Abstract
This study aimed to develop a modified technique for spermatogonial stem cell (SSC) transplantation with the aid of an operating microscope in an infertile mouse model. Male neonatal C57BL/6 (B6) mice served as SSC donors. SSCs labeled with the PKH26-GL marker were detected by flow cytometry to verify purity. Adult B6 males were rendered infertile by busulfan treatment as the recipient. One month later the SSC suspension was delivered into recipient seminiferous tubules by manual microinjection under microscope with 100x magnification. This was compared to the conventional mechanical micromanipulator method via efferent ducts, rete testis, and seminiferous tubules, respectively. The volume injected and time required in the different procedures were compared. The recipient accepted manual microinjection via seminiferous tubules was subjected to histology, confocal laser scanning microscopy, and real-time fluorescent PCR at different checkpoints after transplantation. Positive controls received neither busulfan treatment nor transplantation. Negative controls were injected with an equal amount of transplant medium. The results showed that manual microinjection took 10 minutes per testis for the complete delivery of 50 µl of the SSC suspension, which was significantly less time-consuming and delivered a larger volume of SSC suspension than other methods. Transplanted SSCs demonstrated the earliest transference and colonization in recipient testes 7 days after transplantation. The newly generated germ cell layers appeared to be intact during spermatogenesis 90-days post-transplantation. This manual injection technique under microscope provides an alternative method to deliver the SSCs into the recipient seminiferous tubules.
Introduction
Spermatogenesis is one of the most active and complex processes of cellular reproduction in adult males. Sperm formation requires a precise and functional coordination of the somatic cells, especially Sertoli cells of the seminiferous epithelium, to support the differentiation of germ cells into spermatozoa. Spermatogonial stem cell (SSC) transplantation is a potentially valuable technique offering oncological patients gonadal protection by reinitiating spermatogenesis from stem cells re-infused into the seminiferous tubules.
The technique for SSC transplantation into the seminiferous tubules has been investigated in various studies, and it is valuable and applicable for many species [Brinster and Zimmermann Citation1994; Schlatt et al. Citation1999; Ogawa et al. Citation1997; Ogawa et al. Citation1999]. Many researchers use injection pipettes that are controlled by a mechanical micromanipulator to transplant stem cells into the seminiferous tubules. The injection micropipette is secured in a pipette holder. The cell solution to be injected is deposited in the syringe, which is then screwed onto the metal end of an Eppendorf capillary holder attached by flexible tubing to a pressure injector. Although this method has been applied in some rodents, it is considered time-consuming and laborious because the micromanipulator needs to move to and focus on the site for each injection [Brinster and Zimmermann Citation1994; Schlatt et al. Citation1999].
Two additional methods for delivery of donor SSCs into recipient seminiferous tubules were later developed, which introduce the donor SSCs into the recipient testis via efferent ducts and rete testis [Ogawa et al. Citation1997]. However, the resistance and small space of efferent ducts severely limit the required amount of SSC transplantation [Ogawa et al. Citation1999]. Injection to rete testis requires less dissection than that of SSC transplantation via efferent ducts, but the injection angle and the depth to the rete testis are very difficult to control, therefore, leakage from injection site is inevitable [Brinster and Avarbock Citation1994].
Although spermatogonial stem cell injection has three different options, the final goal is to introduce donor germ cells into recipient seminiferous tubules where the donor proliferative spermatogonia could obtain a favorable environment for transference, proliferation, and differentiation. This study aimed to modify the SSC transplantation technique with the aid of an operating microscope for the more efficient injection of donor SSC suspension into the recipient seminiferous tubules in mice.
Results and Discussion
Purification of enriched SSC suspension
The experimental groups are summarized in . After isolation and purification, SSCs were labeled with PKH26-GL dye staining intact cell membranes. Fluorescence intensity was evaluated by flow cytometry. Positive staining rates of the purified group and the negative control were 96.95 ± 2.74% and 2.43 ± 0.56%, P < 0.05. The fluorescence intensity of the purified group and the negative control was 2135.54 ± 179.9 and 19.20 ± 1.13, P < 0.05. The purity of spermatogonial stem cell in cell suspensions was detected by flow cytometry. Using c-kit as the cell marker, the positive rate was 68.33 ± 2.45%. FF05;. Using α6-Integrin as the cell marker, the positive rate was 72.04 ± 3.65%.
Table 1. Transplantation of the donor SSCs transplantation into the recipient efferent ducts, rete testis, seminiferous tubules by mechanical micromanipulator, and seminiferous tubules by manual microinjection.
Comparison of different SSC transplantation techniques
For SSC transplantation via the recipient efferent duct, smaller volumes (15 µl per testis, 15-20 minute) of donor cell suspension were needed to fill the recipient tubules. Microsurgical penetration encountered excessive intratesticular pressure, however, it is impossible to precisely evaluate the intratesticular pressure during injection. Overfilling the testicular capsule with SSC suspension was also observed. Only one or two penetrations were attempted before leakage occurred. Injection of donor cells into the recipient rete testis required less dissection than that needed to expose the recipient efferent ducts (). However, only one or two rete testis penetrations could be performed before leakage occurred preventing effective filling of the tubules. A total of 10 µl could be injected per testis at a speed of 15 to 20 min. Even so, the injection angle and the injection depth into the rete testis were difficult to precisely determine. The injection volume via recipient seminiferous tubules using the mechanical micromanipulator could be 30 µl per testis, respectively () and the procedure is summarized in . After a tiny cut (2 mm length) on the testicular tunica under the operating microscope, seminiferous tubules were exposed to the surface of the cut by slow extrusion (Fig.1A). The pipette was filled with transplant SSC suspension. The injected SSC fluid could be observed filling the seminiferous tubules as they turned blue because of the blue dye in transplant suspension (Fig.1B). Same procedure was repeated in other unfilled seminiferous tubules at the same incision, in order to achieve a precise SSC microinjection into a maximal number of seminiferous tubulues (Fig.1C). At the end of each procedure, one third of the total testicular surface area turned blue. The testis for SSC transplantation received three incisions in total, one incision at the middle of the anterior-lateral testicular envelope, another incision along the posterior-lateral axis of the testicular envelope, and the last in the middle of the internal testicular envelope. Approximately 50 µl of transplant SSC suspension was injected at the speed of 10 minutes per testis (Fig.1D). For manual microinjection of SSC suspension via recipient seminiferous tubules, the transplant suspension was accurately injected into maximal recipient seminiferous tubules that were exposed at the cut until filling 1/3 of an entire testis. A testis for transplant received a total of three cuts using the above protocol to achieve a maximal transfer. Almost 100% of surface tubules can routinely be filled by this procedure. The injection procedure is highly efficient (average 10 minutes per testis). The health of recipients was assessed by inspection on the day after busulfan treatment and transplantation, and then regularly inspected until recipient testes were collected. All were healthy with no occurrence of inflammation on the testicular tissue. Comparison of outcomes of the different transplantation methods is shown in .
Figure 1. Microinjection into the seminiferous tubules. A) Performing a small incision under microscope. B) SSC suspension was injected into the seminiferous tubules. C) The single cell suspension was transplanted via pipettes into the maximal seminiferous tubules through the same incision. D) Overview of the entire testicular tissue after the microinjection.

Transference, proliferation, and differentiation of transplanted SSCs
Transplanted PKH26-GL labeled SSCs were studied by CLSM at day 7 (B), SSCs taken from the transplantation group showed fluorescent spermatogonia filling the lumina of seminiferous tubules, and portions of the corresponding fluorescence was dispersed to the basal membrane of the recipient seminiferous tubules. It was the earliest report of the SSC colonization. The fluorescence was detected from the basal membrane, and no fluorescence was observed in the lumina of the seminiferous tubules at 30 days post-transplantation. Portions of the stain disappeared from the cell membrane due to cell division after migration into the basal membrane of the seminiferous tubules. At day 90, spots of scattered fluorescence indicated that the transplanted spermatogonial stem cells colonized in the recipient testis and formed ample spermatids in lumen of the seminiferous tubules (C and 2D).
Figure 2. Observation of the recipient seminiferous tubules by PKH26–GL and DAPI fluorescence photomicrograph after transplantation. A) The negative control (CLSM × 100). B) The fluorescence showed the earliest occurrence of spermatogonia dispersed at the basal membrane of seminiferous tubules (CLSM ×100) 7 days after SSC transplantation. C) The PKH26–GL fluorescence could be seen at the basal membrane 90 days post-transplantation (CLSM ×400). D) The transplanted spermatogonial stem cells colonized the recipient testis and formed plenty of spermatids in lumen of the seminiferous tubules 90 days post-transplantation observed by PKH26–GL and DAPI fluorescence (CLSM × 400).
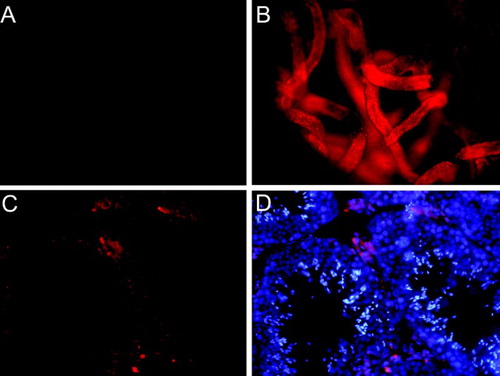
We noticed that substantial red fluorescence appeared in the interstitial space (D) and deduced that it might be caused by: i) leakage of transplant SSC suspensions into interstitial space; ii) not the frontal section of seminiferous tubules in the tissue slicing; iii) transplant SSCs were devoured by Leydig cells. No red fluorescence appeared in the interstitial space in negative control (A).
H&E stain showed that nearly all the endogenous spermatogonia disappeared and sertoli cells decreased after establishment of the infertility model with busulfan treatment. In the positive control, spermatogonia in different stages were aligned on the seminiferous epithelium orderly and spermatozoa were formed in lumina of the seminiferous tubules during three months. With the extension of observation time, Sertoli cells increased and few endogenous spermatogonia emerged in the seminiferous epithelium in the negative control. In the transplant group, spermatogonia in different stages were aligned gradually in the seminiferous epithelium orderly, spermatozoa were formed in lumina of the seminiferous tubules, and Sertoli cells increased ().
Figure 3. Manifestations of the positive control, the negative control, and the transplantation group at different checkpoints (H&E Staining ×400). The positive control showed autologous SSCs proliferated at all checkpoints. The negative control showed limited spermatogenesis at all checkpoints. The transplantation group, donor SSCs colonized nearly filled all lumina of the seminiferous tubules at the first checkpoint, and thereafter multiplied dominantly.
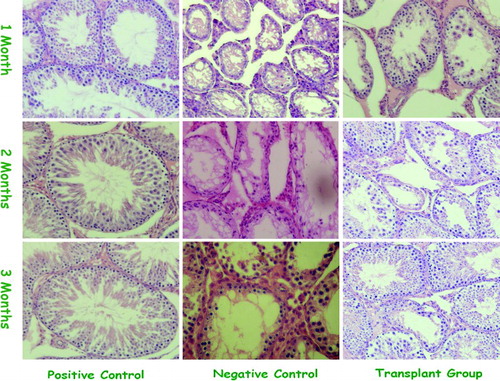
Transplanted SSCs generated different stages of spermatogonia in recipient seminiferous tubules, as well as primary and secondary spermatocytes. Spermatocytes were detected from the seminiferous tubules-cavity surface to the bottom, and sperm were formed in the lumina in the positive control (). The negative control showed few spermatogenic cells and limited spermatogenesis at the lumina of seminiferous tubules at the second checkpoint. However, the transplantation group demonstrated donor SSC colonization on nearly all lumina of the seminiferous tubules by 30 days post-transplantation, and thereafter multiplied dominantly and formed numerous spermatozoa. By 90 days post-transplantation, sperm were also observed in lumina of the seminiferous tubules. No morphological abnormalities in spermatogenesis, missing layers of germ cells, or abnormalities in the elongation phase of spermatogenesis were found in the germ cell colonies at day 90 ().
The c-kit and α6-Integrin mRNA expression were assessed by RFQ-PCR. The sequence of c-kit and α6-Integrin were amplified and yielded 115 bp and 97 bp product, respectively. Target PCR product content was corrected using the internal standard GAPDH gene (method, target gene copies equaled to 106 internal standard gene copies), using the standard curve and amplification curve as parameters for the mRNA analysis. All PCR products were quantitated against GAPDH (Tables and ). At each checkpoint, the levels of c-kit and α6-Integrin mRNA from the transplantation group were lower than those of the positive control, and higher than those of the internal control, all p < 0.05. Additionally, the level of marker mRNA increased along each group (all p < 0.01).
Table 2. C-kit mRNA expression of the positive control, negative control, and transplantation groups at different checkpoints, RFQ–PCR.
Table 3. α6-Integrin mRNA expression of the positive control, negative control, and transplantation groups at different checkpoints, RFQ–PCR.
SSC transplantation may provide potential treatment for specific male infertilities [Poirot and Schubert Citation2011; Yuan et al. Citation2009]. The mouse model of SSC transplantation provides a model system for basic and clinical research [Yuan et al. Citation2009]. Spermatogonia transplantation was accomplished in 1994 when Brinster and Zimmermann [1994] published their landmark findings of SSC transplantation. Following their discovery, transplantation of donor SSCs via the recipient efferent ducts and via the recipient rete testis were successfully developed [Ogawa et al. Citation1997].
Injection of donor spermatogonia into recipient efferent ducts is a difficult technique that requires careful surgical dissection to expose the delicate efferent ducts [Ogawa et al. Citation1997]. Nevertheless, it was reported that the injection of donor spermatogonia into recipient efferent ducts is more quickly performed than seminiferous tubular injection [Brinster and Zimmermann Citation1994]. In this study, efferent ducts were also selected as the injection site for SSC transplantation, following a pressure increase in the pipette the cell suspension would flow first into the rete and then began to fill the seminiferous tubules. However, less volume of donor cell suspension was injected into recipient efferent ducts compared with microinjection into the seminiferous tubules. The microsurgical penetration encountered excessive intratesticular pressure during injection via the recipient efferent duct. Overfilling the testicular capsule may result in intratesticular ischemia with a subsequent detrimental effect on donor cell viability and on the recipient tubular environment [Ogawa et al. Citation1997]. However, it is impossible to precisely evaluate the intratesticular pressure during injection. Injection via rete testis required less dissection than that needed to expose the recipient efferent ducts. Therefore, it is known as a more rapid approach. Our experience showed that the injection angle and the depth of injection into the rete testis were difficult to precisely determine. The injection pipette easily penetrated through the wall, dispersing the donor SSC suspension into interstitial tissue. Only one or two rete testis penetrations could be attempted per testis, the leakage from the first penetration site often escalated during the second penetration preventing larger volume transfer. However, the injection volume determined the quantity of proliferative spermatogonia that could be transferred. It is a key element to evaluate SSC transplantation efficiency. Direct microinjection of donor SSCs into the recipient seminiferous tubules is less invasive and allows injection of donor SSCs into several tubules at various sites on the testicular surface [van Pelt et al. Citation1996]. The conventional method for donor SSC transplantation is the penetration of seminiferous tubules by two needles, guided by two mechanical micromanipulators under the microscope [Tanaka et al. Citation1997]. Although the method is appropriate for the immature, mature, and post-mature rat and mouse testis, the depth of field and focus may change during injection. Therefore, when using a mechanical micromanipulator, the depth of field and focal length needs to be adjusted. On average it takes 30 minutes/testis to complete an entire testis, it is time-consuming and laborious [Brinster and Zimmermann Citation1994; Schlatt et al. Citation1999]. In this study, we could microinject 50 µl SSC suspension into seminiferous tubules by this manual technique within 10 minutes per testis, which demonstrated that this modified technique was quick and feasible.
As shown above, we employed a new modified technique for SSC transplantation with the aid of an operating microscope via the manual microinjection of SSC suspension into the mouse seminiferous tubule. Comparing the techniques of microinjection via efferent ducts and rete testis, the main advantages for this new technique are: i) that once a penetration was achieved, the cell suspension could be immediately transferred into seminiferous tubules; ii) that the cell suspension could be precisely injected into multiple seminiferous tubules until the SSC suspension filled the whole testicle; and iii) that approximately 50 µl of transplant SSC suspension was delivered at an average speed of 10 minutes per testis. Compared with the conventional method for donor SSC transplantation via seminiferous tubules by mechanical micromanipulator, the advantages of the new technique are: i)that during the injection, the depth of field and focus could be flexibly adjusted, and ii) that it was time-saving, as in a surgical status under anesthesia by sodium pentobarbital, the longer the narcotic period for the experimental animals, the greater detrimental impact on their physical condition. However, it was not easy to perform manual microinjection at 100 x magnification. Practice is required to master this microsurgical technique.
With this modified technique for SSC transplantation, we evaluated the outcomes through three cycles of mouse spermatogenesis. By tracking the red fluorescence PKH26-GL dye with CLSM, the photomicrographs demonstrated that the spermatogonia filled in all the lumina of seminiferous tubules, and portions of the corresponding fluorescence also dispersed into the basal membrane of seminiferous tubules at 7 days post-transplantation, which became the earliest occurrence of the colonization of the migrating donor cells. Both results from H&E and CLSM demonstrated that the transplanted SSCs showed the earliest occurrence of transference, and colonization in recipient testes. The fluorescence photomicrographs showed spots of scattered fluorescence 90 days after transplantation, which indicated the formation of plenty of spermatogonia in the lumina of seminiferous tubules. After high-dose chemotherapy, there were a certain number of spermatogonial stem cells and sertoli cells in the recipient seminiferous epithelium, the number of the recipient SSCs and sertoli cells increased when the toxic effect faded away, which suggested that both the donor-derived spermatogenesis and the recipient-derived spermatogenesis came forth after the donor stem cells were transferred to the recipient testis. However, the purified donor SSCs were able to proliferate vividly and form plenty of spermatozoa in lumina of the seminiferous tubules. Neither morphological abnormalities in spermatogenesis, nor missing layers of germ cells, nor abnormalities in elongating phase of spermatogenesis were found in the germ cell colonies 90 days post-transplantation. Histological analysis of the grafted tissue showed that first elongating spermatids appeared at 30 days post-transplantation, and up to 20% of the seminiferous tubules contained the elongated spermatids which was consistent with the findings of Honaramooz et al. [2002]. A key indicator for premeiotic germ cell migration, spermatogonial adhesion and proliferation, early differentiation, and the inhibition of apoptosis of germ cells in the testis is c-kit [Yoshinaga et al. Citation1991; Loveland and Schlatt Citation1997; Vincent et al. Citation1998]. We monitored the SSC transference, proliferation, and differentiation using c-kit as a molecular marker. By RFQ-PCR analysis, we found that c-kit expression in the transplantation group was lower than that in the positive control and higher than that in the negative control three months after SSC transplantation, which was consistent with observation of the results of H&E staining. As a laminin receptor, α6-Integrin has been verified as a molecule marker to identify SSC [Shinohara et al. Citation1999]. We used α6-Integrin as the molecular marker of SSC to monitor the SSC transference, proliferation, and differentiation and the results were the same with that of c-kit as the molecular marker. Several morphological abnormalities in spermatogenesis after syngeneic spermatogonial transplantation, such as missing layers of germ cells or abnormalities in the elongating phase of spermatogenesis [Russell et al. Citation1996] were originally reported; in this study, however, the linage characteristic of the transplantation group, and the close linage of the c-kit and α6-Integrin expression between the positive control and the transplantation group, indicated that the abnormalities rarely existed after transplantation within three complete mouse spermatogenesis cycles, which is a key indicator of an improved technique for SSC transplantation.
Full implementation of the transplantation technology depends largely on the development of recipient preparation, enrichment of undifferentiated spermatogonia within the transplant solution, and technical improvement of the transplantation procedure [Khaira et al. Citation2005]. In this study, microinjection of SSCs into efferent ducts and testicular rete had been performed using a glass pipette with a sharp tip of 30-40 µm. Movement of the pipette was controlled by a mechanical micromanipulator. We tried to inject the donor cells into the seminiferous tubules through the efferent ducts. Following increasing pressure in the pipette, the cell suspension flowed first into the testis rete and then began to fill the seminiferous tubules. After successful attempts, small amounts of colored solution could be detected in the intratesticular rete and seminiferous tubules. Before efferent duct injection, one must carefully dissect the area between testis and the head of the epididymis. The fragile efferent ducts are hard to recognize in the fat and connective tissue present in this area. In addition, filling was not even when the suspension spread from testis rete. This is probably because of the variation of the resistance of tubules. Once the pressure in the pipette increased sharply, we noticed that blood circulation would disappear on the testicular surface or the tunica became ruptured, which might result in an ischemic testis. As for the technique of SSCs injection via testis rete, we also found that manipulation was even more difficult. The reasons are first, the depth of testis rete is only 0.2 mm or less in mouse, if the injection angle deviated or the pipette went beyond the limit, the intratesticular boundary of testis rete would be penetrated, thus, the donor cell suspension would leak into intertubular tissue rather than filling into the tubules. And second, the injector tip cannot dislodge from the rete because reentry into the same opening is almost impossible, and an additional penetration may result in leakage from the original puncture site. Compared to the above methods, the modified technique with the aid of an operating microscope allowed the precise microinjection of an efficient volume of donor SSC suspension into recipient seminiferous tubules. Using this new technique we demonstrated that donor SSCs could transfer, proliferate, and differentiate in recipient testis. Therefore, it might be an alternative approach for SSC transplantation.
The use of transgenic donor mice would be more ideal to identify spermatogenic cells in transplanted testis rather than using cell surface marker. In this study, we used PKH26-GL red fluorescence to label the transplanted cells, which can be incorporated into the cell membrane, combined with RFQ-PCR technique to trace donor SSC transference, differentiation, and proliferation. In long-term observation this was unable to differentiate between the restored endogenous spermatogenesis and the transplanted SSCs. However, this pilot study emphasizes the modified technique of SSC transplantation. It was demonstrated that cell surface labeling had sufficed to determine SSCs colonization after delivery of SSCs into the recipient seminiferous tubules. Further studies using transgenic donor mice are required to determine the efficacy of SSC differentiation following transplantation.
Materials and Methods
Ethics statement
All experimental procedures were approved by the animal care and use committee of Fudan University in accordance with the guide for care and use of laboratory animals of the national academy of sciences (Permit No. 2007-0002).
Donor and recipient mice
Neonatal male C57BL/6 (B6) mice (6-10 d-old) served as SSC donors (n = 20). Adult B6 males (six-w-old) (n = 40) were randomly selected as positive control. As recipient (n = 130) for SSC tranplantation and negative control, infertility was induced in adult B6 mice by intra-abdomen injection of busulfan (Sigma Chemical Company, St. Louis, MO, USA) at the dosage of 40 mg/kg one month before SSC transplantation [van Pelt et al. Citation1996]. The recipients were maintained in the Shanghai Experimental Animal Center, Chinese Academy of Science. The animal health was assessed by inspection on the day after busulfan treatment and transplantation, and then regularly inspected until the testes were collected.
Separation and purification of SSCs
Dulbecco′s Modified Eagle Medium: Nutrient Mixture F12 (DMEM/F12%(Sigma) was prepared according to the product instructions, containing 10% fetal bovine serum (FBS; Gibco, Lifetechonology, Paisley, Scotland, UK), 50 U/ml penicillin, 50 µg/ml streptomycin, 0.12% NaHCO3, 4 mmol/L L-glutamine, 0.5mmol/L sodium pyruvate, and 15 mmol/L 4-(2-hydroxyethyl)-piperazineethanesulfonic acid (HEPES) buffer (pH 7.4).
Donors were anaesthetized with 1% sodium pentobarbital injection. Testes were collected aseptically. Single cell suspensions were prepared as previously described [Van Pelt et al. 1996]. Cell concentration was adjusted to 5 × 106 cells/ml after discarding the supernatant. For purification, the dissociated SSCs were pelleted by centrifugation and separated by size using equilibrium density gradients of 10%, 25%, 35%, and 50% Percoll (Pharmacia Biotech AB, Uppsala, Sweden). After centrifugation at 600 g at 18 °C for 30 min, purified cell suspension was washed three times with Ca2+ and Mg2+ free Hank's balanced salt solution (HBSS; Gibco, Lifetechonology, Paisley, Scotland, UK ). For transplantation, the purified suspension was adjusted to a concentration of 2 × 107 cells/ml by DMEM/F12 medium [Wang et al. Citation2008], containing 72% spermatogonia with abundant SSCs. Cell viability was above 95%. It was preserved on ice. Immediately before transplantation, SSC suspension was adjusted to a final concentration of 0.04% trypan blue to assess the location of the transplant SSC suspension in the recipient seminiferous tubules.
Flow cytometric detection on purity of SSC suspension
Donor SSCs were washed with HBSS, and then were centrifuged at 500 g for 5 min. SSC cells were resuspended in C kit dilution provided by PKH26-GL kit (Sigma). PKH26-GL is a fluorescent cell linker compound that is incorporated into the cell membrane and is found to be useful for cell labeling. PKH26-GL was diluted in C kit solution to a concentration of 4 × 0−6 mol/ml. Cell suspension were gently mixed with an equal volume of PKH26-GL staining solution to a final concentration of 1 × 107 cells/ml. An equal volume of 4% fetal bovine serum (FBS; Gibco, Lifetechonology, Paisley, Scotland, UK) was added to terminate the staining and further incubated for 1 min, and then adjusted to a concentration of 2 × 107 cells/ml after thrice wash with HBSS. Samples were taken for evaluation of fluorescence intensity and positive staining, and a cell suspension of the same concentration but without PKH26-GL staining served as the negative control.
Experimental groups
The first experiment was demonstrated in . For evaluation of transplantation efficiency and outcomes, recipients were divided into four groups to receive microinjection of SSC suspension via efferent ducts (n = 30), rete testis (n = 30), seminiferous tubules by mechanical micromanipulator (n = 30), and seminiferous tubules by manual microinjection (n = 40), respectively.
After manual microinjection via seminiferous tubules with the aid of the operating microscope was identified to be most optional, it underwent further investigation for evaluation on the donor SSC transference, differentiation, and proliferation by hematoxylin and eosin (H&E) staining, confocal laser scanning microscopy (CLSM), and real-time fluorescent PCR. The second experiment was divided as the positive control (n = 40), the negative control (n = 40, a lateral testis per mouse), and the transplantation group (n = 40, a lateral testis per mouse). Ten mice were randomly selected from each group respectively, at 30, 60, and 90 d post-transplantation. In addition, an extra 10 mice from each group were employed 7 d post-transplantation, and were subjected to CLSM examination for the exploration on the earliest donor SSC colonization.
SSC transplantation
Transplantation of donor SSCs into recipient via efferent ducts [Ogawa et al. Citation1997], rete testis [Ogawa et al. Citation1997], seminiferous tubules using mechanical micromanipulator [Brinster and Zimmermann Citation1994], and seminiferous tubules using manual microinjection were explored respectively. For manual microinjection of donor SSC suspension into recipient seminiferous tubules, the preparation of injection pipette was similar to that originally described for the SSCs injection [Wang et al. Citation2008]. The transplant medium consisted of 0.4g/L KCL, 0.06g/L KH2PO4, 8.0g/L NaCl, 0.35g/L NaHCO3, 0.048g/L Na2HPO4, 1.0g/L D-glucose, 0.037g/L EDTA, 5g/L BSA, and 0.1g/L DNase (Sigma) [Sofikitis et al. Citation2003]. One lateral testis of each recipient was subjected for SSC transplantation. After a tiny cut (2 mm length) on the testicular tunica under the operating microscope, seminiferous tubules were exposed to the surface of the cut by slow extrusion (). The pipette was filled with transplant SSC suspension. When selecting a seminiferous tubule, the injection pipette was held parallel to the ordinate axis of the seminiferous tubule. Using a SMZ1000 Zoom Stereomicroscope (Nikon, Torrance, CA, USA) equipped with TK-C 1481 EG High Resolution Colour Camera (JVC, Yokohama, Japan) at 100 x magnification, SSC suspension was gently injected into the seminiferous tubules. Approximately 50 µl of transplant SSC suspension was injected at the speed of 10 min per testis . The contralateral testis of recipient was injected with an equal amount of transplant medium by the same method, serving as the negative control. The incisions on the testicular tunica were closed with one stitch of 5-0 absorbable suture. After the testis was returned to the body cavity, the peritoneum layer was closed continually with 4-0 absorbable suture and finally the skin wound was closed with interrupted 3-0 silk stitches.
CLSM
After harvest of testicular tissue samples, they were decapsulated with fine forceps in HBSS for full separation of seminiferous tubules. The seminiferous tubules were fixed with 4% paraformaldehyde for 30 min. In order to trace the transplanted SSCs in recipient seminiferous tubules and to rebuild the three-dimensional vision, a red fluorescence PKH26-GL was applied, which can be activated by a 551nm wavelength argon ion laser and collected through a λ emission max 567nm long pass filter. The testis sample were embedded in O.C.T and frozen by liquid nitrogen, and then sliced into 10 µm in thickness by Leica Ultracut E ultramicrotome. The slices were fixed in 4% paraformaldehyde for 20 min. After washing in PBS, they were incubated in 5 mg/ml 4', 6-Diamidino-2-phenylindole (DAPI, Sigma) solution for 10 min at room temperature. A blue fluorescence was observed by CLSM, which can be activated by a 364 nm wavelength xenon ion laser and collected through a λ emission max 454nm long pass filter.
H&E staining
The testicular tissues were collected and fixed in Bouin's fixative for 24 h, dehydrated, and embedded in paraffin. Sample tissues were sectioned transversely to obtain 10 µm slices by Leica Ultracut E ultramicrotome. Slices was stained by hematoxylin for 10 min and followed by eosin staining for 1 min, and then examined by inverted light microscopy (Zeiss, Bayer, Germany).
RFQ-PCR
Recipient testicular tissue (1 mg) was sampled, total RNA was isolated and purified, and cDNA was amplified from RNA using RT-PCR. The expression level of c-kit and α6-Integrin mRNA was analyzed respectively. Briefly, the c-kit gene was amplified using upstream primer 5'-CTTCTGTGTCACCATTTGGAAAAC-3', and downstream primer 5'- CGTTGGAGGCCTTACACTCC-3', respectively. PCR primers were designed by primer 3 software (PubMed GenBank). Mouse cDNA sequences were synthesized. For detection of the target gene sequence, the TaqMan probe 5'-FAM-TGGTTCAGAGTTCCATAGACTCCAGCGTC-TAMARA-3' was purchased from Takara Bio Inc. (Takara, Shiga, Japan). The α6-Integrin gene was amplified using upstream primer 5'-GTCTGTAACAGCAACCTTAAGCTAGAGT-3', and downstream primer 5'-ATCTTTTAGGACTAATTCTGGGATGC-3', respectively. PCR primers were designed by primer 3 software (PubMed GenBank). Mouse cDNA sequences were synthesized. For detection of the target gene sequence, the TaqMan probe 5'-FAM–TTGGTACCCGAGAAGGAAATCAAGACAAATTC-TAM ARA-3' was purchased from Takara Bio Inc. (Takara, Shiga, Japan). The intensity of the digested mRNA product was quantified using iCycler version 3.0 analysis software (Bio-Rad, Hercules, California, USA). The amplification product was quantified through an ultraviolet spectrophotometer (Beckman, Fullerton, California, USA) with standard curves obtained from 106 copies of cDNA template.
Statistical analysis
Representative data are shown as the mean with corresponding standard deviation (means ± SD). Statistical analysis was conducted using SPSS 11.5 Statistical Software. Where indicated, the t test and single factor analysis of variance were used in data analysis, with a significance level of p < 0.05.
Declaration of interest: This work is supported by the National Natural Science Foundation of China, No. 30860282, and the National Basic Research Program (973) of China, No. 2011CB944503. The authors report no conflicts of interest. The authors are fully responsible for the content and writing of the paper.
Author contributions: Conceived and designed the experiments: LM, BL, XW; Performed the experiments: LM, BL, LL; Analyzed the data: LM, BL, LL; Wrote the paper: LM, BL, LL, XW, CL, QD.
Abbreviations
| SSC: | = | spermatogonial stem cell |
| CLSM: | = | confocal laser scanning microscopy |
| H&E: | = | hematoxylin and eosin |
| PCR: | = | polymerase chain reaction |
| RT-PCR | = | reverse transcription PCR |
| RFQ-PCR: | = | real-time fluorescence quantitative PCR. |
References
- Brinster, R.L. and Zimmermann, J.W. (1994) Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA 91:11298–11302.
- Brinster, R.L. and Avarbock, M.R. (1994) Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA 91:11303–11307.
- Honaramooz, A., Snedaker, A., Boiani, M., Schöler, H., Dobrinski, I. and Schlatt, S. (2002) Sperm from neonatal mammalian testes grafted in mice. Nature 418:778–781.
- Khaira, H., McLean, D., Ohl, D.A. and Smith, G.D. (2005) Spermatogonial stem cell isolation, storage, and transplantation. J Androl 26:442–450.
- Loveland, K.L. and Schlatt, S. (1997) Stem cell factor and c-kit in the mammalian testis: lessons originating from Mother Nature's gene knockouts. J Endocrinol 153: 337–344.
- Ogawa, T., Aréchaga, J.M., Avarbock, M.R. and Brinster, R.L. (1997) Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol 41:111–122.
- Ogawa, T., Dobrinski, I. and Brinster, R.L. (1999) Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell 31:461–472.
- Poirot, C. and Schubert, B. (2011) Fertility preservation in prepubertal children. Bull Cancer 98:489–499.
- Russell, L.D., Franca, L.R. and Brinster, R.L. (1996) Ultrastructural observations of spermatogenesis in mice resulting from transplantation of mouse spermatogonia. J Androl 17:603–614.
- Schlatt, S., Rosiepen, G., Weinbauer, G.F., Rolf, C., Brook, P.F. and Nieschlaq E. (1999) Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod 14:144–150.
- Sofikitis, N., Kaponis, A., Mio, Y., Makredimas, D., Giannakis, D., Yamamoto, Y., (2003) Germ cell transplantation: a review and progress report on ICSI from spermatozoa generated in xenogeneic testes. Hum Reprod Update 9:291–307.
- Shinohara, T., Avarbock, M.R. and Brinster, R.L. (1999) β1- and α6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA 96:5504–5509.
- Tanaka, A., Nagayoshi, M., Awata, M., Mawatari, Y., Tanaka, I.and Sofikitis, N. (1997) Conclusions from the transplantation of human or hamster spermatogonia/primary spermatocytes to rat or mouse testis. Fertil Steril 68:S61.
- van Pelt, A.M., Morena, A.R., van Dissel–Emiliani, F.M., Boitani, C., Gaemers, I.C., de Rooij, D.G., . (1996) Isolation of the synchronized A spermatogonia from adult vitamin A-deficient rat testes. Biol Reprod 55:439–444.
- Vincent, S., Segretain, D., Nishikawa, S., Nishikawa, S.I., Sage, J., Cuzin, F., (1998) Stage-specific expression of the Kit receptor and its ligand (KL) during male gametogenesis in the mouse: a Kit-KL interaction critical for meiosis. Development 125:4585–4593.
- Wang, X., Ding, Q., Zhang, Y., Wang, H., Ma, L. and Xie, X. (2008) Two allogeneic descendents derived from the high-dose busulfan-treated infertile mouse model after freeze-thawed spermatogonial stem cell transplantation. Fertil Steril 90:1538–1549.
- Yoshinaga, K., Nishikawa, S., Ogawa, M., Hayashi, S., Kunisada, T., Fujimoto, T., (1991) Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 113:689–699.
- Yuan, Z., Hou, R. and Wu, J. (2009) Generation of mice by transplantation of an adult spermatogonial cell line after cryopreservation. Cell Prolif 42:123–131.