Abstract
This study compared the sperm nuclear vacuoles and semen quality in the evaluation of male infertility. One hundred and forty-two semen samples were obtained from patients who visited the Male Infertility Clinic at Toyama University Hospital. Semen samples were evaluated by conventional semen analyses and the Sperm Motility Analysis System (SMAS). In addition, spermatozoa were analyzed at 3,700-6,150x magnification on an inverted microscope equipped with DIC/Nomarski differential interference contrast optics. A large nuclear vacuole (LNV) was defined as one or more vacuoles with the maximum diameter showing > 50% width of the sperm head. The percentage of spermatozoa with LNV (% LNV) was calculated for each sample. Correlations between the % LNV and parameters in SMAS and conventional semen analyses were analyzed. Processed motile spermatozoa from each sample were evaluated. The mean age of patients was 35 years old. Semen volume was 2.9 ± 1.6mL (0.1-11.0; mean ± standard deviation, minimum-maximum), sperm count was 39.3 ± 54.9 (x106/mL, 0.01-262.0), sperm motility was 25.1 ± 17.8% (0-76.0), and normal sperm morphology was 10.3 ± 10.1% (0-49.0). After motile spermatozoa selection, we could evaluate % LNV in 125 ejaculates (88.0%) and at least one spermatozoon with LNV was observed in 118 ejaculates (94.4%). The percentage of spermatozoa with LNV was 28.0 ± 22.4% (0-100) and % LNV increased significantly when semen quality decreased. The correlation between the % LNV and the semen parameters was weak to moderate; correlation coefficients were -0.3577 in sperm count (p < 0.0001), -0.2368 in sperm motility (p = 0.0084), -0.2769 in motile sperm count (p = 0.019), -0.2419 in total motile sperm count (p = 0.0070), and -0.1676 in normal sperm morphology (p = 0.0639). The % LNV did not show a significant correlation with the SMAS parameters except for weak correlation to beat/cross frequency (r = -0.2414, p = 0.0071). The percentage of spermatozoa with LNV did not have a strong correlation with parameters in conventional semen analysis and SMAS in the patients with male infertility; however, a certain level of negative influence of LNV to sperm quality cannot be excluded.
Introduction
Semen analysis remains the main tool for the evaluation of male infertility because semen parameters can predict the fertility potential [ Guzick et al. 2001; Jedrzejczak et al. 2008]. Conventional microscopic examination of semen is simple and inexpensive, yet can be prone to high variability and lack of standardization [Akashi et al. 2010]. In this context, the World Health Organization (WHO) provided a laboratory manual for the examination and processing of human semen [WHO Citation2010] to improve the standardization of the analysis of semen and to avoid inter-operator variability. To obtain objective results, the automated computer-assisted semen analysis (CASA) system still has some advantages over manual methods if appropriate procedures are followed. Some reports have shown a good correlation of the sperm motility estimated by the CASA system with the fertilization rates [Hirano et al. 2001; Parinaud et al. 1996]. We have previously reported the usefulness of a new computer-assisted sperm analysis, the Sperm Motility Analysis System (SMAS). This system showed high reliability in estimating the sperm concentration, sperm motility, velocity, and linearity to screen for semen quality during the management of male infertility [Akashi et al. 2010]. However, morphological assessment of spermatozoa cannot be performed on this system.
In the field of assisted reproduction technology (ART), Bartoov et al. [2001] developed a novel method to assess the detailed morphology of motile spermatozoa in real-time at a magnification of up to 6,600 × , the motile sperm organelle morphology examination (MSOME). This has yielded a definition of normal spermatozoa, and new abnormalities, especially sperm head vacuoles, have been described [Bartoov et al. 2002]. The high-magnification observation technique has been adapted to select the best spermatozoa for oocyte injection, introducing a new technique named intracytoplasmic morphologically selected sperm injection (IMSI). Numerous vacuoles have been identified inside the sperm nucleus under a light microscope at high magnifications and a significant increase in pregnancy rate and a reduction in the abortion rate were observed by excluding this morphologically abnormal sperm from IMSI [Bartoov et al. 2003; Berkovitz et al. 2005; Berkovitz et al. 2006]. Vanderzwalmen et al. [2008] also confirmed that the size and number of nuclear vacuoles exerted a significant negative effect upon embryo development to the blastocyst stage. Using a prospective randomized study Balaban et al. [2011] demonstrated that IMSI did not provide a significant improvement in the clinical outcome compared with ICSI although there were trends for higher implantation, clinical pregnancy, and live birth rates in the IMSI group. Severe male factor patients benefited from the IMSI procedure with significantly higher implantation rates compared to the conventional ICSI group. Recent meta-analysis demonstrated no significant difference in fertilization rate between ICSI and IMSI groups [Souza Setti et al. 2010]. However, a significantly improved implantation and pregnancy rate was observed in IMSI cycles with a significantly decreased miscarriage rate as compared with ICSI cycles. On the contrary, Tanaka et al. [2012] reported human sperm head vacuoles did not affect ICSI outcomes. After injection of a motile spermatozoon with large and small vacuoles selected by IMSI, 60.9% and 85.7% of metaphase II oocytes could be normally fertilized, respectively. However, 50.0% and 51.9% of the zygotes developed to the blastocyst stage, respectively, which were not different from the outcome by conventional ICSI (51.5%). The WHO laboratory manual for the examination and processing of human semen [WHO Citation2010], advises that the acrosomal region should contain no large vacuoles, and not more than two small vacuoles, which should not occupy more than 20% of the sperm head. Therefore, it is generally accepted that vacuolated spermatozoa are classified as having abnormal morphology.
The etiology of sperm nuclear vacuoles remains unclear. The nuclear vacuoles are thought to be derived from either the nuclear membrane, cytoplasmic membrane, or both, as indicated by transmission electron microscopy studies. These could be the remnants of unnecessary cytoplasm and organelles, which should have been eliminated during spermiogenesis [Toshimori and Ito 2008; Toshimori 2009]. More recently, Perdrix et al. [2011] showed that vacuoles were located inside the nucleus by transmission electron microscopy observation. Excess residual membrane constituents can be the source of reactive oxygen species (ROS). ROS exposes sperm to excessive oxidative stress resulting in DNA damage [Atkin et al. 2006]. Several studies also reported that large vacuoles were in link with chromatin condensation failure [Gopalkrishnan et al. 2000; Perdrix et al. 2011; Boitrelle et al. 2011; Franco et al. 2012].
There is limited information on evaluating the sperm nuclear vacuoles to screen the semen quality during the management of male infertility. Recently, Perdrix et al. [2012] reported that sperm head vacuoles were significantly larger in abnormal semen samples, and that large nuclear vacuoles were negatively correlated with sperm morphology (r = -0.53, strong correlation) as well as vitality (r = -0.18, weak correlation), sperm concentration (r = -0.27, moderate correlation), and progressive motility (r = -0.31, moderate correlation). Sperm head vacuoles were detected in 99.4% spermatozoa in an abnormal group, and 98.4% spermatozoa in a normal group. However, the relationship between the CASA parameters and sperm nuclear vacuoles has never been evaluated. The objective of the present study was to compare sperm nuclear vacuoles and semen quality in the evaluation of male infertility. Sperm nuclear vacuoles were observed by high-magnification microscopy in semen samples from Japanese male infertility patients, and the state of sperm nuclear vacuoles were compared to the parameters of SMAS [Akashi et al. 2010] and a conventional semen analysis.
Results
Patient Population
The mean age of the patients was 34.5 years old. Semen samples were obtained from 72 consecutive subjects who had possible causes of male infertility. Nearly half of the patients showed idiopathic male infertility and 37.5% had palpable varicoceles (). Sperm motility was 0% in 8 ejaculates and we could not obtain any motile spermatozoa in 17 ejaculates (16.5%) after motile spermatozoa selection by PureCeption™ Determination Kits. The number of spermatozoa counted was 27 ± 23 (mean ± s.d.; 0-102, minimum-maximum). shows the representative pictures of sperm heads captured by 3,700x or 6,150x magnification using an inverted microscope equipped with Nomarski differential interference contrast optics (IX71, Olympus, Tokyo). Among 125 ejaculates in which % LNV could be evaluated, at least one spermatozoon with a large nuclear vacuole was observed in 118 ejaculates (94.4%). The percentage of spermatozoa with LNV was 28.0 ± 22.14% (mean ± s.d.).
Figure 1. High magnification by an inverted microscope equipped with Nomarski differential interference contrast optics and video system. Arrows indicate nuclear vacuoles observed through a 100x (1.40 numerical aperture) objective lens. Dotted lines indicate the width of the sperm head (3.3 µM) and the maximum diameter of a nuclear vacuole (2.5 µM).
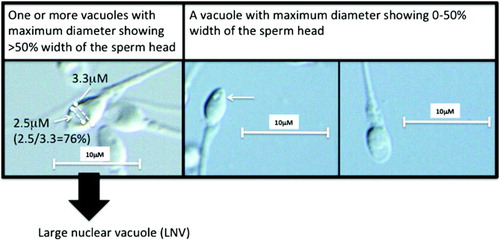
Table 1. Background of the patients.
Comparison of % LNV
The mean % LNV in processed motile spermatozoa between patients with idiopathic male infertility and varicoceles was 25.8% and 31.8%, respectively, which were not significantly different (). Parameters in conventional semen analysis were also not different between these two groups.
Table 2. Comparison of the % LNV between patients with idiopathic male infertility and varicocele.
The % LNV in processed motile spermatozoa showed low to moderate correlations with decreased values in the sperm count, sperm motility, total sperm count, motile sperm count, and total motile sperm count by a conventional semen analysis (). However, no correlation was observed between the % LNV in motile spermatozoa and the semen volume, morphology, or with the parameters of the SMAS except for beat/cross frequency. Multiple logistic regression analysis revealed that the sperm count, curvilinear velocity, and amplitude of lateral head displacement were independent variables to predict the % LNV.
Table 3. Correlation between the % LNV and the parameters of the conventional semen analysis and SMAS.
Discussion
The present study revealed large vacuoles in the sperm nuclear area among 94.4% of ejaculates with processed motile spermatozoa. The mean % LNV was 28.0%. The correlation between the % LNV and the semen parameters was weak to moderate and the correlation coefficients were 0.0734 in semen volume (p = 0.4161), -0.3577 in sperm count (p < 0.0001), -0.2368 in sperm motility (p = 0.0084), -0.2769 in motile sperm count (p = 0.019), -0.2419 in total motile sperm count (p = 0.0070), and -0.1676 in normal sperm morphology (p = 0.0639). The % LNV did not show a significant correlation with the SMAS parameters except for a weak correlation to beat/cross frequency (r = −0.2414, p = 0.0071). From these findings, one cannot exclude that large nuclear vacuoles indicate the existence of possible damage to spermatozoa. This is the first study to compare the state of sperm nuclear vacuoles and semen quality evaluated by conventional semen analysis and SMAS, one of the CASA systems, in the evaluation of male infertility.
Recent results showed that the presence of sperm nuclear vacuoles negatively correlates with fertilization, pregnancy, and implantation [Bartoov et al. 2001; Bartoov et al. 2002; Berkovitz et al. 2005; Berkovitz et al. 2006]. A significant increase in pregnancy rate and a significant reduction in abortion rate are observed by excluding this type of morphologically abnormal sperm from IMSI [Bartoov et al. 2003; Berkovitz et al. 2005 Berkovitz et al. 2006]. Sperm DNA damage is thought to negatively affect reproductive performance [Fuse et al. 2006; Practice Committee of American Society for Reproductive Medicine Citation2008]. There is clinical evidence that the spermatozoa of infertile males have substantially more DNA damage than those of fertile subjects [Zini and Libman 2006]. The mechanisms proposed to resolve the etiology of DNA damage include apoptosis, oxidative stress, and abnormal chromatin packaging [Ménézo et al. 2010; Miller et al. 2010]. Many studies have indicated that there is a positive relationship between sperm DNA fragmentation and large nuclear vacuoles in the sperm nuclear area [Oliveira et al. 2010a; Skowronek et al. 2012; Sakkas and Alvarez 2010]. Furthermore, several studies reported that large vacuoles were linked with chromatin condensation failure [Gopalkrishnan et al. 2000; Perdrix et al. 2011; Boitrelle et al. 2011; Franco et al. 2012; Cassuto et al. 2012]. Gopalkrishnan et al. [2000] demonstrated that percent morphologically normal sperm did not differ significantly between the groups of proven-fertile men and patients whose partners presented with early pregnancy loss, but increased sperm head abnormalities were seen. The ultrastructural studies showed defects of chromatin condensation and irregular nuclei with vacuoles as possible contributing factors from males to early pregnancy loss. Perdrix et al. [2011] reported that DNA fragmentation was significantly higher in unselected spermatozoa than those selected with large vacuoles. However, aneuploidy was increased and chromatin condensation was significantly altered in selected spermatozoa with large vacuoles. Boitrelle et al. [2011] showed that the rate of non-condensed chromatin was higher for vacuolated spermatozoa than for normal spermatozoa, and that normal and vacuolated spermatozoa did not differ significantly in terms of DNA fragmentation. Cassuto et al. [2012] demonstrated a negative correlation between abnormal sperm-head morphology and sperm parameters. No correlation was found with DNA fragmentation although the sperm chromatin decondensation rate was twice as high as the controls. Therefore, the observation of sperm nuclear vacuoles may indirectly indicate spermatozoa carrying possible DNA alterations, especially chromatin condensation failure, with real-time optical microscopy without denaturation. Of course, this is not always the case; Watanabe et al. [2011] reported that large vacuoles were not responsible for DNA damage in patients who produce high-quality semen.
This type of study is usually carried out using motile spermatozoa to improve the treatment success rate of in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). The frequency of motile spermatozoa with large nuclear vacuoles ranged from 25.9 to 38.0%, and varied according to the definition of large vacuoles, the sample size, and the patient population [Oliveira et al. 2010b; Franco et al. 2012; Perdrix et al. 2011; Perdrix et al. 2012]. The present study found that the % LNV in motile spermatozoa was 28.0%, which was consistent with previous reports. To date, there is no clear definition of large vacuoles. Relative vacuole area to sperm head or sperm nuclear vacuole was judged as abnormal if occupying more than 13% [Perdrix et al. 2011], 25% [Boitrelle et al. 2011], or 50% of the sperm head area [Franco et al. 2008; Mauri et al. 2010; Oliveira et al. 2010a; 2010b], or vacuoles with a diameter of more than 1.5 µm and visible at 400x magnification [Watanabe et al. 2011], or vacuoles considered as large without any precise criterion [Garolla et al. 2008]. Because the calculation of vacuole area and sperm nuclear area is time-consuming, we used a simplified definition according to that proposed by Boitrelle et al. [2011] and Watanabe et al. [2011], obtaining similar frequency of large nuclear vacuoles.
Although large sperm nuclear vacuoles are reported to be associated with sperm DNA alterations, their etiology is still unclear. In the present study, 37.5% of the patients had palpable varicoceles. This population of patients did not differ significantly from the previous reports as varicoceles were present in about 35% of males with primary infertility [Pryor and Howards 1987]. Varicoceles are known to alter sperm morphology and nuclear quality [Baccetti et al. 2006]. Therefore, there is a possibility that the large population of varicoceles introduce a bias to the results. Oliveira et al. [2010b] reported that males with varicoceles presented more spermatozoa with LNV in their study using semen samples from 240 patients (OR = 1.02; 95% CI = 1.00 to 1.03). The semen parameters were not significantly different between those with varicoceles and those with idiopathic male infertility in our clinical practice [Komiya et al. 2012]. In addition, the % LNV was not significantly different if compared between the patients with varicoceles and idiopathic causes of male infertility in the current series. From these findings, the patients' backgrounds do not seem to affect the state of sperm nuclear vacuoles significantly in the present study. However, this could be due to sample size or study design. The nuclear vacuoles could be the remnants of cytoplasm and organelles, which should have been eliminated during spermiogenesis [Toshimori and Ito 2008]. Vacuoles observed by the IMSI system also could be the result of dips or thumbprints, which are difficult to identify by optical microscopes. This is unlikely as they do not have any elements from the nuclear membrane or cytoplasm. Boitrelle et al. [2011] examined 100 ‘vacuolated’ spermatozoa, and described that the nucleus exhibited a large, abnormal ‘thumbprint’-like concavity at the site of a large vacuole linked to failure of chromatin condensation.
There is limited information comparing sperm nuclear vacuoles and semen quality. In this context, Perdrix et al. [2012] similarly analyzed the relationship between conventional sperm parameters and MSOME. Sperm nuclear vacuoles were assessed objectively with digital imaging system software. MSOME assessment (sperm head length, width, and area, as well as vacuole number, vacuole area, and relative vacuole area to sperm head [RVA (%) = 100x vacuole area (μm2) / head area (μm2)]) were performed for 440 semen samples. This sample size was much larger than the present study. They considered spermatozoa with vacuoles occupying more than 12.4% of the nuclear area as abnormal. Sperm head vacuoles were detected in 99.4% of the spermatozoa in the abnormal group, which was comparable to the results presented above. Sperm head vacuoles were significantly larger in the abnormal group compared with the normal group (7.88 ± 0.16% vs. 5.88 ± 0.19%, p < 0.0001). RVA increased significantly when sperm concentration (p < 0.0001), vitality (p = 0.0002), progressive motility (p < 0.0001), and normal forms (p < 0.0001) decreased. Correlations were weak with vitality (r = -0.18), moderate with sperm concentration (r = -0.27) and progressive motility, (r = -0.31), but strong with sperm morphology parameters [normal forms and acrosomes (r = -0.53)]. We found no relationship between LNV and normal sperm morphology although weak to moderate correlations were found to sperm count and sperm motility. The difference between the studies by Perdrix et al. [2012] and reported here may be due to sample size, the causes of male factors (which were not clear in Perdrix's study), or the methods of morphological examination, sperm selection, and vacuole evaluation. For example, Perdrix et al. [2012] assessed sperm morphology (% normal forms) according to David's modified classification [David et al. 1975; Auger et al. 2001] and particularly focused on the percentage of spermatozoa presenting acrosome abnormalities. This could make a difference in the results with respect to sperm morphology.
A conventional semen analysis does not evaluate additional motion characteristics such as velocity, linearity, or amplitude of lateral head displacement (ALH). The CASA systems, like SMAS in the present study, provide a rapid, accurate and objective assessment of the attributes of sperm motion. The CASA system provides a reliable estimate of the fertilization capacity of human sperm in the penetration assay [Fetterolf and Rogers 1990]. The present study found that % LNV increased significantly when SMAS parameters decreased. The % LNV in processed motile spermatozoa showed some association to linearity (r = -0.1987, p = 0.0276) and beat/cross frequency (r = -0.2414, p = 0.0071) as parameters of objective sperm motility in the SMAS system. In addition, multiple logistic regression analysis revealed that curvilinear velocity and ALH were the independent variables to predict % LNV. ALH was recently reported to be significantly increased in association with the fertilization rate promoted by pituitary adenylate cyclase-activating polypeptide (PACAP)-mediated sperm–cumulus cell interaction [Tanii et al. 2011].
There are limitations associated with this study. The sample size is relatively small in comparison to other studies. The evaluation of motile sperm was not real time observation but reviews of electrically recorded movie files, which cannot be translated directly to clinical practice like ICSI. However, this method could be more accurate than real time observation when judging the presence of sperm nuclear vacuoles. Magnification by the video system was up to 6,150x, which was relatively small in comparison to recent studies with 6,600x to 10,000x magnification [Bartoov et al. 2001; Boitrelle et al. 2011; Franco et al. 2012]. Higher magnification seems to have advantages in MSOME. The resolution of the microscopes depends on the numerical aperture of the lens. The current study used lenses with numerical apertures of 1.40-1.42, which were the best available for that microscope. The endpoint of the present study was only the semen variables with no information regarding fertility potential. However, a number of studies have shown the predictive value of the semen analysis parameters, provided that the parameters were not studied separately from each other. It is necessary to evaluate whether the % LNV alone or in combination with the semen parameters can discriminate between fertile and infertile males.
In conclusion, large nuclear vacuoles in the sperm head were observed in the majority of ejaculates, and mean % LNV was 28.0%. The percentage of spermatozoa with LNV increased significantly when semen quality decreased. LNV did not show a strong correlation with parameters in conventional semen analysis and SMAS in the patients with male infertility. However, a negative influence of LNV to sperm quality cannot be excluded.
Materials and Methods
This study was approved by the Institutional Review Board of the University of Toyama. Ethical consent for the work to be carried out was given and informed consent was obtained from each patient. This study followed principles in the Declaration of Helsinki. One hundred and forty-two semen samples were obtained by masturbation from infertile male patients who visited the Male Infertility Clinic at Toyama University Hospital. The definition of male infertility was the failure to conceive following twelve months of unprotected intercourse due to possible male factors. Semen samples were evaluated within one hour of collection by conventional semen analyses [WHO Citation1999] and SMAS (Kaga Electronics, Tokyo, Japan), which were performed as described previously [Akashi et al. 2010; Tanii et al. 2011]. In addition, spermatozoa were analyzed at 3,700x or 6,150x magnification by an inverted microscope equipped with Nomarski differential interference contrast optics (IX71, Olympus, Tokyo) and a video system (FX630, Olympus, Tokyo). A 60x (1.42 numerical aperture) or 100x (1.40 numerical aperture) objective lens was used with oil. The Two-Step Discontinuous PureCeption™ Gradient Technique was used for selection of motile spermatozoa according to the manufacturer's manual of PureCeption™ Determination Kits as follows: 2.0 mL of PureCeption 40% Upper Phase solution was transferred into a sterile disposable centrifuge tube. Then, 2 mL of PureCeption 80% Lower Phase solution was placed under the upper layer. A 1.5-2.0 mL aliquot of fresh liquefied semen was layered gently on top of the gradient. Samples were warmed to 37°C, then the tubes were centrifuged at 500 x g for 20 min. The PureCeption solutions and seminal fluid were removed without disturbing the sperm pellet, leaving a small amount (approximately 0.3 mL) of PureCeption 80% Lower Phase solution over the sperm pellet. The sperm pellet was then transferred in this residual medium to a clean conical centrifuge tube for further washing. A total of 3-5 mL of Quinn's Sperm Washing Medium was added, and the pellet resuspended. The tubes were centrifuged at 500 x g for 5 min to wash away residual PureCeption 80% Lower Phase solution. The supernatant was removed, and the sperm pellet re-suspended in a suitable volume of appropriate medium. The images of spermatozoa were captured and stored by image-filing software, FlvFs (Flovel, Tokyo) on a video system. Images in each ejaculate were captured and analyzed within 30 to 60 min. A nuclear vacuole was defined as ‘large’ if the maximum diameter of the vacuole was more than 50% of the width of the sperm head (). We used this definition modifying those by Boitrelle et al. [2011] and Watanabe et al. [2011] to simplify the observation. Boitrelle et al. [2011] used the definition of large vacuoles accounting for more than 25% of the head's cross-sectional area. Therefore, the diameter is roughly equivalent to 50% if the area is 25%. Watanabe et al. [2011] classified vacuoles with a diameter of >1.5 µm and visible at 400× magnification as ‘large’. According to the WHO laboratory manual [WHO Citation2010], the head dimensions of 77 Papanicolaou-stained spermatozoa, measured by a computerized system (coefficient of variation for repeated measurements 2–7%) had the following dimensions: median length 4.1 µm, 95% CI 3.7–4.7; median width 2.8 µm, 95% CI 2.5–3.2; median length-to width ratio 1.5, 95% CI 1.3–1.8. Therefore, the diameter of vacuole is more than 50% of sperm head width if the vacuole has the diameter of >1.5 µm (). It is easier to judge spermatozoa with LNV using our definition. The percentage of spermatozoa with LNV was calculated for each ejaculate. Correlations between % LNV and parameters in SMAS and conventional semen analyses were analyzed. Processed motile spermatozoa from each ejaculate were evaluated.
Statistical analysis
The statistical analysis of the data was carried out with the JMP 8.0.1 statistical software package (SAS Institute Japan, Tokyo). The results are shown as the mean ± SD. A value of p < 0.05 was defined to be significantly different. Spearman's rank correlation coefficient was used to find the correlations between % LNV and parameters of conventional semen analyses and SMAS. The unpaired t-test was used in analyzing the % LNV between two groups. Multiple logistic regression analysis was used to evaluate independent variables to predict % LNV.
Declaration of interest: The authors declare no conflicts of interest. The authors alone are responsible for the contents of this paper.
Author contributions: Patient recruitment and treatment: AK, AW, HF; Performed conventional semen analyses: YK; Conducted observations of LNV and data analyses: AK; Principle investigator: HF.
Abbreviations:
| SMAS: | = | Sperm Motility Analysis System |
| LNV: | = | large nuclear vacuole |
| % LNV: | = | percentage of spermatozoa with LNV |
| WHO: | = | World Health Organization |
| CASA: | = | computer-assisted semen analysis |
| ART: | = | assisted reproduction technology |
| MSOME: | = | motile sperm organelle morphology examination |
| IMSI: | = | intracytoplasmic morphologically selected sperm injection |
| ROS: | = | reactive oxygen species |
| IVF: | = | in vitro fertilization |
| ICSI: | = | intracytoplasmic sperm injection |
| ALH: | = | amplitude of lateral head displacement. |
References
- Aitken, R.J., Wingate, J.K., De Iuliis, G.N., Koppers, A.J. and McLaughlin, E.A. (2006) Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa. J Clin Endocrinol Metab 91:4154–4163.
- Akashi, T., Watanabe, A., Komiya, A. and Fuse, H. (2010) Evaluation of the Sperm Motility Analyzer System (SMAS) for the Assessment of Sperm Quality in Infertile Men. Syst Biol Reprod Med 56:473–477.
- Auger, J., Eustache, F., Andersen, A.G., Irvine, D.S., Jorgensen, N., Skakkebaek, N.E., (2001) Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum Reprod 16:2710–2717.
- Baccetti, B.M., Bruni, E., Capitani, S., Collodel, G., Mancini, S., Piomboni, P., (2006) Studies on varicocele III: ultrastructural sperm evaluation and 18, X and Y aneuploidies. J Androl 27:94–101.
- Balaban, B., Yakin, K., Alatas, C., Oktem, O., Isiklar, A. and Urman, B. (2011) Clinical outcome of intracytoplasmic injection of spermatozoa morphologically selected under high magnification: a prospective randomized study. Reprod Biomed Online 22:472–476.
- Bartoov, B., Berkovitz, A. and Eltes, F. (2001) Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med 345:1067–1068.
- Bartoov, B., Berkovitz, A., Eltes, F., Kogosowski, A., Menezo, Y. and Barak, Y. (2002) Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl 23:1–8.
- Bartoov, B., Berkovitz, A., Eltes, F., Kogosovsky, A., Yagoda, A., Lederman, H., (2003) Pregnancy rates are higher with intracytoplasmic morphologically selected sperm injection than with conventional intracytoplasmic injection. Fertil Steril 80:1413–1419.
- Berkovitz, A., Eltes, F., Yaari, S., Katz, N., Barr, I., Fishman, A., (2005) The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod 20:185–190.
- Berkovitz, A., Eltes, F., Ellenbogen, A., Peer, S., Feldberg, D. and Bartoov, B. (2006) Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod 21:1787–1790.
- Boitrelle, F., Ferfouri, F., Petit, J.M., Segretain, D., Tourain, C., Bergere, M., (2011) Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprints linked to failure of chromatin condensation. Hum Reprod 26:1650–1658.
- Cassuto, N.G., Hazout, A., Hammoud, I., Balet, R., Bouret, D., Barak, Y., et al. (2012) Correlation between DNA defect and sperm-head morphology. Reprod Biomed Online 24:211–218.
- David, G., Bisson, J.P., Czyglik, F., Jouannet, P. and Gernigon, C. (1975) Anomalies morphologiques du spermatozoïde humain: proposition pour un système de classification. J Gynecol Obstet Biol Reprod 4:17–36.
- Fetterolf, P.M. and Rogers, B.J. (1990) Prediction of human sperm penetrating ability using computerized motion parameters. Mol Reprod Dev 27:326–331.
- Franco, J.G. , Baruffi, R.L., Mauri, A.L., Petersen, C.G., Oliveira, J.B., and Vagnini, L. (2008) Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod Biomed Online 17:42–45.
- Franco, J.G. Jr., Mauri, A.L., Petersen, C.G., Massaro, F.C., Silva, L.F., Felipe, V. (2012) Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int J Androl 35:46–51.
- Fuse, H., Akashi, T., Mizuno, I., Nozaki, T. and Watanabe, A. (2006) Postoperative changes of sperm chromatin heterogeneity, using acridine orange staining, in varicocele patients. Arch Androl 52:223–226.
- Gopalkrishnan, K., Padwal, V., Meherji, P.K., Gokral, J.S., Shah, R. and Juneja, H.S. (2000) Poor quality of sperm as it affects repeated early pregnancy loss. Arch Androl 45:111–117.
- Garolla, A., Fortini, D., Menegazzo, M., De Toni, L., Nicoletti, V., Moretti, A., (2008) High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online 17:610–616.
- Guzick, D.S., Overstreet, J.W., Factor-Litvak, P., Brazil, C.K., Nakajima, S.T., Coutifaris, C., (2001) Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 345:1388–1393.
- Hirano, Y., Shibahara, H., Obara, H., Suzuki, T., Takamizawa, S., Yamaguchi, C., (2001) Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet 18:213–218.
- Jedrzejczak, P., Taszarek-Hauke, G., Hauke, J., Pawelczyk, L. and Duleba, A.J. (2008) Prediction of spontaneous conception based on semen parameters. Int J Androl 31:499–507.
- Komiya, A., Watanabe, A., Kawauchi, Y. and Fuse, H. (2012) Testicular volume discrepancy is associated with decreased semen quality in infertile Japanese males with varicoceles. Reprod Med Biol 11:117–121.
- Mauri, A.L., Petersen, C.G., Oliveira, J.B., Massaro, F.C., Baruffi, R.L. and Franco, J.G. (2010) Comparison of day 2 embryo quality after conventional ICSI versus intracytoplasmic morphologically selected sperm injection (IMSI) using sibling oocytes. Eur J Obstet Gynecol Reprod Biol 150:42–46.
- Ménézo, Y., Dale, B. and Cohen, M. (2010) DNA damage and repair in human oocytes and embryos: a review. Zygote 21:1–9.
- Miller, D., Brinkworth, M. and Iles, D. (2010) Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 139:287–301.
- Oliveira, J.B., Massaro, F.C., Baruffi, R.L., Mauri, A.L., Petersen, C.G. and Silva, L.F. (2010a) Correlation between semen analysis by motile sperm organelle morphology examination and sperm DNA damage. Fertil Steril 94:1937–1940.
- Oliveira, J.B., Petersen, C.G., Massaro, F.C., Baruffi, R.L., Mauri, A.L., and Silva, L.F. (2010b) Motile sperm organelle morphology examination (MSOME): intervariation study of normal sperm and sperm with large nuclear vacuoles. Reprod Biol Endocrinol 8:56.
- Parinaud, J., Richoilley, G., Moutaffian, H., Vieitez, G. and Mieusset, R. (1996) Are the characteristics of spermatozoa in the insemination medium useful for predicting in-vitro fertilization results? Int J Androl 19:103–108.
- Perdrix, A., Travers, A., Chelli, M.H., Escalier, D., Do Rego, J.L., Milazzo, J.P., (2011) Assessment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod 26:47–58.
- Perdrix, A, Saïdi, R., Ménard, J.F., Gruel, E., Milazzo, J.P., Macé, B., (2012) Relationship between conventional sperm parameters and motile sperm organelle morphology examination (MSOME). Int J Androl 35:491–498.
- Practice Committee of American Society for Reproductive Medicine. (2008) The clinical utility of sperm DNA integrity testing. Fertil Steril 90 ( Suppl):S178–180.
- Pryor, J.L. and Howards, S.S. (1987) Varicocele. Urol Clin N Amer 14:499–513.
- Sakkas, D. and Alvarez, J.G. (2010) Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 93:1027–1036.
- Skowronek, F., Casanova, G., Alciaturi, J., Capurro, A., Cantu, L., Montes, J.M., (2012) DNA sperm damage correlates with nuclear ultrastructural sperm defects in teratozoospermic men. Andrologia 44:59–65.
- Souza Setti, A., Ferreira, R.C., Paes de Almeida Ferreira Braga, D., de Cássia Sávio Figueira, R., Iaconelli, A. and Borges, E. Jr. (2010) Intracytoplasmic sperm injection outcome versus intracytoplasmic morphologically selected sperm injection outcome: a meta-analysis. Reprod Biomed Online 21:450–455.
- Tanaka, A., Nagayoshi, M., Tanaka, I. and Kusunoki, H. (2012) Human sperm head vacuoles are physiological structures formed during the sperm development and maturation process. Fertil Steril 98:315–320.
- Tanii, I., Aradate, T., Matsuda, K., Komiya, A. and Fuse, H. (2011) PACAP-mediated sperm-cumulus cell interaction promotes fertilization. Reproduction 141:163–171.
- Toshimori, K. and Ito, C. (2008) Human sperm ultrastructures and fertility. J Mamm Ova Res 25:232–239.
- Toshimori, K. (2009) Dynamics of the mammalian sperm head: modifications and maturation events from spermatogenesis to egg activation. Adv Anat Embryol Cell Biol 204:5–94.
- Vanderzwalmen, P., Hiemer, A., Rubner, P., Bach, M., Neyer, A., Stecher, A., (2008) Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod Biomed Online 17:617–627.
- Watanabe, S., Tanaka, A., Fujii, S., Mizunuma, H., Fukui, A., Fukuhara, R., (2011) An investigation of the potential effect of vacuoles in human sperm on DNA damage using a chromosome assay and the TUNEL assay. Hum Reprod. 2011 26:978–986.
- WHO (1999) World Health Organization Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge University Press; New York, USA.
- WHO (2010) WHO laboratory manual for the examination and processing of human semen, 5th. ed. Part I. Semen Analysis, Chapter 2 Standard procedures, World Health Organization, Switzerland pp.7–115.
- Zini, A. and Libman, J. (2006) Sperm DNA damage: clinical significance in the era of assisted reproduction. CMAJ 175:495–500.