Abstract
Studies on functional characteristics of the regressing primate corpus luteum (CL) to luteotrophic stimulus on day 1 of the non-fertile menstrual cycle are scarce. Recombinant human luteinizing hormone (rhLH) (20 IU/Kg BW; n = 10) or human chorionic gonadotropin (hCG) (180 IU; n = 6) were administered intravenously to female bonnet monkeys on day 1 of menses. Exogenous treatment of rhLH or hCG caused a significant increase in circulating progesterone (P4) levels 2-4 hours post treatment (P < 0.05). Lutectomy prior to onset of menses confirmed that CL is the site of the increased P4 concentrations. Increased levels of phosphorylated P44/42 MAPK, MKK3/6 activation and concomitant histological changes were observed within 4 hours in CL of monkeys receiving hCG treatment. The results from this study demonstrate the acute progesterone synthesizing capacity of regressing monkey CL after LH or hCG challenge. This has potential implications for interpreting the steroidogenic response after gonadotropin stimulation tests in the early follicular phase of the normal ovulatory and anovulatory women undergoing controlled ovarian stimulation protocols as part of assisted reproductive technology (ART) and in women with polycystic ovarian syndrome.
Introduction
In higher primates, the molecular mechanisms underlying functional CL regression is one of intense areas of investigation. Existing data on CL regression suggest many forms of cell death i.e., apoptosis, autophagy, and necrosis play a role during regression in the primate CL (reviewed by [ Devoto et al. Citation2009]). It is thought that the decreased downstream signaling efficiency of the LH/hCG receptor but not the decrease in LH levels or decline in LH receptors in the late CL account for its regression [Zeleznik Citation1998; reviewed by Bishop et al. Citation2011]. A differential steroidogenic response has been observed in regularly menstruating women stimulated with gonadotropins after an ovulatory cycle or after a GnRH agonist treatment in the anovulatory cycle due to reactivation of the regressing CL [Jarvela et al. Citation2007]. Studies involving baboons reported the rescue of late CL after gonadotropin stimulation during the follicular phase [Castracane et al. Citation1998]. However, it is not known whether the regressing primate CL on day 1 of menses can acutely respond to the stimulating doses of exogenous LH or hCG i.e., higher levels of the hormone than the episodic stimuli provided by endogenous LH level. Since CL responds differently to LH and hCG depending on its developmental stage, we evaluated the acute responses of the regressing CL to both LH and hCG.
In view of the above information, the aims of the present study were to: 1) examine the acute responses (P4 and estradiol (E2) secretion) of regressing monkey CL on day 1 of the menses to stimulating doses of LH or hCG, 2) confirm the source of steroidogenic response, if any, after gonadotropin stimulation in monkeys subjected to lutectomy before day 1 of the menstrual cycle, and 3) address the signaling mechanisms underlying the acute response to gonadotropin treatment. This is the first study to address the important issue of functional capacity of luteal tissue during regression.
Results
Hormone responses, morphological changes, and ERK activation in regressing CL after stimulation
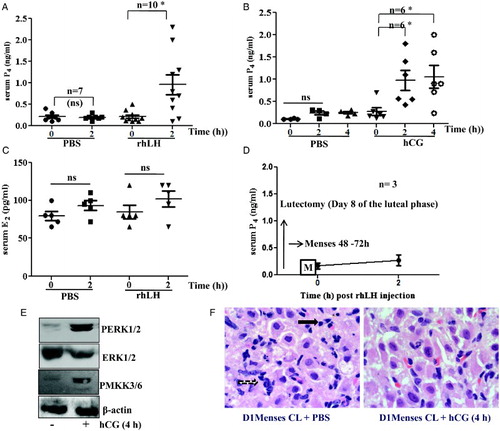
Exogenous administration of LH and hCG to female bonnet monkeys on day 1 of the menses showed a significant increase in serum P4 levels around 2 hours and up to 4 hours post stimulation, respectively (P < 0.05). A and B represent the serum concentrations of P4 after LH/phosphate buffered saline (PBS) and hCG/PBS administration, respectively. We have previously reported the circulating levels of P4 throughout the normal menstrual cycle and also 2 days after menses in the bonnet monkey [Suresh and Medhamurthy Citation2009]. The data suggested no reactivation of bonnet monkey CL following menses in the natural cycle [Suresh and Medhamurthy Citation2009]. No significant increase in serum E2 was observed after LH administration as presented in C. The effect of LH administration on serum concentration of P4 on day 1 of the menstrual cycle in animals which have undergone lutectomy is shown in D. No significant increase in P4 concentration was observed suggesting that the source of P4 was the result of CL reactivation, and not the growing follicles. Immunoblots for PERK1/2, ERK1/2, PMKK3/6 and β-actin loading control for pooled samples of vehicle and hCG treated CL are presented in E. CL from monkeys treated with hCG showed the highest abundance of active dually phosphorylated ERK1/2 and also the upstream activator of P38, phospho MKK3/6. Histological sections of CL tissue from day 1 of menses without and with hCG treatment are presented in F. A decreased volume of cells, pkynotic nuclei in regressing CL appears to slightly change along with the presence of numerous red blood cells after hCG treatment for 4 hours.
Figure 1. Steroidogenic response, ERK1/2 activation, and morphological changes of bonnet macaque CL after luteotrophic stimulus on day 1 of the menses. A and B) Scatter graphs (mean ± SEM) concentrations of serum P4 after LH and hCG administration. C) Scatter graph (mean ± SEM) concentrations of serum E2 levels after hCG administration. D) Mean ± SEM concentration of P4 after LH stimulus on day 1 of the menses in lutectomized animals. E) Western blots of PERK1/2, ERK1/2, PMKK3/6 and β-actin loading control for pooled samples of untreated and hCG treated CL. F) Haematoxylin and eosin stained regressing CL on day 1 of the menses before and after hCG treatment. The black arrow indicates pyknotic nuclei and the black dotted arrow indicates the cells with decreased cell volume in representative images (100x magnification). *: P < 0.05 when analyzed with paired t-test in comparison to vehicle treated control animals; P4: progesterone; PBS: phosphate buffered saline; rhLH: recombinant human luteinizing hormone; hCG: human chorionic gonadotropin; E2: estradiol; CL: corpus luteum; D1: day 1
Discussion
Differences between in vitro and in vivo studies regarding the reactivation of regressing primate CL to hCG challenge during late luteal phase have been observed [Castracane et al. Citation1998; Auletta et al. Citation1995]. In this study, we report for the first time, the ability of regressing CL to respond acutely and secrete P4 in response to exogenous LH or hCG treatment. These observations are important when analyzing the steroidogenic response to gonadotropin stimulation testing in normal ovulatory women and also during hCG stimulation tests in women with polycystic ovary syndrome. In the present study, the magnitude of the progestational response was varied among different monkeys and this suggests that P4 biosynthesis is acutely influenced by the luteotropic stimulus even in regressing CL of some monkeys. The divergence in the magnitude of response observed presumably reflects different functional states of CL on day 1 of the menses in different monkeys. Castracane et al. [1998] reported the rescue of baboon CL in the next follicular phase after gonadotropin stimulation, and it has been demonstrated that the regressing CL was responsive to the hCG when performing ovarian stimulation tests in the early follicular phase of regularly menstruating women [Jarvela et al. Citation2007]. Together with these studies, the findings in the present study confirm that the luteolytic mechanisms underlying the demise of the CL of each cycle are not complete on day 1 of the menstrual cycle. This observation is in contrast to the report by Auletta and co-workers who showed, in vitro, the unresponsiveness of rhesus monkey luteal tissue collected on day of menses to hCG and dibutyryl cAMP [Auletta et al. Citation1995]. It is possible that certain steroidogenic cells escape from regression and maintain their ability to acutely respond to the luteotrophic stimulus only in vivo. As CL is a mixed population of cells, it is possible that the lack of response in vitro indicate the absence of other cell types, dose of hCG, or conditions in culture.
Furthermore, the findings in this study revealed that downstream signaling efficiency of LH receptors was intact in the monkey CL on day 1 of the menses. Although morphological changes are seen after hCG treatment on day 1 of menses, further studies are required to confirm and quantify the histological changes including apoptotic nuclei. Dewi et al. [2002], described the requirement of ERK1/2 activation in production of P4 by human granulosa-lutein cells, and our findings demonstrate an association of CL reactivation and PERK1/2 levels after hCG stimulation. Our data confirm the previous report that the functional status of monkey CL was associated with activation status of MAPKs [Yadav and Medhamurthy Citation2006]. These results suggest that the integrity of progestational response of CL to gonadotropin stimulation is not completely lost by the day 1 of menses. In conclusion, our findings indicate that the regressing CL on day 1 of the menses can be reactivated by stimulating higher doses of LH or hCG, and this acute progestational response of the CL is associated with ERK1/2 activation and PMKK3/6 levels.
Materials and Methods
Reagents
Human CG (hCG, Profasi®) and recombinant human LH (rhLH) were obtained from Ares Serono, Aubonne, Switzerland. The E2 (#244) and P4 (#337) antisera were kindly provided by Prof. G. D. Niswender, Colorado State University, Fort Collins, Colorado, USA. Phospho- p42/44 MAPK (9101), p42/44 MAPK (9102), pMKK3/6 (9231), and Phototype-HRP Western detection system with horseradish peroxidase-coupled anti-rabbit IgG (7071) were purchased from Cell Signaling Technology, Inc. Danvers, MA, USA. All other reagents were purchased from Sigma-Aldrich Chemicals Pvt. Ltd, Bommasandra, Bangalore, India.
Animals and experimental design
The study was approved by the Institutional Animal Ethics Committee of the Indian Institute of Science, Bangalore. Information related to the animal husbandry, diet, and health maintenance of monkeys has been previously reported [Suresh and Medhamurthy Citation2009]. Age ranged from 8-13 y. The experiments were carried out during the months of August to January, a period considered as breeding season in the bonnet monkey.
Effects of hCG or LH treatment on day 1 of menses
Monkeys exhibiting at least two consecutive regular menstrual cycles were recruited for the experiment. Animals were monitored daily for the onset of menses and on day 1 of menses, a single i.v. injection of hCG (180 IU) was administered to 6 monkeys, and this dose was selected based on results in another study that involved simulation of early pregnancy in non mated cycling bonnet monkeys [Yadav and Medhamurthy Citation2006]. A single i.v. injection of rhLH (20 IU/kg BW) was administered to 10 monkeys on day 1 of the menses, and this dose has been shown to stimulate CL of monkeys subjected to GnRH antagonist induced luteolysis at the mid luteal phase [Priyanka et al. Citation2009]. Blood samples were collected post rhLH (up to 2 h) or hCG (up to 4 h) treatments. Injection of phosphate buffered saline (PBS) served as control (n = 7).
Lutectomy and hCG stimulation on day 1 of the menses
To confirm the source of progestational response of the regressing CL, lutectomy (n = 3) was performed on day 8 of the luteal phase by accessing the ovaries through mid-ventral laparotomy. Details of lutectomy have been provided in earlier publication [Suresh and Medhamurthy Citation2009]. Menses was observed 48-72 h post lutectomy and monkeys on day 1 of menses was subjected to hCG stimulation similar to experiments with monkeys with spontaneous menses.
Histology, immunoblotting, and hormone assays
CL tissue sections were prepared and stained with hematoxylin and eosin to observe for morphological changes as described earlier [Yadav et al. Citation2002].Immuno blot analyses of CL tissue lysates were carried out as per published procedures [Yadav and Medhamurthy Citation2006].
Estradiol and P4 concentrations in serum were determined by specific radioimmunoassays as described earlier [Suresh and Medhamurthy Citation2009].
Statistical analysis
Serum concentrations of E2 and P4 are presented as scatter graphs with mean ± SEM. Paired t-test was used to analyze the differences in the concentrations of E2 and P4. P < 0.05 was considered statistically significant.
Abbreviations
| CL: | = | corpus luteum; rhLH: recombinant human luteinizing hormone |
| hCG: | = | human chorionic gonadotropin |
| ART: | = | assisted reproductive technology |
| P4: | = | progesterone |
| E2: | = | estradiol |
| PBS: | = | phosphate buffered saline. |
Acknowledgments
We are grateful to Dr. Basavanagouda and staff of the Primate Research Laboratory for assistance with surgeries. We thank Dr. Jayachandra Kadathur for his help in histology work.
Declaration of interest: Financial support from the Department of Biotechnology, Government of India, to conduct these studies is gratefully acknowledged. The authors declare that they have no competing interests.
Author contributions: Conceived and designed the experiments: PSS, RM; Performed the experiments: PSS, RM; Analyzed the data: PSS, RM; Contributed reagents/materials/analysis tools: RM; Wrote the manuscript: PSS.
References
- Auletta, F.J., Kelm, L.B. and Schofield, M.J. (1995) Responsiveness of the corpus luteum of the rhesus monkey (Macaca mulatta) to gonadotrophin in vitro during spontaneous and prostaglandin F2 alpha-induced luteolysis. J Reprod Fertil 103:107–113.
- Bishop, C.V., Bogan, R.L., Hennebold, J.D. and Stouffer, R.L. (2011) Analysis of microarray data from the macaque corpus luteum; the search for common themes in primate luteal regression. Mol Hum Reprod 17:143–151.
- Castracane, V.D., Stevens, V., Knickerbocker, J., Powell, J., Randolph, M. and Gimpel, T. (1998) Late luteal rescue in the baboon (Papio cynocephalus). Hum Reprod Update 4:383–388.
- Devoto, L., Kohen, P., Munoz, A. and Strauss, J.F., 3rd, (2009) Human corpus luteum physiology and the luteal-phase dysfunction associated with ovarian stimulation. Reprod Biomed Online 18 Suppl 2:19–24.
- Dewi, D.A., Abayasekara, D.R. and Wheeler-Jones, C.P. (2002) Requirement for ERK1/2 activation in the regulation of progesterone production in human granulosa-lutein cells is stimulus specific. Endocrinology 143:877–888.
- Jarvela, I.Y., Niinimaki, M., Martikainen, H., Ruokonen, A. and Tapanainen, J.S. (2007) Ovarian response to the human chorionic gonadotrophin stimulation test in normal ovulatory women: the impact of regressing corpus luteum. Fertil Steril 87:1122–1130.
- Priyanka, S., Jayaram, P., Sridaran, R. and Medhamurthy, R. (2009) Genome-wide gene expression analysis reveals a dynamic interplay between luteotropic and luteolytic factors in the regulation of corpus luteum function in the bonnet monkey (Macaca radiata). Endocrinology 150:1473–1484.
- Suresh, P.S. and Medhamurthy, R. (2009) Dynamics of circulating concentrations of gonadotropins and ovarian hormones throughout the menstrual cycle in the bonnet monkey: role of inhibin A in the regulation of follicle-stimulating hormone secretion. Am J Primatol 71:817–824.
- Yadav, V.K. and Medhamurthy, R. (2006) Dynamic changes in mitogen-activated protein kinase (MAPK) activities in the corpus luteum of the bonnet monkey (Macaca radiata) during development, induced luteolysis, and simulated early pregnancy: a role for p38 MAPK in the regulation of luteal function. Endocrinology 147:2018–2027.
- Yadav, V.K., Sudhagar, R.R. and Medhamurthy, R. (2002) Apoptosis during spontaneous and prostaglandin F(2alpha)-induced luteal regression in the buffalo cow (Bubalus bubalis): involvement of mitogen-activated protein kinases. Biol Reprod 67:752–759.
- Zeleznik, A.J. (1998) In vivo responses of the primate corpus luteum to luteinizing hormone and chorionic gonadotropin. Proc Natl Acad Sci USA. 95:11002–11007.