 30-60% [Chong et al. Citation1984; Hirai et al. Citation2001]. One of the most important problems of semen assessment is associated with the fact that many in vitro sperm parameters are poorly correlated with fertilizing potential of evaluated semen samples [Kosiniak-Kamysz et al. Citation2007]. The experience and skills of the evaluator are of considerable importance in the case of motility and morphology assessment [Oettle Citation1993]. To overcome variability, computer assisted sperm analysis (CASA) was successfully introduced over 30 years ago in human and subsequently in veterinary diagnostic and infertility treatment centers [Hirai et al. Citation2001]. The automatic computer systems allow for the objective evaluation of motility properties and sperm morphological analysis in fresh [Niżański et al. Citation2009; Rijsselaere et al. Citation2002], chilled [Niżański et al. Citation2009;], and cryopreserved semen [Niżański Citation2006]. It has been shown that there is a significant relationship between CASA motility parameters and fertility in pigs [Holt et al. Citation1997, Broekhuijse et al. Citation2012].
Unfortunately, the CASA system also has its own disadvantages. The analysis performed by CASA requires careful attention to the technical settings and semen processing. CASA systems require standardization, optimization, and validation of setups that are necessary to avoid misleading or erroneous data [Rijsselaere et al. Citation2003].
Computer automated sperm morphology analysis (ASMA) has been previously described in boars [Hirai et al. Citation2001]. The authors revealed predictive value of ASMA outcomes on boar fertility. However, ASMA is the method based on evaluation of stained smear. In contrast Real Time Morphology (RTM) is a part of the software that helps its user to promptly perform a morphological analysis, directly after motility evaluation, without the necessity of staining the preparation.
The aim of the present study was to compare two different techniques for sperm morphology evaluation in teratozoospermic boars. Morphological analysis was performed by means of the Real Time Morphology (Sperm Analysis System IVOS ver. 12.2, Hamilton Thorne Bioscence, Beverly, MA, USA) and a conventional technique of morphology assessment.
Results
The results of the computer assisted sperm concentration and motility parameters analysis are shown in . The percentage of morphologically normal spermatozoa and the percentage of particular defects assessed by RTM and Giemsa staining are shown in . To further examine whether there was agreement between both sperm morphology evaluation methods, the Bland and Altman [1986] plot of the mean versus the difference of the values obtained from these methods was conducted and is shown in . The bias between the percentage of morphologically normal spermatozoa assessed by the two methods was 2.9% (95% confidence interval: 0.49 to 5.3; A). The limits of agreement were from –9.7% to 15.5%. The Bland-Altman plot indicated a general agreement between computer assisted and Giemsa staining of sperm cells morphology.
Figure 1. Comparison of two different techniques of sperm cell morphology evaluation in teratozoospermic boars. Computer assisted sperm morphology analysis using the Real Time Morphology software (RTM) and conventional assessment of semen smears stained with Giemsa stain were compared. The Bland and Altman plot assessed agreement between A) percentage of normal sperm, B) percentage of head anomalies, C) midpiece anomalies, D) proximal droplet, E) distal droplet, F) free head, G) looped tail, and H) bent tail detected by RTM and Giemsa staining. The difference in results obtained measured by the RTM and Giemsa staining methods is plotted against their average.
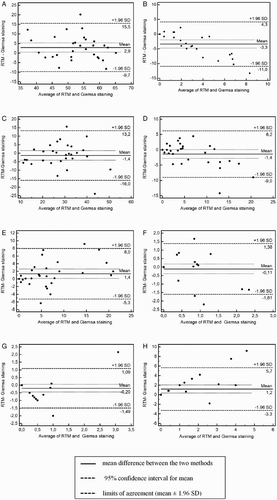
Table 1. Results of computer assisted sperm concentration and motility parameters analysis (n = 30) of diluted ejaculates.
Table 2. Percentage of morphological defects assessed by computer assisted Real Time Morphology software (RTM) and Giemsa staining method of sperm cells morphology evaluation (mead ± SD, n = 30).
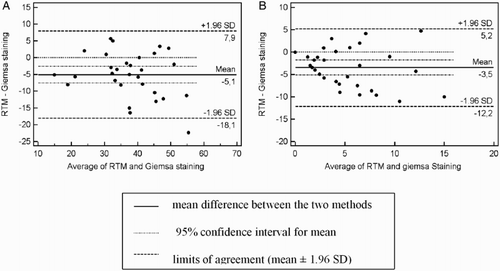
In case of particular defects, plots of agreement ( B, C, D, E, F, G, H ) showed that the differences in most defects were distributed according to the mean of the two morphological evaluation methods. The bias between RTM and Giemsa staining was -3.3, -1.4, -1.4, 1.4, -0.11, -0.2 and 1.2 for the percentage of head anomalies, midpiece anomalies, proximal and distal droplet, free head, looped tail, and bent tail, respectively. The widest limits of agreement (mean ± 1.96 SD) was obtained for the percentage of midpiece anomalies (from −16 to 13.2), the narrowest was obtained for the percentage of looped tail (from −1.49 to 1.09). The Bland Altman plot also indicated general agreement between RTM and Giemsa staining in the case of the percentage of major and minor defects (). The mean of the difference between the two methods was -5.1 and -3.5 and the ‘limits of agreement’ (mean difference + 2SD) or the 95% confidence limits were from −18.1 to 7.9 and -12.2 to 5.2 for the percentage of major and minor defects, respectively. It was not possible to evaluate acrosomes using RTM.
Figure 2. Comparison of two different techniques of sperm cell morphology evaluation in teratozoospermic boars. Computer assisted sperm morphology analysis using the Real Time Morphology software (RTM) and conventional assessment of semen smears stained with Giemsa stain. The Bland and Altman plots were used to assess the agreement between measures. Plot of the percentage of major (A) and minor defects (B) detected by the RTM and Giemsa staining. The difference in results obtained measured by the RTM and Giemsa staining methods is plotted against their average.
Discussion
This study was performed to investigate the usefulness of RTM software for boar sperm morphology evaluation. The results obtained showed that the RTM outcomes are comparable with the Giemsa staining method. The main difference observed in this study between the two techniques concerned the head anomalies and acrosome evaluation.
Among multiple parameters of semen quality, sperm morphology is probably one of the best indicators of a man's fertility potential, as it has been shown to be the most stable parameter and has the advantage of being predictive of fertility success [Gago et al. Citation1998]. For this reason, sperm morphology and its relation to fertilization ability in vivo and in vitro has been studied intensively. Studies have suggested that sperm morphology assessment by relatively simple and inexpensive methods can provide prognostic information about male fertility similar to that obtained from some of the more elaborate sperm function tests [Buendía et al. Citation2002].
In our study, we used the Giemsa staining, which was described by Watson [1975] as a very common method used for many years in veterinary practice [Bielas et al. Citation2003]. The other method used in our study was the computer assisted sperm morphology analysis using Real Time Morphology software. According to the knowledge of the authors, there is no information concerning the efficiency of the morphological evaluation of boar sperm with the use of the RTM which enables the user to perform a rapid morphological analysis directly after motility evaluation, without the necessity of staining.
Some studies claim that different staining techniques are both effective and reliable [Menkveld et al. Citation1997; Marnet et al. Citation2000; Graves et al. Citation2005]. However, other studies have shown marked differences between stains with regard to stain intensity, differentiation, and contrast, but more importantly sperm size and shape, all of which may significantly influence the outcomes of morphology evaluation [Coetzee et al. Citation2001].
In our study, we detected general agreement between RTM and Giemsa staining in the percentage of morphologically normal spermatozoa and major and minor defects. Our data were similar to previous studies that reported equal clinical relevance in the conventional and computer assisted techniques of morphology assessment [Marnet et al. Citation2000].
The lack of a specific recommended staining technique, especially in veterinary andrology centers, availability of many different stains, and the stain's influence on the sperm cell morphology causes considerable difficulty in their comparison and interpretation of the results obtained. Ombelet et al. [1997] showed that the morphological evaluation is the most diversified of all parameters determined during the process of the sperm quality evaluation. For this reason, many authors [Brito et al. Citation2011; Coetzee, et al. Citation2001; Dott et al. 1972; Rijsselaere et al. Citation2003; Sekoni et al. Citation1981] have tried to develop a new, standardized method for sperm morphology evaluation. Several [Brito et al. Citation2011; Harasymowycz et al. Citation1976; Sekoni et al. Citation1981] have described a method of assessment, without staining. The computer assisted sperm analyzers allow one to perform the morphological evaluation without the use of any dyes. In theory, this would reduce the errors arising from the applied dyes. Unfortunately, the evaluation of sperm morphology in unstained, fixed preparation is also not perfect, as has been recently reported [Brito et al. Citation2011]. In our study we were not able to detect the acrosomes using RTM. However Sekoni et al. [1981] showed that on slides fixed in buffered formol saline (BFS) it is possibile to observe the acrosome defects, nuclear vacuoles, and cytoplasmic droplets [Sekoni et al. Citation1981]. Unfortunately, Brito et al. [2011] showed that the use of wet-mount preparations fixed in BFS increased the introduction of some artifacts (bent/coiled midpieces). Similar results were obtained by Harasymowycz et al. [1976]. They revealed higher counts (P < 0.01) of head abnormalities in wet, unstained preparations fixed with buffered glutaraldehyde and buffered formol saline in comparison with the traditional stained smear. Conversely, we obtained a higher percentage of sperm head anomalies in Giemsa staining. Nevertheless, the results of the present study confirmed the deficiency of sperm morphology assessment techniques without staining, as we observed obvious difficulties in detailed sperm head structure assessment using RTM. Therefore, these data suggest that Giemsa staining is required for more precise visualization of gamete structure.
The methods of preparation for the computer assisted sperm morphology evaluation and the Giemsa staining slides varies considerably. In the first method, the gametes are immobilized at 50⁰C and evaluated with the use of phase contrast optics. The manufacturer recommended temperature range to immobilize sperm is 75⁰C to 90⁰C. In our study we used a lower temperature and according to our clinical experience it does not influence sperm morphology. The second method is based on the analysis of stained slides with the use of a light microscope. In spite of the considerable differences in the preparation of the samples examined, the results of the Bland Altman plot, regarding the morphologically normal cells in teratozoospermic boar semen, were in agreement between both methods.
An advantage of the morphological evaluation of the sperm cells with the use of RTM is a significant time saving, as the analysis may be carried out directly after motility evaluation, without the necessity of fixing and staining slides. In reproduction centers of bulls and boars, where hundreds of ejaculates are evaluated each day, saving time is very important. A main disadvantage of RTM is the inability to store the specimen and perform another analysis to possibly verify previous results, however, the RTM system allows for storing digital images that could be a more efficient way to maintain records for re-evaluation and quality control than storing glass slides. A much greater problem regarding RTM assessment, is the inability to evaluate acrosome quality. It is widely known that the acrosome and the enzymes it contains play a key role in the process of the corona radiata and zona pellucida penetration [Rijsselaere et al. Citation2002]. Therefore, the accurate assessment of this structure may provide important information about semen quality. According to the manufacturer's information this device is currently unable to properly visualize the acrosomes, and is a limitation.
The RTM computer-assisted sperm morphology analysis proved to be an effective method to evaluate morphology of normal spermatozoa for routine work in swine AI centers. Although RTM is a useful, beneficial, quick, and alternative choice compared to the traditional time consuming assessment, a more detailed description may still be worthwhile using conventional staining and semen smear evaluation. The traditional technique permits for a highly precise analysis of the subtle morphological defects, in particular those concerning the spermatozoon head, which is currently beyond the scope of the computer assisted RTM semen morphology assessment.
Material and Methods
Animals and semen collection
The experiment was approved by the Local Ethics Committee of the Faculty of Veterinary Medicine, Wroclaw University of the Environmental and Life Sciences. Semen samples were collected from 30 boars (18 Landrace and 12 Yorkshire), aged over 18 months and of weight 160-200 kg with reduced fertility. Selection of boars for this experiment was based on the results of traditional sperm morphology evaluation in slides stained with Giemsa stain. The criterion for selection was a percentage of normal spermatozoa below 70% [Pena et al. 2005]. The boars were housed in Insemination Centers in the area of Lower Silesia and Greater Poland. All animals were kept under the same conditions and received the same diet.
The semen samples were collected with the use of the manual gloved hand method [Bielas et al. Citation2003]. Only a sperm-rich fraction was collected. Immediately after collection of the samples, the sperm underwent a subjective assessment of motility (light microscopy, magnification 400x) [Bielas et al. Citation2003] and concentration (SpermaCue Microcuvette, Minitube, GmbH, Germany) [Purdy et al. Citation2010]. After evaluation the semen samples were diluted in a 33⁰C commercial diluent (Beltsville Thawing Solution: BTS, Munitub GmbH, Germany) to a concentration of 3x109 per AI dose (80-100 ml), cooled to 17⁰C and shipped in thermobox to the laboratory of the Department of Reproduction and Clinic of Farm Animals within 5 h after collection. Semen evaluation was performed immediately after delivery.
Giemsa staining
The conventional evaluation of sperm cell morphology was performed using Giemsa stain and microscopic evaluation of smears. The preparations were made by placing 10 µl of sperm at the edge of the slide. By means of a ground-edged glass the drop was spread over the whole surface of the slide. The material was left to dry and then the preparations were stained with Giemsa stain according to the method described by Watson [1975]. Such preparations underwent a microscopic examination at l,000 × under oil immersion with the use of a Nikon E200 light microscope. The morphological evaluation involved at least 200 spermatozoa per slide. Classification of morphological changes followed the system proposed by Blom [1981].
Computer Assisted Morphology Assessment
Computer assisted sperm cell morphological assessment was performed with the use of the Real Time Morphology software - RTM (Sperm Analysis System IVOS ver. 12.2, Hamilton Thorne Bioscence, Beverly, MA, USA), upon the detailed evaluation of sperm concentration and motility by the use of Animal Motility software (Sperm Analysis System IVOS ver. 12.2; Hamilton Thorne Bioscence, Beverly, MA, USA). The computer assisted morphological evaluation did not require slide preparation and staining. The evaluation took place in a Leja4 chamber (Leja, Netherlands), with a 4 µl capacity and a depth of 20 µm. The sperm was immobilized at a temperature of 50°C, generated by the heat probe (Hamilton Thorne Heat Probe) after the preliminary motility and concentration analysis. The sample evaluation was performed by using the phase contrast microscope at the magnification of 20 x 3.8. The image was transferred to a computer which enabled the data record and analysis. Each time a minimum of 200 gametes were evaluated. Classification of the morphological changes followed the system proposed by Blom [1981].
Statistical analysis
Statistical analyses were carried out using STATISTICA software (version 9.1 for Windows, StatSoft Inc., Tulsa, OK, USA). A Bland-Altman [Bland and Altman 1986] assessment for agreement was used to compare the two semen morphology evaluation methods. A range of agreement was defined as mean bias ±2SD.
Declaration of interest: The authors report no conflict of interest. This study was supported by the Polish Ministry of Science and Higher Education, grant no. N308 008 31/3067.
Author contributions: Conceived and designed the experiments: WN; Performed the experiments: AA; Analyzed the data: AP; Contributed reagents/materials/analysis tools: HM; Wrote the manuscript: AA, AP, MO.
Abbreviations
| RTM: | = | Real Time Morphology software |
| CASA: | = | computer assisted sperm analysis |
| ASMA: | = | automated sperm morphology analysis |
| MOT: | = | total motility |
| PMOT: | = | progressive motility |
| VAP: | = | average path velocity |
| VSL: | = | straight line velocity |
| VCL: | = | curvilinear velocity |
| ALH: | = | amplitude of lateral head displacement. |
References
- Bielas, W., Dubiel, A. and Niżański, W. (2003) Effects of cryopreservation methods, packaging systems and the thermoresistance test on the post-thaw quality of boar semen. Med Wet 59:172–175.
- Björndahl, L., Söderlund, I., Johansson, S., Mohammadieh, M., Pourian, M.R. and Kvist U. (2004) Why the WHO Recommendations for Eosin-Nigrosin Staining Techniques for Human Sperm Vitality Assessment Must Change. J Androl 25:671–678.
- Bland, J.M. and Altman, D.G. (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 8(1):307–310.
- Blom, E. (1981) Ocena morfologiczna wad plemników buhaja. II. Propozycja nowej klasyfikacji wad plemników. Morphological evaluation of bull spermatozoa abnormalities. II. Proposal of a new classification of spermatozoa abnormalities. Med Wet 37:239–242.
- Brito, L.F., Greene, L.M., Kelleman, A., Knobbe, M. and Turner, R. (2011) Effect of method and clinician on stallion sperm morphology evaluation. Theriogenology 76:745–750.
- Broekhuijse, M.L., Sostaric, E., Feitsma, H. and Gadella, B.M. (2012) Application of computer-assisted semen analysis to explain variations in pig fertility. J Anim Sci 90:779–789.
- Buendía, P., Soler, C., Paolicchi, F., Gago, G., Urquieta, B., Pérez-Sánchez, F., (2002) Morphometric characterization and classification of alpaca sperm heads using the sperm-class analyzer computer-assisted system. Theriogenology 57:1207–1218.
- Chong, A.P., Walters, C.A. and Weinneb, S. (1984) The neglected laboratory test. J Androl 4:280–282.
- Coetzee, K., Bermes, N., Krause, W. and Menkveld, R. (2001) Comparison of normal sperm morphology outcomes from two different computer-assisted semen analysis systems. Andrologia 33:159–163.
- Dott, H.M. and Foster, G.C. (1972) A technique for studying the morphology of mammalian spermatozoa which are eosinophilic in a differential ‘live/dead’ stain. J Reprod Fertil 29:443–445.
- Gago, C., Pérez-Sánchez, F., Yeung, C.H., Tablado, L., Cooper, T.G. and Soler, C. (1998) Standardization of sampling and staining methods for the morphometric evaluation of sperm heads in the Cynomolgus monkey (Macaca fascicularis) using computer-assisted image analysis. Int J Androl 21:169–176.
- Graves, J.E., Higdon, H.L., Boone, W.B. and Blackhurst, D.W. (2005) Developing techniques for determining sperm morphology in today's andrology laboratory. J Assisted Reprod and Genet 22:219–225.
- Harasymowycz, J., Ball, L. and Seidel, G.E. (1976) Evaluation of bovine spermatozoal morphologic features after staining or fixation. Am J Vet Res 37:1053–1057.
- Hirai, M., Boersma, A., Hoeflich, A., Wolf, E., Foil, J., Aumuller, T.R., (2001) Objectively measured sperm motility and sperm head morphometry in boars (Sus scrofa): relation to fertility and seminal plasma growth factors. J Androl 22:104–111.
- Holt, C., Holt, W.V., Moore, H.D., Reed, H.C. and Curnock, R.M. (1997) Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials. J Androl 18:312–323.
- Iguer-ouada, M. and Verstegen, J.P. (2001) Evaluation of the “Hamilton Thorn computer-based automated system” for dog semen analysis. Theriogenology 55:733–749.
- Kosiniak-Kamysz, K., Bittmar, A. and Podstawski, Z. (2007) Alkaline phosphatase activity as a marker of dog semen freezability. ucrari Stiintifice: Zootehnie si Biotehnologii 40:131–135.
- Kozdrowski, R. and Dubiel, A. (2004) Morphological changes in wild boar (Sus Scrofa L.). Semen in annual cycle. Electronic Journal of Polish Agricultural Universities (EJPAU). Website: http://www.ejpau.media.pl/volume7/issue2/veterinary/art-10.html 2004.
- Marnet, B., Vieitez, G., Milhet, P., Richoilley, G., Lesourd, F. and Parinaud, J. (2000) Computer assisted assessment of sperm morphology: comparison with conventional method. Inter J Androl 23:22–28.
- Menkveld, R., Lacquet, F.A., Kruger, T.F., Lombard, C.J., Sanchez Sarmiento C.A. and Villiers, A. (1997) Effects of different staining and washing procedures on the results of human sperm morphology evaluation by manual and computerised method. Andrologia 29:1–7.
- Niżański, W. (2006) Intravaginal insemination of bitches with fresh and frozen-thawed semen with addition of prostatic fluid: Use of an infusion pipette and the Osiris catheter. Theriogenology 66:470–483.
- Niżański, W., Klimowicz, M., Partyka, A., Savic, M. and Dubiel, A. (2009) Effects of the inclusion of equex STM into tris-based extender on the motility of dog spermatozoa incubated at 5⁰C. Reprod Dom Anim 44( Suppl 2):363–365.
- Oettle, E.E. (1993) Sperm morphology and fertility in the dog. J Reprod Fertil Suppl 47:257–260.
- Ombelet, W., Menkveld, R., Kruger, T.F. and Steeno, O. (1995) Sperm morphology assessment: historical review in relation to fertility. Hum Reprod Update 1:543–557.
- Ombelet, W., Pollet, H., Bosmans, E. and Vereecken, A. (1997) Results of a questionnaire on sperm morphology assessment. Human Repro 12:1015–1020.
- Peña, F.J., Saravia, F., García-Herreros M, Núñez-Martínez, I., Tapia, J.A., Johannisson, A., (2005) Identification of sperm morphometric subpopulations in two different portions of the boar ejaculate and its relation to postthaw quality. J Androl 26:716–723.
- Purdy, P.H., Tharp, N., Stewart, T., Spiller, S.F. and Blackburn, H.D. (2010) Implications of the pH and temperature of diluted, cooled boar semen on fresh and frozen-thawed sperm motility characteristics. Theriogenology 74:1304–1310.
- Rijsselaere, T., Van Soom, A., Maes, D. and de Kruif, A. (2002) Use of the sperm quality analyzer (SQAII-C) for the assessment of dog sperm quality. Reprod Dom Anim 37:158–163.
- Rijsselaere, T., Van Soom, A., Maes, D. and de Kruif, A. (2003) Effect of technical settings on canine semen motility parameters measured by the Hamilton-Thorne analyzer. Theriogenology 60:1553–1568.
- Ron-el, R., Nachum, H., Herman, A., Golan, A., Caspi, E. and Soffer, Y. (1991) Delayed fertilization and poor embryonic development associated with impaired semen quality. Fertil Steril 55:338–344
- Sekoni, V.O., Gustafsson, B.K. and Mather, E.C. (1981) Influence of wet fixation, staining techniques, and storage time on bull sperm morphology. Nord Vet Med 33:161–166.
- van der Horst, G. and Maree, L. (2009) SpermBlue: a new universal stain for human and animal sperm which is also amenable to automated sperm morphology analysis. Biotech Histochem 84:299–308.
- Watson, P.F. (1975) Use of a Giemsa stain to detect changes in acrosomes of frozen ram spermatozoa. Vet Rec 97:12–15.