Abstract
Disruption in the normal timing of female puberty, such as in pre-pubertal cancer treatments, can cause abnormal somatic development. We sought to evaluate the impact of cyclophosphamide (CTX) on the somatic, uterine, and ovarian, development of pre-pubertal mice. Pre-pubertal (day 18 of life) C57BL/6J female mice were randomized to receive placebo (group 1A and 1B), 200 mg/kg CTX (group 2A), or 120 mg/kg CTX (group 2B). Mice were euthanized on day 56 (A groups) or 95 (B groups) of life. Body weight and length, uterine and ovarian weight and right femur length and weight were measured, and ovarian insufficiency was assessed. Data were analyzed using ANOVA and t-test. Body weight and length did not differ among groups at time of euthanasia. The femur was shorter and weighed less in mice treated with CTX than in controls. Uterine weight was lower in group 2B than 1B (46.1 mg, 95% CI: 42.9-49.4, vs. 62.2 mg, 95% CI: 58.5-65.8, respectively; p = 0.005) and was lower in mice that developed ovarian insufficiency than in mice that did not (p < 0.05). Ovarian weight was lower in mice treated with CTX, regardless of whether they developed ovarian insufficiency. Even with no observable effect on adult body length and weight, CTX treatment in pre-pubertal mice appears to negatively affect femur, uterine, and ovarian development. However, uterine development seems to be dependent on the hormonal status created by CTX more than on its direct effect.
Introduction
Puberty is a physiologic process characterized by profound physical, skeletal, and psychological changes. Pubertal onset in humans [Frish and Revelle 1970] and rodents [Frish et al. 1975] is associated with attainment and maintenance of particular hormone levels. Defective skeletal and physical development in adults who are childhood cancer survivors has been reported [Crofton et al. Citation2000]. In addition, states associated with a disruption in the normal hormonal production in the gonads, such as chemotherapy, are associated with abnormal timing of puberty, abnormal development of uterus, ovaries, or testicles, and breast.
Gonadotoxicity is a well-known side effect of cancer therapy in children and adult survivors [Chemaitilly et al. Citation2006; Green et al. Citation2009; Sarafoglou et al. Citation1997]. Female survivors of childhood cancer are at a high risk for ovarian failure and infertility. The risk is up to 22% for patients exposed to alkylating agents [Oeffinger et al. Citation2006]. However, ovarian damage seems to be age-dependent, as in 50% of the pre-pubertal girls younger than 10 years ovarian function may be retained in contrast to girls older than 10 years who uniformly experience acute ovarian failure after chemo/radiotherapy [Sarafoglou et al. Citation1997]. Ovarian failure, better described as primary ovarian insufficiency (POI), is defined as absence of menstrual periods accompanied by menopausal follicle-stimulating hormone (FSH) levels. Even if ovarian damage is not severe enough to cause POI, it can still cause diminished ovarian reserve (DOR), which can be identified by the presence of menstrual cycles with moderately elevated FSH levels, and is usually associated with infertility and a low response to fertility treatments.
In humans, the alkylating agent cyclophosphamide (CTX) was shown to be the agent most often implicated in causing damage to oocytes and granulosa cells in a dose-dependent manner [Oktem and Oktay Citation2007a; Wallace et al. Citation2005]. CTX is often used in the treatment of blood cancers, which affect children more frequently than other cancers [Cancer Facts and Figures Citation2011]. Animal studies have been instrumental in learning that CTX causes apoptosis of various cell types, including ovarian and bone. In the ovary, CTX causes apoptosis of granulosa cells and oocytes, while in the bone it causes apoptosis in the metaphyseal growth plate and trabecular bone, thus affecting bone elongation potential [Oktem and Oktay Citation2007a; Meirow et al. Citation1999; Xian et al. Citation2007]. While it is known that uterine irradiation at a young age hampers the final adult uterine development [Critchley and Wallace Citation2005], the impact of chemotherapy on uterine development is uncertain despite reports of a higher incidence of fetal growth restriction and preterm delivery among those exposed. These studies were conducted in human and animal adult subjects and no study to date has evaluated the impact of CTX in pre-pubertal subjects. The aim of this study was to recreate in mice the same conditions associated with childhood cancer treatment with different chemotherapy doses and then evaluate their somatic development through puberty and adulthood.
Results
The hypothesis that chemotherapy would prevent the normal pubertal development of the uterus, ovaries, and bones, independent of causing ovarian insufficiency was directly tested as outlined in . In the mouse strain C57BL/6J, puberty is characterized by vaginal opening at approximately 25 days of life, estrus 3-4 days later, and subsequently by regular cycling every 5-6 days, which culminates with mating. A total of 34 mice were randomized to the 2 treatment groups. Body weight and length and BMI did not differ between groups euthanized at age 56 and 95 days (). At 56 days, the femur was shorter in mice treated with CTX (p = 0.01). However, femur weight was not different between groups 1A and 2A. Similarly, uterine weight was not different in groups 1A and 2A. At 56 days, ovarian weight was not measured independent of the uterus ().
Figure 1. Study design. The animals were divided into placebo and test groups. The effect of CTX was assessed as a function of both dose and age of exposure. Animals were euthanized and tissue was harvested and examined.
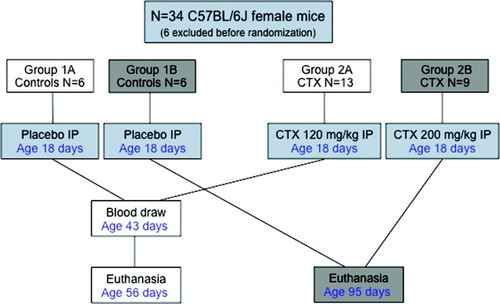
Table 1. BMI, femur length and weight, uterine and ovarian weight, means and confidence intervals (95% CI) in the control and CTX-exposed groups.
At 95 days, the femur was shorter in group 2B than 1B (p = 0.002). Similarly, the femur weighed less in group 2B than 1B (p = 0.009). Uterine weight was less in group 2B than 1B (p = 0.005). Similarly, ovarian weight was less in group 2B than 1B (p < 0.001) (). The cutoffs used for FSH identified a total of 6/22 POI (27.3%) and 8/22 DOR (36.4%) in pre-pubertal mice exposed to the two CTX doses. Combined, 63.7% of the mice showed ovarian function impairment (DOR or POI) after CTX exposure. FSH mean values and 95% CI in the four different groups are reported in . As expected, FSH levels were higher in the CTX-exposed groups that developed DOR or POI (p < 0.04). The CTX-exposed mice that did not develop DOR or POI (group 2A non-DOR and group 2B non-DOR) showed higher mean FSH levels than the non-exposed (groups 1A and 1B), despite this the difference was not significant.
Table 2. Follicle stimulating hormone (FSH) means and confidence intervals (95% CI) in controls and the three mouse groups identified by the FSH cut-off values.
Comparisons between the non-treated (groups 1A and 1B) and CTX-treated (groups 2A and 2B) mice that did and did not develop DOR and POI are displayed in . Femurs were shorter in all CTX-treated mice than in the untreated groups, and no difference was found among the CTX-treated groups (normal FSH, DOR, and POI). There was no difference in femur weight between the groups of mice euthanized at 56 days of life. However, femurs weighed less in all CTX-treated mice euthanized at 95 days of life (p < 0.03). Uteri weighed less in the CTX-treated mice that developed POI than in the mice that did not develop POI and those that developed both DOR and POI. Uterine weights in group 2B were significantly higher in those with POI than DOR. The ovaries weighed less in the mice treated with CTX, regardless of whether they developed DOR or POI (p < 0.02).
Table 3. Femur length and weight, uterine and ovarian weight, means and confidence intervals (95% CI) in the three groups identified by the FSH cut-off values.
Discussion
CTX treatment in pre-pubertal mice appears to negatively affect femur, uterine, and ovarian development, yet with no observable effect on adult body length, weight, and BMI. The results presented in this study show that CTX treatment impairs both long bone growth and bone mass accrual. The groups of CTX-treated mice had a shorter femur than controls. This seems to be due to a CTX-induced stromal collagen, as well as calcified matrix, apoptosis which affects elongation and trabecular thickness, as reported in previous studies [Xian et al. Citation2007]. In our study, we cannot assume malnutrition from CTX as a cause of defective bone development, since we observed a similar BMI in all groups. Human studies have evaluated skeletal development as assessed by height and the presence of deformities [Crofton et al. Citation2000; Schriock et al. Citation1991]. Schriock et al. [1991] found that growth retardation was significantly greater in children who had earlier disease onset and that growth deceleration occurred during chemotherapy and also after a temporarily improved growth period. Indeed, we observed a difference in bone elongation in both our follow-ups through late puberty and adulthood (groups 2A and B). However, in our follow-up through late puberty (group 2A) we did not observe a difference in bone weight, likely because of the early timing of euthanasia.
Endogenous estrogen production in the mice that did not develop POI was only partially and not significantly able to recoup bone length and weight in adult mice. The impairment of skeletal development in human subjects could be the result of a combination of corticosteroids use and chemotherapy. However, as shown above CTX has a negative impact on bones independent of corticosteroids. An argument could be made as to whether the impairment is a direct effect of CTX on bone development, a growth hormone deficiency from pituitary damage, or a combination of the two. However, a growth hormone deficiency would be reflected in a long-term deficiency in height and weight, which was not observed in this study or human studies [Caruso-Nicoletti et al. 1993]. Femur length does not contribute to the mouse length as conventionally measured. This could explain the lack of difference in length, weight, and BMI among all groups in our study, contrary to what was found by follow-up studies of adult childhood cancer survivors [Crofton et al. Citation2000; Caruso-Nicoletti et al. Citation1993].
In our study 63.7% of pre-pubertal mice exposed to CTX, independent of the dose used, had increased FSH reflective of a decreased endogenous estrogen production. This figure is higher than what was reported in human studies [Saragoflu et al. 1997; Meirow and Nugent Citation2001; Sanders et al. Citation1996]. This is probably due to the different assessment methods of ovarian function in those studies (presence or absence of puberty or menses). However, we believe that even a DOR, not just POI, can have a major impact on the subject's health and fertility and, therefore, should be assessed along with POI. Ovarian weight was significantly lower in CTX-treated mice than in the untreated mice. Remarkably, the weight was also lower in CTX-treated mice that did not develop DOR or POI. We presume that those mice would develop DOR or POI at a later time, since the decreased weight reflects an ovarian tissue loss for which the function is not yet apparent in the acute or subacute stages.
In humans, uterine irradiation at a young age hampers final adult uterine development, and uterine volume has been shown to correlate with age at irradiation [Critchley and Wallace Citation2005]. The impact of pre-pubertal administration of chemotherapy on uterine development is uncertain. As described above (see ) significantly smaller uteri in adult mice (group 2B), but not in late puberty (group 2A) was apparent. This could be possibly due to early euthanasia in this last group, before a difference would have time to manifest. Most of the mice in group 2B developed DOR or POI as estimated by FSH and as such, had little or no ovarian estrogen production through puberty and into adulthood. Based on these results it appears that uterine development depends on the hormonal status (estrogen) created by CTX treatment more than its direct effect on myometrium and endometrium, as endogenous estradiol production was able to recoup a normal uterine weight, despite CTX treatment. Histology studies currently underway and longer follow-up of animal and/or human subjects should substantiate these findings.
In conclusion, we confirmed a deleterious role for CTX on ovarian and bone development that was dose-independent and independent from the development of ovarian insufficiency. We also found that endogenous estrogen's ability to reverse bone mass loss in pre-pubertal mice is only partial and we reinforced the notion that uterine development through puberty is hormone-dependent and that CTX treatment does not hamper its final growth.
Materials and Methods
The study was approved by the IACUC office at the University of Tennessee Health Science Center at Memphis (UTHSC) (protocol number 1980, initially approved May 2011). Pre-pubertal (postnatal d 18) C57BL/6J females (Jackson Laboratory, Bar Harbor, Maine, USA) were randomized to receive placebo (controls, groups 1A and 1B), or CTX in two different doses, 120 mg/kg in group 2A and 200 mg/kg in group 2B, which was administered with a single intraperitoneal injection (see for a design flowchart). The dosages of CTX used were based on previous studies, which demonstrated a significant dose-dependent ovarian toxicity [Meirow et al. Citation1999; Ohnemus et al. Citation2004; Oktem and Oktay Citation2007b]. Mice were then euthanized at different time points to evaluate for possible age-dependent changes in the variables studied.
Mice were housed in the Animal Center at UTHSC under a 12-h light/dark cycle with food and water ad libitum. At age 42 d, the mice in groups 1B and 2B underwent blood collection from the retro-orbital plexus venosus and were euthanized at 95 d of life as adults. Groups 1A and 2A underwent blood collection at the time of euthanasia, at age 56 d, when in late puberty. Serum testing for FSH levels was performed in triplicate with ELISA in a specialized laboratory (Mott Center for Human Growth and Development, Wayne State University, Detroit, MI, USA). We used the FSH measurements to identify cut-off values to define POI and decreased ovarian reserve DOR. POI assumes that the ovaries have little or no follicles, or that the follicles are not functional. DOR assumes a residual ovarian hormonal production from the reduced, but still present, follicles. We chose an FSH value greater than 30 mIU/mL to identify the mice that developed POI, and a value between 15 and 30 mIU/mL to identify DOR. The cut-offs were based on the values obtained in the control groups (1A and 1B) where the maximum FSH value was 9.8 mIU/mL, and on standardized human cut-offs. We considered a value between 1.0 and 15 mIU/mL as normal.
Euthanasia was performed by cervical dislocation after sedation in an isoflurane chamber. Body weight and length (crown-rump) measurements were obtained at that time. BMI was calculated as g/mm2. Uterine and ovarian weight, and right femur length and weight were obtained from each mouse after dissection.
Femur, uterine, and ovarian development of the mice that developed DOR and POI after CTX administration were compared to those that did not develop those conditions and the control groups. Since the variables examined were normally distributed, we used t-test and ANOVA (SPSS 19.0 statistical package) to compare means in the different groups. We used 95 percent confidence intervals (95% CI) to define internal estimates associated with each probability.
Abbreviations
| CTX: | = | cyclophosphamide |
| FSH: | = | follicle-stimulating hormone |
| POI: | = | primary ovarian insufficiency |
| DOR: | = | decreased/diminished ovarian reserve. |
Declaration of interest: This study was supported by an Institutional grant from the University of Tennessee Health Science Center, Memphis, Tennessee, to LD. There are no conflicts of interest.
Author contributions: Conceived and designed the experiments: LD; Performed the experiments: LD, RAU, NS-L; Analyzed the data: LD, RAU; Contributed reagents/materials/analysis tools: LD, LJW, RWW, DCM; Wrote the manuscript: LD, LJW, RAU.
References
- Cancer Facts and Figures 2011. (2011) http://www.cancer.org/Cancer/CancerinChildren/DetailedGuide/cancer-in-children-key-statistics. Accessed April 13, 2012.
- Caruso-Nicoletti, M., Mancuso, M., Spadaro, G., Dibenedetto, S.P., DiCataldo, A. and Schiliró, G. (1993) Growth and growth hormone in children during and after therapy for acute lymphoblastic leukaemia. Eur J Ped 152:730–733.
- Chemaitilly, W., Mertens, A.C., Mitby, P., Whitton, J., Stovall, M., Yasui, Y., (2006) Acute ovarian failure in the childhood cancer survivor study. J Clin End Metab 91:1723–1728.
- Critchley, H.O. and Wallace, W.H. (2005) Impact of cancer treatment on uterine function. J Nat Canc Inst Mon 34:64–68.
- Crofton, P.M., Ahmed, S.F., Wade, J.C., Elmlinger, M.W., Ranke, M.B., Kelnar, C.J., (2000) Bone turnover and growth during and after continuing chemotherapy in children with acute lymphoblastic leukemia. Ped Res 48:490–496.
- Frisch, R.E. and Revelle, R.. (1970) Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 169:397–399.
- Frisch, R.E., Hegsted, D.M. and Yoshinaga, K. (1975) Body weight and food intake at early estrus of rats on a high-fat diet. Proc Nati Acad Sci USA 72:4172–4176.
- Green, D.M., Sklar, C.A., Boice, J.D., Mulvihill, J.J., Whitton, J.A., Stovall, M., (2009) Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 27:2374–2381.
- Meirow, D., Lewis, H., Nugent, D. and Epstein, M. (1999) Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod 14:1903–1907.
- Meirow, D. and Nugent, D. (2001) The effects of radiotherapy and chemotherapy on female reproduction. Hum Repr Update 7:535–543.
- Oeffinger, K.C., Mertens, A.C., Sklar, C.A., Kawashima, T., Hudson, M.M., Meadows, A.T., (2006) Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572–1582.
- Ohnemus, U., Unalan, M., Handjiski, B. and Paus, R. (2004) Topical estrogen accelerates hair regrowth in mice after chemotherapy-induced alopecia by favoring the dystrophic catagen response pathway to damage. J Invest Dermat 122:7–13.
- Oktem, O. and Oktay, K. (2007a) A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Canc Res 67:10159–10162.
- Oktem, O. and Oktay, K. (2007b) Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 110:2222–2229.
- Sanders, J.E., Hawley, J., Levy, W., Gooley, T., Buckner, C.D., Deeg, H.J., (1996) Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 87:3045–3052.
- Sarafoglou, K., Boulad, F., Gillio, A. and Sklar, C. (1997) Gonadal function after bone marrow transplantation for acute leukemia during childhood. J Ped 130:210–216.
- Schriock, E.A., Schell, M.J., Carter, M., Hustu, O. and Ochs, J.J. (1991) Abnormal growth patterns and adult short stature in 115 long-term survivors of childhood leukemia. J Clin Oncol 9:400–405.
- Wallace, W.H., Thomson, A.B., Saran, F. and Kelsey, T.W. (2005) Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Rad Oncol Biol Phys 62:738–744.
- Xian, C.J., Cool, J.C., van Gangelen, J., Foster, B.K. and Howarth, G.S. (2007) Effects of etoposide and cyclophosphamide acute chemotherapy on growth plate and metaphyseal bone in rats. Canc Biol Ther 6:170–177.