Abstract
The object of this study was to investigate the effect of molybdenum on the development of mouse preimplantation embryos cultured in vitro. Zygotes were flushed from one outbred mouse strain (Kunming), and then were cultured in potassium simplex optimized medium (KSOM) containing 0, 5, 10, 20, 40, 80, 120, and 160 µg/ml of molybdenum for 5 days until the mid-blastocyst stage. The addition of ≤ 20 µg/ml molybdenum did not affect the blastocyst and birth rates. Molybdenum at doses of 40 µg/ml and higher significantly decreased the cleavage, blastocyst and birth rates, the average cell number, and significantly increased the proportion of degenerative blastocysts. At 120 µg/ml molybdenum inhibited the blastocysts development to birth. At 160 µg/ml molybdenum caused overall developmental arrest (up to 16-cells) of embryos and their massive degeneration. In conclusion, molybdenum negatively affected the development of embryos in a dose-dependent manner. With lower doses (≤ 20 µg/ml), mouse embryos were not apparently damaged. With very high doses (≥ 40 µg/ml), embryo quality significantly decreased. This assessment of the effect of molybdenum on the preimplantation embryo is an initial survey of toxicological risk.
Introduction
Environmental contamination is a serious problem around the world [Ishaq et al. Citation2010]. Heavy metals are a major source of pollution and have caused several adverse health effects [Järup Citation2003]. Molybdenum, as a heavy metal, has been broadly used in industrial manufacturing [Pandey and Singh Citation2002]. Animals exposed to molybdenum in drinking water or while foraging for plants are likely to suffer from molybdenosis or reflect copper deficiency [Raisbeck et al. Citation2006]. Humans exposed to molybdenum in food [Vyskocil and Viau Citation1999] or circumstances releasing high levels of molybdenum [Titenko-Holland et al. Citation1998; Kargar et al. Citation2011; Yu et al. Citation2011] are likely to suffer from anemia, anorexia, profound diarrhea, gout-like disease, reduced sexual activity [EVM 2000], and neural tube defects [Huang et al. Citation2011].
Excessive amounts of molybdenum can cause adverse reproductive effects. Previous studies have mainly investigated the adverse effects of this metal on male reproductive function [Pandey and Singh Citation2002; Thomas and Moos Citation1951; Lyubimov et al. Citation2004; Meeker et al. Citation2008; Wirth and Mijal Citation2010]. In comparison, few studies on the adverse effects on female reproductive health have been presented and have conflicting information. For example, Huang et al. [2011] suggested that molybdenum at a high-dose (8.51 µg/mg average content levels in soil) during human pregnancy was related to the increased risk of neural tube defects. Furthermore, Fungwe et al. [1990] indicated that a high level of molybdenum significantly prolonged estrous cycle and caused several adverse effects on rat embryogenesis. In contrast, other studies showed no effects on the estrous cycle and reproductive indices in rats [Lyubimov et al. Citation2004], or the estrous cycles and conception rates in guinea pigs [Howell et al. Citation1993].
The correlation of cord blood levels of molybdenum in newborns with maternal concentrations of molybdenum suggests that molybdenum compounds easily cross the placental barrier [Bougle et al. Citation1988; Bougle et al. Citation1989]. Moreover, mammalian embryo development is highly sensitive to environment, presenting as embryo loss and pregnancy rate reduction [Pampfer and Donnay Citation1999; Fleming et al. Citation2004; Fabian et al. Citation2011]. To our knowledge there is no data on in vitro embryo toxicity of molybdenum in mammals.
The purpose of the study was to investigate the possible toxicity of molybdenum on mouse preimplantation embryos. The embryos were cultured in media containing various concentrations of molybdenum, and their quality and subsequent developmental potential were used to evaluate the effects of molybdenum on the development of the mouse preimplantation and post-implantation embryo development.
Results
As indicated by the average number of cells, birth rate, and degenerate rate molybdenum at ≤ 10 µg/ml did not significantly affect the embryo quality (). Morphological evaluation of cultured blastocysts showed that at ≥ 40 µg/ml molybdenum negatively affected blastocyst quality by significantly decreasing average number of cells and birth rate, and significantly increasing degenerate rate (). Few blastocysts were obtained from 120 µg/ml and none were observed at a concentration of 160 µg/ml, which similarly showed a significant increase in the incidence of both degenerate embryos () and arrest.
Figure 1. Analysis of developmental capacities of embryos. Embryos were in vitro cultured in media supplemented with 0 (control) to 160 µg/ml Mo for 5 d. The developmental quality of cultured embryos (normal blastocysts, degenerate blastocysts, and arrested at the preblastocyst (2-cell to morula) stage with abnormal morphology or lysed cellular content) were evaluated using stereomicroscopy and differences in the distribution of embryonic stages were analyzed using chi-square test (**P < 0.01). Mo: molybdenum
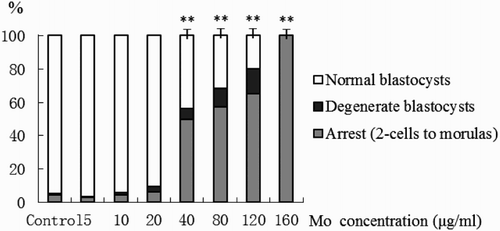
Table 1. Effects of sodium molybdate on the developmental capacity of mouse embryos cultured in vitro.
Embryos cultured in molybdenum at ≥ 40 µg/ml were of poor quality showing highly retarded development (). Even at lower concentrations, where cell numbers were significantly lower, neither blastocyst development nor birth rates were affected. At a concentration of 40 µg/ml and higher molybdenum significantly decreased the proportion of in vitro derived blastocysts, and their quality was poor (). None of blastocysts from the 120 µg/ml treatment was able to develop to birth. Developmental arrest (up to 16-cells) and massive degeneration was observed at 160 µg/ml.
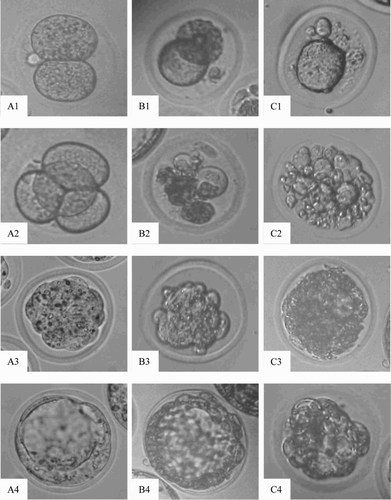
Figure 2. Morphological images of embryo (×400). A1-A4) normal embryos from control group; B1-B4 and C1-C4) degenerate embryos from the tested groups exposure to sodium molybdate dihydrate at dose 40 µg/ml and 120 µg/ml, respectively. Arabic numerals 1-4: 2-cell, 4-cell, morula and blastocyst stages of embryo development, respectively.
Discussion
Only limited information is available on female reproductive toxicity of molybdenum compounds in mammals, poultry, and invertebrates [Jeter and Davis Citation1954; Lepore and Miller Citation1965; Suttle and Field Citation1969; Van Gestel et al. 2011a; Van Gestel et al. Citation2011b]. These early studies mainly focused on in vivo toxicity of molybdenum. To address this limitation, in vitro toxicity of molybdenum on mouse preimplantation embryo development was evaluated.
The results presented in this study show that the presence of molybdenum can negatively affect the development of embryos in a dose-dependent manner. When molybdenum levels were equal to or greater than 40 µg/ml, embryonic development was significantly reduced, and the quality of in vitro cultured blastocysts was also negatively affected. This was shown by significant decrease of the average cell number and significant increase of the incidence of degenerated blastocysts. Most of the blastocysts which appeared morphologically normal did not develop to birth after embryo transfer. Similar phenomena were observed in other types of cells cultured in vitro. For example, ammonium tetrathiomolybdate at 10 µg/ml resulted in a marked reduction in cellular clumping, and at 100 µg/ml and higher in absence of clumping [Kendall et al. Citation2003]. This showed that ammonium tetrathiomolybdate can prevent induced differentiation of bovine granulosa cells cultured in vitro. A follow-up study showed that 5-100 µg/ml of ammonium tetrathiomolybdate can prevent induced differentiation of bovine theca cells in culture, but apparently not affect cellular morphology and cell number [Kendall et al. Citation2006]. In addition, ammonium molybdate at 330 µg/ml can inhibit cell division and differentiation in vitro [Kolesarova et al. Citation2009]. The results in the present study are consistent with the above studies in aspects of dose of molybdenum and their effects on the differentiation of cells cultured in vitro.
Our results also show that the preimplantation embryos might have some degree of tolerance to molybdenum. Embryos exposed to lower concentrations (≤ 20 µg/ml) of molybdenum develop to term without significant difference from the control, which is probably due to the element(s) (such as copper [Lawits and Biggers Citation1993]) that are in excess and thus still available. However, this is limited; as no offspring were obtained from the embryos treated with 120 µg/ml and higher concentrations of molybdenum. Whether molybdenum negatively affects full term development of the embryos and whether the damage is reversible requires further study. The in vitro embryotoxicity model reported in this study might be also used for investigating other biological functions of molybdenum on the embryos. The use of an in vitro culture environment has allowed us to isolate the effects of a single element, molybdenum, on the development of the mouse embryo removing potential confounding factors.
In conclusion, molybdenum negatively affects the development of preimplantation embryos in a dose-dependent manner. Mouse embryos were able to survive in ≤ 20 µg/ml molybdenum without apparent developmental damage. However, at 40 µg/ml or higher molybdenum significantly decreased embryo quality.
Materials and Methods
Chemicals
Unless otherwise stated, all media and components were purchased from Sigma–Aldrich Corp. (St. Louis, MO, USA).
Animals
This study was approved by the Animal Care and Use Committee at the Henan University of Science and Technology. Kunming (KM) and ICR strain female mice, aged 4 to 8 w, were used as embryo donors and recipients, respectively. All mice used in this study were maintained under Good Laboratory Practice (GLP) conditions. The mice had free access to drinking water and commercial standard pellet diet.
Embryos and treatment
Mouse embryos were generated from KM females that were superovulated by intraperitoneal injection of 10 IU PMSG followed 48 h later by 10 IU of hCG. Zygotes were flushed from oviducts 18 h post-hCG injection, removed their cumulus cells by a brief exposure to 0.01% hyaluronidase, and then cultured in 50 µl droplets of potassium simplex optimized medium (KSOM) [Lawits and Biggers Citation1993] with 0, 5, 10, 20, 40, 80, 120, and 160 µg/ml of molybdenum (sodium molybdate dihydrate) covered under mineral oil at 377°C and 5% CO2 in air for 5 d. The selection of those doses were based on previous reports [Kendall et al. Citation2003; Kendall et al. Citation2006; Kolesarova et al. Citation2009] and the concentration that humans may be exposed [Huang et al. Citation2011].
Evaluation of embryo quality
The quality of blastocysts was assessed with morphological fluorescence staining [Fabian et al. Citation2007]. Briefly, blastocysts of day 5 were stained with 20 µg/ml of Hoechst dye 33342 for 5 min at 37°C and were then prepared as wet mounts on a glass microscope slide under a coverslip. The number of nuclei in the blastocysts was then recorded under fluorescence microscope at x 400 magnification (TE2000 Nikon). Blastocysts were morphologically classified () as normal (containing blastocyst cavity, round or oval nuclei, without morphological changes), degenerate (embryos with non-standard morphology, characterized usually by massive cytoplasmic fragmentation or lysed cellular content; cell nuclei with typical fragmented or condensed morphology), and arrest (embryos which were not able to develop to blastocyst stage, 2-cell to morula). The percentages of normal, degenerate, and embryo arrest were calculated relative to the total number of cleaved embryos.
Embryo transfer
Normal blastocysts in morphology from various groups were used in embryo transfer experiments. Groups of 10 blastocysts were surgically transferred to each uterine horn on Day 2.5 in pseudopregnant ICR recipient female mice.
Statistical analysis
Five replicates for each experiment were conducted. All percentage data were pooled and then tested by chi-squared analysis using the DPS Data Processing System 6.01 software (Refine Information Tech. Co. Ltd, Shanghai, China). Cell numbers of individual groups were compared using the general linear model (SAS Genmod procedure). Differences between experimental groups were considered significant at P <0 .05.
Acknowledgments
Dr. Ming-Tao Zhao, Xing-Qi Chen, and Dr. Shi-Qiang Zhang are thanked for assistance in preparing the manuscript.
Declaration of interest: This work was supported by the National Natural Science Foundation of China (Project No. 31040081) and the Higher Education Finance Scheme for Young Backbone Teachers in Henan Province (Project No. 2010GGJS-071), China. The authors report no declarations of interest
Author contributions: Conceived and designed the experiments: SW,C-MB; Performed the experiments: C-MB,Y-LZ,T-ZZ,Z-JY,S-YG; Analyzed the data: S-DW,X-LC; Contributed reagents/ materials/ analysis tools: X-WZ,X-GM,L-JJ; Wrote the manuscript: SW,C-MB,F-JL.
References
- Bougle, D., Bureau, F. and Foucault, P. (1988) Molybdenum content of term and preterm human milk during the first two months of lactation. Am J Clin Nutr 48:652–654.
- Bougle, D., Voirin, J. and Bureau, F. (1989) Molybdenum normal plasma values at delivery in mothers and newborns. Acta Paediatr Scand 78:319-320.
- EVM (Expert Group on Vitamins and Minerals). (2000) Review of Molybdenum. Report prepared for the United Kingdom Food Standards Agency. Report EVM/00/09P:8-11. Website: http://archive.food.gov.uk/committees/evm/papers/evm9.pdf
- Fabian, D., Juhás, S., Il'ková, G. and Koppel, J. (2007) Dose- and time-dependent effects of TNFalpha and actinomycin D on cell death incidence and embryo growth in mouse blastocysts. Zygote 15:241–249.
- Fabian, D., Bystriansky, J., Burkuš, J., Rehák, P., Legáth, J. and Koppel, J. (2011) The effect of herbicide BASTA 15 on the development of mouse preimplantation embryos in vivo and in vitro. Toxicol In Vitro 25:73–79.
- Fleming, T., Kwong, W., Porter, R., Ursell, E., Fesenko, I., Wilkins, A., (2004) The embryo and its future. Biol Reprod 71:1046–1054.
- Fungwe, T.V., Buddingh, F., Demick, D.S., Lox, C.D., Yang, M.T. and Yang, SP. (1990) The role of dietary molybdenum on estrous activity, fertility, reproduction and molybdenum and copper enzyme activities of female rats. Nutr Res 10:515–524.
- Howell, J.M., Shunxiang, Y. and Gawthorne, J.M. (1993) Effect of thiomolybdate and ammonium molybdate in pregnant guinea pigs and their offspring. Res Vet Sci 55:224–230.
- Huang, J., Wu, J., Li, T., Song, X., Zhang, B., Zhang, P., (2011) Effect of exposure to trace elements in the soil on the prevalence of neural tube defects in a high-risk area of China. Biomed Environ Sci 24:94–101.
- Ishaq, M., Khan, M.A., Jan, F.A. and Ahmad, I. (2010) Heavy metals in brick kiln located area using atomic absorption spectrophotometer: a case study from the city of Peshawar, Pakistan. Environ Monit Assess 166:409–420.
- Järup, L. (2003) Hazards of heavy metal contamination. British Medical Bulletin 68:167–182.
- Jeter, M.A. and Davis, G.K. (1954) The effect of dietary molybdenum upon growth, hemoglobin, reproduction and lactation of rats. J Nutr 54:215–220.
- Kargar, M., Khorasani, N., Karami, M., Rafiee, G.R. and Naseh, R. (2011) Study of aluminum, copper and molybdenum pollution in groundwater sources surrounding (Miduk) Shahr-E-Babak copper complex tailings dam. World Academy of Science, Engineering and Technology 76:412–416.
- Kendall, N.R., Marsters, P., Scaramuzzi, R.J. and Campbell, B.K. (2003) Expression of lysyl oxidase and effect of copper chloride and ammonium tetrathiomolybdate on bovine ovarian follicle granulosa cells cultured in serum-free media. Reproduction 125:657–665.
- Kendall, N.R., Marsters, P., Guo, L., Scaramuzzi, R.J. and Campbell, B.K. (2006) Effect of copper and thiomolybdates on bovine theca cell differentiation in vitro. J Endocrinol 189:455–463.
- Kolesarova, A., Capcarova, M., Sirotkin, A. and Massanyi, P. (2009) Insulin-like growth factor-I and progesterone release by ovarian granulosa cells of hens after experimental lead and molybdenum administrations in vitro. Inter J Poult Sci 8:890-895.
- Lawits, J.A. and Biggers, J.D. (1993) Culture of preimplantation embryos. In: Guide to Techniques in Mouse Development, Methods in Enzymology. Wassarman, P.M.and DePhamphilis, M.L. eds, Academic Press, San Diego, 153–164.
- Lepore, P.D. and Miller, R.F. (1965) Embryonic viability as influenced by excess molybdenum in chicken breeder diets. Proc Exp Biol Med 118:p155-157.
- Lyubimov, A.V., Smith, J.A., Rousselle, S.D., Mercieca, M.D., Tomaszewski, J.E., Smith, A.C. (2004) The effects of tetrathiomolybdate (TTM, NSC-714598) and copper supplementation on fertility and early embryonic development in rats. Reprod Toxicol 19:223–233.
- Meeker, J.D., Rossano, M.G., Protas, B., Diamond, M.P., Puscheck, E., Daly, D. (2008) Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116:1473–1479.
- Pampfer, S. and Donnay, I. (1999) Apoptosis at the time of embryo implantation in mouse and rat. Cell Death Differ 6:533–545.
- Pandey, R. and Singh, S.P. (2002) Effects of molybdenum on fertility of male rats. Biometals 15:65–72.
- Raisbeck, M.F., Siemion, R.S. and Smith, M.A. (2006) Modest copper supplementation blocks molybdenosis in cattle. J Vet Diagn Invest 18:566–572.
- Suttle, N.F. and Field, A.C. (1969) Effect of intake of copper, molybdenum and sulphate on copper metabolism in sheep. IV. Production of congenital and delayed swayback. J Comp Pathol 79:453–464.
- Thomas, J.W. and Moos, S. (1951) The effect of orally administered molybdenum on growth spermatogenesis and testes histology of young dairy bulls. J Dairy Sci 34:929–934.
- Titenko-Holland, N., Ahlborn, T., Lowe, X., Shang, N., Smith, M.T. and Wyrobek, A.J. (1998) Micronuclei and developmental abnormalities in 4-day mouse embryos after paternal treatment with acrylamide. Environ Mol Mutagen 31:206–217.
- van Gestel, C.A., Borgman, E., Verweij, R.A. and Ortiz, M.D. (2011a) The influence of soil properties on the toxicity of molybdenum to three species of soil invertebrates. Ecotoxicol Environ Saf 74:1–9.
- Van Gestel, C.A., Ortiz, M.D., Borgman, E. and Verweij, R.A. (2011b) The bioaccumulation of Molybdenum in the earthworm Eisenia andrei: influence of soil properties and ageing. Chemosphere 82:1614–1619.
- Vyskocil, A. and Viau, C. (1999) Assessment of molybdenum toxicity in humans. J Appl Toxicol 19:185–192.
- Wirth, J.J. and Mijal, R.S. (2010) Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med 56:147–167.
- Yu, C., Xu, S., Gang, M., Chen, G. and Zhou, L. (2011) Molybdenum pollution and speciation in Nver River sediments impacted with Mo mining activities in western Liaoning, northeast China. Int J Environ Res 5:205–212.