Abstract
The mammalian sperm nucleus contains an unusually condensed chromatin, due to replacement of the majority of histones by protamines. However, soon after the spermatozoon penetrates the ooplasm at fertilization, decondensation of this densely packed chromatin must occur to allow formation of the male pronucleus and syngamy. Decondensation is accomplished by protamine disulfide bond reduction by oocyte glutathione and replacement of protamines by oocyte histones with the aid of an acceptor molecule. Previous results from our laboratory have demonstrated that heparan sulfate (HS) present in the ooplasm functions as protamine acceptor during human sperm decondensation in vivo. In the present paper, we analyze the role of heparin, structural analogue of HS, and dermatan sulfate (DS) in murine sperm chromatin decondensation in vitro, including the possibility of a synergistic effect between both glycosaminoglycans. Decondensation was assessed under phase contrast microscopy following incubation of murine spermatozoa with glutathione and either heparin, DS, or a combination of both. Ultrastructural changes taking place during decondensation were analyzed by transmission electron microscopy. Both glycosaminoglycans were able to promote the decondensation of murine spermatozoa in vitro but the decondensing ability of heparin was significantly higher. Use of both glycosaminoglycans together revealed the existence of a synergistic effect. Transmission electron microscopy analysis of decondensing spermatozoa supported these findings. Synergism between heparin and DS was observed both in capacitated and non-capacitated spermatozoa but decondensation kinetics was faster in the former. The results obtained indicate a new potential role for dermatan sulfate in murine sperm decondensation at fertilization and provide evidence of differences in the degree of chromatin condensation throughout the murine sperm nucleus.
Introduction
The nucleus of the mature mammalian spermatozoa is unique, both in nucleoprotein composition and chromatin organization [McLay and Clarke Citation2003]. During spermatogenesis the majority of nuclear histones have been replaced by protamines [Florman and First Citation1988; Eddy and O'Brien Citation1994], relatively small and highly basic proteins, rich in arginine and cysteine, rendering a highly condensed sperm nucleus. There are two types of protamines: P1, present in all species, which possesses an arginine-rich central domain [Wouters-Tyrou et al. Citation1998], and P2, present in some species, which contains less cysteine and more histidine, and may thus be expected to sustain the formation of fewer disulfide bonds [Sanchez-Vazquez et al. Citation1996]. There are two general models for the association of protamines with DNA, both implying that chromatin is stabilized by the formation of covalent disulfide linkages between protamines on adjacent DNA strands. In those species where P1 and P2 are present, as is the case with mouse and human, both protamines are critical for fertility, and nuclear formation and sperm DNA stability are disrupted by a change in their ratio [Burgess and Kelly Citation1987; Stevens Citation1993].
Upon entry into the oocyte, the sperm nucleus undergoes marked morphological changes which lead to extensive decondensation and formation of the male pronucleus, in synchrony with oocyte chromatin decondensation into the female pronucleus. This morphological remodeling involves the replacement of sperm protamines by oocyte histones which then organize into nucleosomes. Evidence in the literature indicates that protamine replacement by histones in the paternal chromatin requires the reduction of protamine disulfide bonds by reducing agents present in the egg cytoplasm, and the participation of other egg components that help protamine - histone exchange [Zirkin et al. Citation1989]. For example, inhibition of the meiotic maturation-associated increase in oocyte glutathione (GSH) in mice [Perreault et al. Citation1988] prevents sperm chromatin decondensation, and in amphibian, fish, and Drosophila eggs, another oocyte protein, nucleoplasmin, facilitates protamine removal, chromatin decondensation, and histone replacement [Ohsumi and Katagiri Citation1991; Arnan et al. Citation2003].
Recently, Romanato et al. [2003] reported that heparan sulphate (HS) and heparin, but not other glycosaminoglycans (GAGs), used at physiological concentrations, were able to release protamines from human capacitated sperm chromatin in vitro, and proposed that HS could be functioning as a protamine acceptor in vivo during human sperm nuclear decondensation. However, the mechanism of action of heparin/HS in this process is still a matter of controversy. The presence of heparin receptors on the sperm plasma membrane has been described by several groups [Carrell and Liu Citation2002; Delgado et al. Citation1982; Lassalle and Testart Citation1992], and Delgado et al. [1982] have proposed that heparin binding to its receptors leads to the destabilization of the sperm plasma membrane, which in turn would allow the incorporation of other molecules, such as GSH, into the sperm nucleus [Romanato et al. Citation2003]. Alternatively, a direct effect of heparin on sperm chromatin has been suggested since heparin has a strong affinity for protamines and can combine with them in vitro to form a highly insoluble complex [Romanato et al. Citation2003]. Direct experimental evidence is lacking, and how heparin/HS is able to decondense human sperm in vitro is not clearly understood.
The first evidence on the presence of HS in the mammalian (murine) oocyte and its requirement for human sperm in vitro decondensation by fresh murine oocytes was reported by our research group [Romanato et al. Citation2005; Romanato et al. Citation2008]. Recently, we have also demonstrated [Julianelli et al. 2012] that HS is present in the human oocyte as well, adding further support to the hypothesis that HS is functioning as protamine acceptor during mammalian sperm decondensation in vivo.
Preliminary data from our laboratory suggested that another glycosaminoglycan (GAG) present in the oocyte cumulus complex [Tirone et al. Citation1993], dermatan sulfate (DS), had the ability to decondense murine spermatozoa in vitro. This behavior, different from that which we had observed and described for human sperm decondensation, prompted us to analyze the involvement of other GAGs in murine sperm decondensation, the possibility of their differential effect on chromatin decondensation, and the possible interaction between them in this process.
The aim of this study was to gain further insight on the molecular mechanisms underlying mouse sperm chromatin decondensation in vitro, particularly regarding the possible involvement of more than one GAG in this process. Accordingly, chromatin decondensation in vitro was analyzed in the presence of GSH and different GAGs, in both capacitated and non-capacitated murine spermatozoa.
Results
Decondensing ability of different GAGs in capacitated spermatozoa
In the search for a putative decondensing agent in vivo the ability of different GAGs, usually present in the oocyte-cumulus complex, to decondense capacitated murine spermatozoa, was assayed in vitro in the presence of GSH (). As shown in , both DS and heparin readily decondensed sperm chromatin after 60 minutes of incubation (Hep: 87 ± 2 % vs. DS: 67 ± 2 %, p < 0.001, ANOVA + Tukey-Kramer Multiple Comparison Test, n = 3) while hyaluronic acid (HA) and chondroitin sulfate (CS) were completely inactive (ANOVA + Tukey-Kramer Multiple Comparison Test, not significant (NS), vs. GSH or heparin alone, n = 3) in the same incubation conditions.
Figure 1. Morphology of decondensed murine spermatozoa. Decondensation of murine sperm nucleus as visualized with Hoechst stain (A, D, G), under phase contrast (B, E, H), and merged image (C, F, I). Panels A,B,C) unchanged; panels D,E,F) moderately decondensed; panels G,H,I) grossly decondensed. Original magnification: 400x. Scale bar: 10 µm.
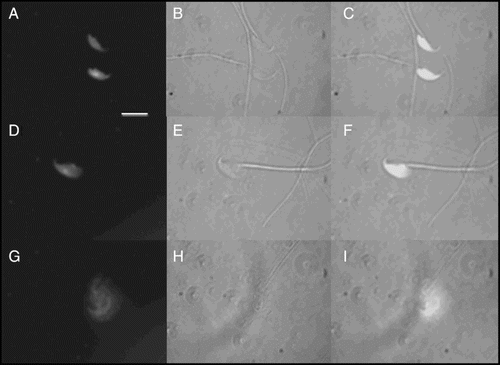
Figure 2. Effect of glycosaminoglycans (GAGs) on sperm decondensation. Capacitated murine spermatozoa were decondensed in the presence of 10 mmol/l glutathione (GSH) and each of the following GAGs (46 µmol/l each): heparin (Hep), chondroitin sulfate (CS), dermatan sulfate (DS), or hyaluronic acid (HA). Decondensation is expressed as %(M + G) and results correspond to mean ± SEM of 3 independent experiments. Decondensation achieved with Hep + GSH was significantly higher than with DS + GSH, and so was decondensation with each pair compared to GSH alone (*p < 0.001, ANOVA + Tukey-Kramer Multiple Comparison Test, n = 3). HA + GSH and CS + GSH were completely inactive (ANOVA + Tukey-Kramer Multiple Comparison Test, NS, vs. GSH or heparin alone, n = 3). Total decondensation (%M + G) was determined as the sum of %M (moderately decondensed) and %G (grossly decondensed) spermatozoa.
Heparin and dermatan sulfate dose-response curves for capacitated and non-capacitated mouse spermatozoa
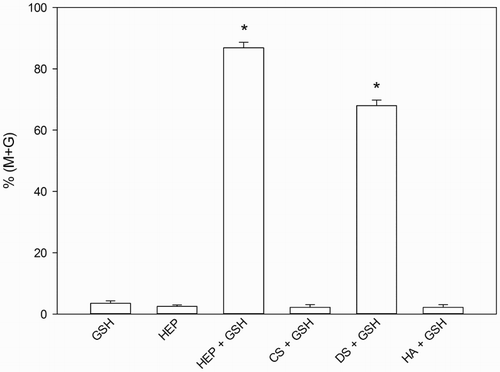
In order to establish the optimum heparin and DS concentrations to be used in the GAG synergism studies, mouse spermatozoa were incubated with 10 mmol/l GSH with increasing concentrations of either heparin or DS (). An incubation time of 15 minutes was chosen for these experiments in order to avoid full decondensation. A shows the heparin dose-response curves obtained with capacitated and non-capacitated spermatozoa. Sperm decondensation increased with heparin concentration until it reached a plateau (at around 5 µmol/l in non-capacitated and 15 µmol/l in capacitated spermatozoa). Percent decondensation achieved was significantly different from the control (GSH alone) at all heparin concentrations tested, both for capacitated and non-capacitated spermatozoa (ANOVA+ Tukey-Kramer Multiple Comparison Test, p < 0.01, n = 4). In comparison, there was virtually no decondensation after incubating either capacitated or non-capacitated spermatozoa with GSH and DS (B). When non-capacitated spermatozoa were used, no significant dose-response curve was obtained (data not shown). At the highest concentration tested (46 µmol/l, p < 0.01 compared to GSH alone) capacitated spermatozoa did show a significant increase in the percentage of decondensation, In view of these results, 0.46 µmol/l heparin and 46 µmol/l dermatan sulfate were chosen for experiments hereafter.
Figure 3. Heparin and dermatan sulfate (DS) dose-response curves for sperm decondensation of capacitated and non-capacitated murine spermatozoa. Dose-response curves were obtained following 15 minute incubation of murine spermatozoa in the presence of different concentrations of heparin or DS and 10 mmol/l glutathione (GSH). Decondensation is expressed as %(M + G) and results correspond to mean ± SEM of 4 independent experiments. Panel A) heparin dose response curve in capacitated (•) and non-capacitated (o) spermatozoa. Decondensation was significantly higher for all heparin concentrations tested when compared to GSH alone (ANOVA+ Tukey-Kramer Multiple Comparison Test, p < 0.01, n = 4). Panel B) DS dose response curve in capacitated spermatozoa. Only the highest DS concentration tested resulted in a significant increase in decondensation compared to GSH alone (ANOVA+ Tukey-Kramer Multiple Comparison Test, p < 0.01, n = 4). Total decondensation (%M + G) was determined as the sum of %M (moderately decondensed) and %G (grossly decondensed) spermatozoa.
Decondensation kinetics for heparin and dermatan sulfate in capacitated and non-capacitated mouse spermatozoa
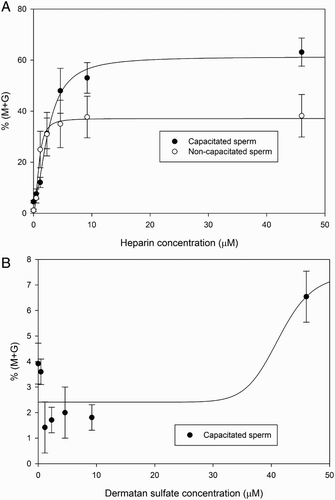
Once the optimum GAG concentration was determined, the time course of decondensation in the presence of heparin, DS, or the combination of both, was analyzed in both capacitated (, left panel) and non-capacitated (, right panel) spermatozoa. Though the shape of the curve was similar for the three experimental conditions, there were differences in t0.5 among them, both in capacitated and non-capacitated spermatozoa. Capacitated spermatozoa (, left panel) decondensed somewhat faster with heparin (t0.5 = 35.45 ± 2.17 minutes) than with DS (t0.5 = 43.55 ± 2.19 minutes) and the combination of both molecules drastically reduced decondensation time to almost half (t0.5 = 17.96 ± 1.20 minutes). However, the three curves reached the same final level of decondensation (Sigmoidal dose-response curve fit, R2= 0.8585, preferred model different EC50). The behavior of non-capacitated spermatozoa was similar (, right panel), although differences in t0.5 were less marked and the final level of decondensation achieved was lower than in capacitated sperm.
Figure 4. Sperm decondensation kinetics in the presence of heparin and dermatan sulfate (DS). Time course of decondensation in capacitated (left panel) and non-capacitated (right panel) murine spermatozoa, was analyzed following incubation with heparin, DS, or a combination of both, in the presence of 10 mmol/l glutathione (GSH). Decondensation is expressed as %(M + G) and results correspond to mean ± SEM of 8 independent experiments. Both capacitated spermatozoa and non-capacitated spermatozoa showed a significant decrease in t0.5 when 0.46 µmol/l heparin and 46 µmol/l dermatan sulfate were used together. Differences were analyzed by Sigmoidal dose-response curve fit, R2= 0.8585, with preferred model being different EC50. Total decondensation (%M + G) was determined as the sum of %M (moderately decondensed) and %G (grossly decondensed) spermatozoa.
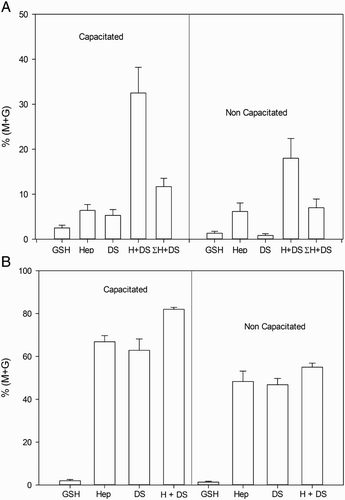
Synergistic effect of heparin and dermatan sulfate on sperm decondensation
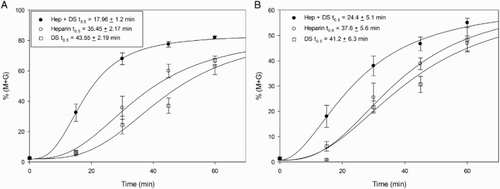
In order to analyze the possible synergism between heparin and DS on murine sperm decondensation, the percent decondensation was evaluated following incubation of both capacitated and non-capacitated spermatozoa with the lowest concentration of heparin (0.46 µmol/l) and the highest concentration of DS (46 µmol/l), alone or combined, for different periods of time. Results are depicted in . Synergism was evident following a 15 minute incubation with capacitated spermatozoa (A, left panel) and a 45 minute incubation with non-capacitated spermatozoa (A, right panel), but was lost after 60 minutes of incubation (B, left and right panels).
Figure 5. Synergistic effect of heparin (Hep) and dermatan sulfate (DS) on murine sperm decondensation. Spermatozoa were incubated with either heparin (0.46 µmol/l), DS (46 µmol/l), or a combination of both, in the presence of 10 mmol/l glutathione (GSH), for 15, 45, or 60 minutes. Decondensation was assessed as %(M + G). A) shows the decondensation achieved by capacitated (15 minutes incubation, left panel, n= 5) and non-capacitated (45 minutes incubation, right panel, n= 4) spermatozoa. The last bar in each panel indicates the sum of the corresponding values for heparin and DS alone. In both types of spermatozoa, incubation with Hep + DS resulted in a significant increase in decondensation compared to incubation with each glycosaminoglycan (GAG) separately (p < 0.01) or to the sum of %(M + G) achieved separately (last bar in each panel, p < 0.05). B) shows that the synergistic effect was lost after 60 minutes of incubation, both in capacitated (left panel, n= 5) and non-capacitated (right panel, n= 4) spermatozoa. (ANOVA+ Tukey-Kramer Multiple Comparison Test). Total decondensation (%M + G) was determined as the sum of %M (moderately decondensed) and %G (grossly decondensed) spermatozoa.
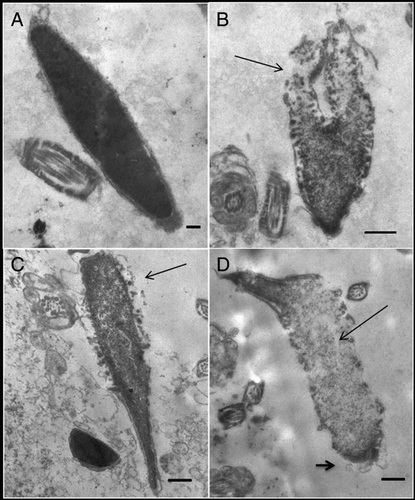
Electron microscopy of decondensed spermatozoa
The effect of GSH, heparin + GSH, DS + GSH, or the combination heparin + DS + GSH on the ultrastructure of decondensing murine spermatozoa was analyzed by transmission electron microscopy (). Following incubation with GSH alone (control, A), fully condensed sperm heads were distinguishable, showing an adequate nuclear envelope conformation. Chromatin decondensation became apparent following incubation with GSH + heparin (B), beginning at the caudal region of the sperm head and presenting granulo-fibrillar areas. When GSH + DS were used (C), decondensation also began at the caudal region of the sperm head, but chromatin appeared more compact. Finally, after the addition of GSH + heparin + DS (D), a significantly higher degree of chromatin decondensation was observed, evidenced by the presence of ‘lacunar’ and granulo-fibrillar areas, as well as the disarray of acrosomal and nuclear membranes. In spermatozoa where chromatin decondensation was incomplete, the cephalic region remained more compact.
Figure 6. Transmission electron microscopy of decondensing murine spermatozoa. The effect of A) 10 mmol/l glutathione (GSH), B) 10 mmol/l GSH + 0.46 µmol/l heparin, C) 10 mmol/l GSH + 46 µmol/l dermatan sulfate (DS), or D) both glycosaminoglycans (GAGs) + 10 mmol/l GSH examined by electron microscopy. Micrographs shown are representative of 200 spermatozoa analyzed for each experimental condition. Sperm nuclei treated with GSH (A) were uniformly electron-dense and fully condensed, with intact nuclear envelope and outer membranes. Following incubation with heparin + GSH (B) decondensation could be observed, starting at the caudal region of the sperm head (arrow); membrane disarray was evident. Incubation with DS + GSH (C) also produced decondensation starting at the caudal region (arrow), but chromatin appeared more condensed than with heparin. When both GAGs were used together (D) a higher degree of decondensation was observed (presence of ‘lacunar’ and granulo-fibrillar areas, thin arrow), with totally disorganized membranes, including acrosomal membranes (short arrow). On the cephalic region, residues of packed chromatin still remain. Scale bars: A, 0.2; B-D, 0.5 µm.
Discussion
Sperm nuclear chromatin is about 6–8 times more condensed than somatic cell chromatin, as a consequence of the replacement of the majority of sperm histones by protamines during spermatogenesis [Ward and Coffey 1991]. After fertilization, however, this compact chromatin must decondense in order to interact with oocyte DNA and attain syngamy.
The process of sperm decondensation in the oocyte involves the reduction of intra and intermolecular protamine disulfide bridges by endogenous GSH, and the removal of reduced protamines with the aid of an acceptor molecule, which our laboratory has found to be heparan sulfate, present in both murine and human ooplasm. The molecular basis of this process is not well understood in many species, including mouse and human [Romanato et al. Citation2008; Julianelli et al. 2012]. Previous reports from our laboratory described the role of glycosaminoglycans in human sperm decondensation and here we describe the effect on chromatin decondensation in murine spermatozoa.
Analysis of the effect of different GAGs on in vitro murine sperm decondensation resulted in an interesting finding. Contrary to previous observations on human sperm decondensation [Romanato et al. Citation2003], DS was shown to be almost as active as heparin in decondensing mouse sperm chromatin, under standard decondensation conditions: 46 µmol/l of the GAGs and a 60 minute incubation period (). One cannot help but wonder whether this differential response in murine and human spermatozoa might be related to the percentage of histones and/or the relative amount of protamine 1 and protamine 2 present in mature spermatozoa in both species. Murine spermatozoa organize about 1–2% of their genome in nucleosomes, whereas up to 15% of human sperm DNA is packaged in this manner [Johnson et al. Citation2011].
Alternatively, this differential decondensing ability of DS and heparin on human and mouse sperm nuclei could be explained in terms of the molecular characteristics of protamines P1 and P2 and the P1/P2 ratio in both species. Mouse and human protamines have similar physicochemical characteristics, such as percentage of the basic amino acid arginine, isoelectric point, and number of total residues (conserved between human and mouse, although differing in sequence), with P2 including histidine in the primary structure. However, a major difference between species is the 1:1 ratio of P1/P2 found in the human sperm nucleus in comparison to a 1:2 ratio in mouse nuclei. We have previously mentioned that P2 has a lower content of cysteine residues than P1, allowing for a diminished possibility of disulfide bond formation. Additionally, the P2 proform contains a larger amount of the acidic amino acid, glutamate, which incorporates a negative charge into its structure and could therefore lessen its interaction with the negatively charged DNA molecule. Considering all these characteristics, it could be expected that DS, a less sulfated glycosaminoglycan compared to heparin, would show a stronger decondensing activity in mouse than in human spermatozoa and that a synergistic effect could occur between both molecules, with DS acting where P2 is more abundant and heparin acting in those regions of the chromatin where the more tightly bound P1 is present.
The results presented in this paper suggest that decondensation does not take place in the same way when heparin/HS or DS are used. The system was more sensitive to heparin than to DS (), but also the maximum level of decondensation achieved was higher with heparin than with DS ( and ), and the time course of the process indicated a faster response to heparin (). Doubtlessly, these differences could well be related to the physicochemical characteristics of the molecules. Heparin and DS not only possess a different distribution of charged groups throughout the molecule, but also DS is less sulfated than heparin, and therefore has a smaller net negative charge. Consequently, a differential ability to diffuse through the sperm membranes and to interact with chromatin could be expected for both GAGs but remains to be directly assessed.
Although murine sperm decondensation occurred somewhat faster in the presence of heparin than in the presence of DS alone, it was almost twice as fast when both GAGs were used together (). This observation prompted us to propose a possible synergism between both molecules. Such synergistic behavior suggests that both GAGs would be acting together and interacting with each other in neighboring regions of the sperm chromatin rather than independently at distant places. If the latter were true, a simple additive effect on decondensation would be expected when both molecules were used simultaneously.
Although the concentrations of heparin and DS used in this experiment differ considerably, the rationale for choosing these experimental conditions was to force the system to reveal a possible synergistic behavior which could not be observed otherwise. Both glycosaminoglycans (DS and HS, the molecular equivalent of heparin and naturally present in both human and mouse oocytes) appear to be synthesized by granulose cells in response to follicle-stimulating hormone (FSH) [Ax and Bellin Citation1988; Tirone et al. Citation1993] but, to our knowledge, there is no data available on the amount present inside the oocyte. Future experiments in our laboratory will focus on the quantitation of both molecules in human and murine oocytes and, until then, the results presented herein represent the first indication of a possible interaction between these glycosaminoglycans and sperm chromatin decondensation.
Results presented here comparing chromatin decondensation in capacitated and non-capacitated spermatozoa are interesting and probably a consequence of changes in membrane composition and protein distribution that are associated with sperm capacitation. Capacitated spermatozoa attained higher levels of decondensation and also showed faster decondensation kinetics than non-capacitated spermatozoa, probably as a reflection of the role of the sperm membrane in regulating the entry of GAGs and/or GSH into the sperm cell [Romanato et al. Citation2005].
Another possible explanation for these differences between nuclear decondensation of capacitated and non- capacitated spermatozoa, not to be ruled out at this point, involves the perinuclear theca, a cytoskeletal structure intercalated between the inner acrosomal membrane and the nuclear envelope of the mammalian sperm head [Sutovsky et al. Citation1997; Oko and Sutovsky Citation2009]. This structure is rich in disulfide bonds and appears to be involved in chromatin stabilization; its disappearance when the sperm head is incorporated into the oocyte would allow for chromatin decondensation and, in this way, the perinuclear theca might play a role in the regulation of this process. Though the perinuclear theca seems to remain unaltered until the spermatozoon enters the oocyte [Ramalho-Santos et al. Citation2000], subtle modifications in its structure could be occurring during sperm capacitation and these might, in turn, alter the spermatozoon's sensitivity to decondensing reagents. This is an interesting hypothesis which will be analyzed in future experiments.
Electron microscopy of decondensing spermatozoa enabled us to gain insight into the ultrastructural changes that take place in the sperm head during nuclear decondensation. The results obtained revealed that decondensation begins at the caudal region of the sperm head, where the tail is inserted, and continues all the way up until full chromatin decondensation is attained. In agreement with decondensation kinetics observed for both GAGs, DS + GSH promoted a lower degree of decondensation than heparin + GSH and the addition of both GAGs + GSH resulted in a fully decondensed chromatin. In somatic cells undergoing cell division, it has been well established that different degrees of condensation along the chromatin are responsible for uneven timing when chromosomes duplicate; our findings could reflect a similar situation for sperm chromatin, although at present this remains pure speculation.
Electron microscopy also revealed that as decondensation progressed, membranes were disorganized and eventually disappeared, leaving high concentrations of condensed chromatin on the periphery of the cell. This observation is in agreement with the findings by Sanchez-Vazquez et al. [1996] who showed similar results with heparin and GSH on mouse spermatozoa.
The fact that the decondensation process starts at the caudal region of the sperm head probably reflects the lack of perinuclear theca overlying this region of the nuclear membrane and is in agreement with the findings of Ramalho-Santos et al. [2000] who showed, using the rhesus monkey model, that persistence of the perinuclear theca after ICSI prevents sperm chromatin decondensation. Once again, these results could be indicative of the involvement of the perinuclear theca in the regulation of the chromatin decondensation process.
Taken together, our findings support the idea of differences in chromatin condensation throughout the murine sperm nucleus, also recognized in other species such as the human, and probably due to the fact that not only protamines but also a certain amount of histones (variable according to the species) remain attached to DNA. This undoubtedly confers very particular and specific characteristics to sperm chromatin, with probably important effects on epigenetics and early embryo development [Pittoggi et al. Citation1999; Arpanahi et al. Citation2009; Miller et al. 2010].
In conclusion, this paper presents, for the first time, evidence that two molecules that share certain physicochemical characteristics and that are normally present in follicular fluid and the cumulus – oocyte complex, heparan sulfate and dermatan sulfate, act synergistically on murine sperm decondensation in vitro. As the presence of DS inside the oocyte has not yet been demonstrated, much less its concentration determined, we cannot truly assess the relative importance of this synergism in vivo. However, it can be speculated that this cooperative behavior in such a crucial event in mammalian reproduction, i.e., sperm decondensation is a prerequisite for male pronucleus formation, is possibly reflecting differential degrees of condensation at different regions of the sperm chromatin. This, in turn, might be of physiological relevance to the developing embryo. Studies are currently under way in our laboratory to determine whether dermatan sulfate is present in the murine ooplasm, as has already been demonstrated for heparan sulfate.
Materials and Methods
Sperm collection and capacitation
Animal care and manipulation was in agreement with institutional guidelines and the Guiding Principles in the Care and Use of Animals (DHEW Publication, NIH 80–23). Animals (3 to 4 eight w old CF-1 male mice per experiment) were fed ad libitum and kept in air-conditioned rooms at 20-28°C with a 12 h light–dark period.
Epididymis from each mouse were removed and transferred to a dish containing 300 µl of In Vitro Fertilization Medium (IVFM, 99.3 mmol/l NaCl, 2.70 mmol/l KCl, 0.50 mmol/l MgSO4.6H2O, 1 mg/ml glucose, 0.31 mmol/l Na2HPO4.2H2O, 1.80 mmol/l CaCl2.H2O). Medium pH was adjusted to 7.3 with 25.07 mmol/l NaOH, and 0.0055 mg sodium pyruvate and 0.35 ml L-Na-lactate (60% syrup) were added to a final volume of 100 ml. Spermatozoa were recovered by cutting the isolated caudae into fragments and allowing mature sperm to ‘swim out’ into IVFM at 37°C for 10 min. Tissue fragments were removed and the remaining sperm suspension incubated in capacitating conditions for 1 h at a concentration of 30-50 x 106 spermatozoa/ml. Sperm motility and viability were checked in each experiment on a 10 µl aliquot of the sperm suspension, under phase contrast, in an Olympus CH2 microscope at 400x magnification.
Evaluation of sperm capacitation
Capacitation status of spermatozoa was assessed by CTC assay and by tyrosine phosphorylation (data not shown) [Barbonetti et al. Citation2010]. A 100 µl aliquot of sperm suspension, containing 10x106 spermatozoa/ml, was mixed rapidly with CTC stock solution and 30 s later fixed by addition of 1.6 µl of 12.5% glutaraldehyde in 1 M Tris buffer (pH 7.8). The mixture was centrifuged for 30 s at 9,500 xg and the resulting pellet washed by centrifugation with 400 µl distilled water (three times). A 50 µl aliquot was placed on a prewarmed slide (37°C) and left to dry overnight in the dark. One drop of DABCO mounting medium was carefully added and a coverslip placed on top. Cells were observed under the fluorescence microscope. Three main patterns of CTC fluorescence could be identified: F, with uniform fluorescence over the entire head, characteristic of non-capacitated, acrosome-intact cells; B, with a fluorescence-free band on the postacrosomal region, characteristic of capacitated, acrosome-intact cells; and AR, with dull or no fluorescence over the sperm head, characteristic of capacitated, acrosome-reacted cells. A bright fluorescence over the midpiece of spermatozoa could be seen at every stage [Kong et al. Citation2009].
Decondensing ability of different GAGs
Capacitated and non-capacitated sperm were decondensed in the presence of 10 mmol/l GSH and 46 µmol/l heparin (13,500 Da, 170 IU/mg) or each of the following GAGs: HS, CS, DS, and HA in IVFM at 37°C for 15, 30, 45, and 60 min [Romanato et al. Citation2003]. Controls consisted of parallel incubations with GAGs or GSH alone. After each time period, a 20 µl aliquot was removed and fixed with an equal volume of 2.5% glutaraldehyde in phosphate-buffered saline (PBS). Two 5 µl aliquots were transferred onto a microscope slide, a coverslip placed on top and nuclear status assessed under phase contrast in an Olympus CH2 microscope at 400x. Spermatozoa were classified [Bedford et al. Citation1973] as unchanged (U), moderately decondensed (M), or grossly decondensed (G) (). At least 200 cells were evaluated in each aliquot. Total decondensation achieved (%M + G) was determined as the sum of %M and %G. Heparin, structural analogue of HS, has been demonstrated to possess the same biological activity in the in vitro decondensation of sperm chromatin [Romanato et al. Citation2003] and, due to its accessibility, has been used as a substitute for heparan sulfate, in the present experiments.
Cooperative effect of heparin and dermatan sulfate on nuclear sperm decondensation
The optimum concentrations of heparin and DS were determined by incubating spermatozoa in 10 mmol/l GSH and increasing concentrations of heparin or dermatan sulfate (0.46, 1.2, 2.3, 4.6, 9.2, 46 µmol/l). Total decondensation was determined as previously described after 15, 30, 45, and 60 min of incubation.
To evaluate the possible cooperative effect between both GAGs, chromatin decondensation kinetics in capacitated and non-capacitated spermatozoa was determined in the presence of 10 mmol/l GSH and 0.46 µmol/l heparin, 46 µmol/l dermatan sulphate, or the combination of both GAGs. Aliquots were drawn after 15, 30, 45, and 60 min of incubation and sperm decondensation determined as previously described.
Electron microscopy
Spermatozoa were decondensed in the presence of heparin and GSH for 60 min at 37°C and prepared for electron microscopy. Samples (heparin, GSH, and heparin + GSH) were diluted 1:4 in 0.1 mol/l PBS (pH 7.4) at room temperature, thoroughly mixed, transferred to conical tubes, and centrifuged at 380 xg for 10 min. Pellets (5 x 106 spermatozoa) were carefully fixed using 3% glutaraldehyde in PBS, at 4°C. After 18 h, fixed samples were treated with osmium tetroxide (1.3%), dehydrated with increasing concentrations of ice-cold ethanol, and washed with propylene oxide at room temperature. Pellets were embedded in Eponate 12 - Araldite (Pelco, Redding, CA, USA) and sliced in an ultramicrotome with a diamond blade. Slices were analyzed with a Zeiss EM 109T electron microscope (Laboratorio Nacional de Investigación y Servicios de Microscopía Electrónica, LANAIS-MIE, Buenos Aires, Argentina) after double staining with uranyl acetate and lead citrate.
Statistical analysis
Data were analyzed using one-way analysis of variance and the corresponding post-test as indicated in each case, using the Instat 3.0 software program or GraphPad Prism 5.0 (GraphPad Inc., La Jolla, CA, USA). A p value < 0.05 was considered significant.
Declaration of interest: This work was supported by the University of Buenos Aires (UBACYT, grant number 20020100101034) and a donation from Fundación Honorio Bigand. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported in the present manuscript.
Author contributions: Performed the research: MCS; Performed the electron microscopy: CAS; Coached MCS with the experiments: VLJ,MR; Helped with the data analysis and thoroughly revised the manuscript: LC; Equal responsibilities in designing the experiments and wrote the paper: VAF, JCC.
Abbreviations:
| GAG: | = | glycosaminoglycan |
| GSH: | = | glutathione |
| HS: | = | heparan sulfate |
| DS: | = | dermatan sulfate |
| HA: | = | hyaluronic acid |
| CS: | = | chondroitin sulfate |
| NS: | = | not significant. |
References
- Arnan, C., Saperas, N., Prieto, C., Chiva, M. and Ausio, J. (2003) Interaction of nucleoplasmin with core histones. J Biol Chem 278:31319–31324.
- Arpanahi, A., Brinkworth, M., Iles, D., Krawetz, S.A., Paradowska, A., Platts, A.E., (2009) Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Res 19:1338–1349.
- Ax, R.L. and Bellin, M.E. (1988) Glycosaminoglycans and follicular development. J Anim Sci 66:32–49.
- Barbonetti, A., Vassallo, M.R.C., Cordeschi, G., Venetis, D., Carboni, A., Sperandio, A., (2010) Protein tyrosine phosphorylation of the human sperm head during capacitation: immunolocalization and relationship with acquisition of sperm-fertilizing ability. Asian J Androl 12:853–861.
- Bedford, J.M., Bent, M.J. and Calvin, H. (1973) Variations in the structural character and stability of the nuclear chromatin in morphologically normal human spermatozoa. J Reprod Fertil 33:19–29.
- Burgess, T.L. and Kelly, R.B. (1987) Constitutive and regulated secretion of proteins. Annu Rev Cell Biol 3:243–293.
- Carrell, D.T. and Liu, L. (2002) Heparin binding sites are present at a higher concentration on sperm of subfertile men than donors of known fertility. Arch Androl 48:147–154.
- Delgado, N.M., Reyes, R., Huacuja, L., Merchant, H. and Rosado, A. (1982) Heparin binding sites in the human spermatozoa membrane. Arch Androl 8:87–95.
- Eddy, E.M. and O'Brien, D.A. (1994) The spermatozoon. In The Physiology of Reproduction ed.Knobil, E., Neill, J. Raven Press, New York, pp. 29–77.
- Florman, H.M. and First, N.L. (1988) The regulation of acrosomal exocytosis. I. Sperm capacitation is required for the induction of acrosome reactions by the bovine zona pellucida in vitro. Dev Biol 128:453–463.
- Johnson, G.D., Lalancette, C., Linnemann, A.K., Leduc, F., Boissonneault, G. and Krawetz, S.A. (2011) The sperm nucleus: chromatin, RNA, and the nuclear matrix. Reproduction 141:21–36.
- Julianelli, V., Farrando, B., Alvarez Sedo, C., Calvo, L., Romanato, M. and Calvo, J.C. (2012) Heparin enhances protamine disulfide bond reduction during in vitro decondensation of human spermatozoa. Hum Reprod 27:1930–1938.
- Kong, M., Diaz, E.S. and Morales, P. (2009) Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biol Reprod 80:1026–1035.
- Lassalle, B. and Testart, J. (1992) Relationship between fertilizing ability of frozen human spermatozoa and capacity for heparin binding and nuclear decondensation. J Reprod Fertil 95:313–324.
- McLay, D.W. and Clarke, H.J. (2003) Remodelling the paternal chromatin at fertilization in mammals. Reproduction 125:625–633.
- Miller, D., Brinkworth, M. and Iles, D. (2010) Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 139:287–301.
- Ohsumi, K. and Katagiri, C. (1991) Characterization of the ooplasmic factor inducing decondensation of and protamine removal from toad sperm nuclei: involvement of nucleoplasmin. Dev Biol 148:295–305.
- Oko, R. and Sutovsky, P. (2009) Biogenesis of sperm perinuclear theca and its role in sperm functional competence and fertilization. J Reprod Immunol 83:2–7.
- Perreault, S.D., Barbu, R.R. and Slott, V.L. (1988) Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol 125:181–186.
- Pittoggi, C., Renzi, L., Zaccagnini, G., Cimini, D., Degrassi, F., Giordano, R., (1999) A fraction of mouse sperm chromatin is organized in nucleosomal hypersensitive domains enriched in retroposon DNA. J Cell Sci 112:3537–3548.
- Ramalho-Santos, J., Sutovsky, P., Simerly, C., Oko, R., Wessel, G.M., Hewitson, L., (2000) ICSI choreography: fate of sperm structures after monospermic rhesus ICSI and first cell cycle implications. Hum Reprod 15:2610–2620.
- Romanato, M., Cameo, M.S., Bertolesi, G., Baldini, C., Calvo, J.C. and Calvo, L. (2003) Heparan sulphate: a putative decondensing agent for human spermatozoa in vivo. Hum Reprod 18:1868–1873.
- Romanato, M., Regueira, E., Cameo, M.S., Baldini, C., Calvo, L. and Calvo, J.C. (2005) Further evidence on the role of heparan sulfate as protamine acceptor during the decondensation of human spermatozoa. Hum Reprod 20:2784–2789.
- Romanato, M., Julianelli, V., Zappi, M., Calvo, L. and Calvo, J.C. (2008) The presence of heparan sulfate in the mammalian oocyte provides a clue to human sperm nuclear decondensation in vivo. Hum Reprod 23:1145–1150.
- Sanchez-Vazquez, M.L., Reyes, R., Delgado, N.M., Merchant-Larios, H. and Rosado, A. (1996) Differential decondensation of class I (rat) and class II (mouse) spermatozoa nuclei by physiological concentrations of heparin and glutathione. Arch Androl 36:161–176.
- Stevens, C.F. (1993) Quantal release of neurotransmitter and long-term potentiation. Cell 72 Suppl:55–63.
- Sutovsky, P., Oko, R., Hewitson, L. and Schatten, G. (1997) The Removal of the Sperm Perinuclear Theca and Its Association with the Bovine Oocyte Surface during Fertilization. Dev Biol 188:75–84.
- Tirone, E., Siracusa, G., Hascall, V.C., Frajese, G. and Salustri, A. (1993) Oocytes preserve the ability of mouse cumulus cells in culture to synthesize hyaluronic acid and dermatan sulfate. Dev Biol 160:405–412.
- Ward, W.S. and Coffey, D.S. (1991) DNA Packaging and Organization in Mammalian Spermatozoa: Comparison with Somatic Cells. Biol Reprod 44:569–574.
- Wouters-Tyrou, D., Martinage, A., Chevaillier, P. and Sautiere, P. (1998) Nuclear basic proteins in spermiogenesis. Biochimie 80:117–128.
- Zirkin, B.R., Perreault, S.D. and Naish, S. (1989) Formation and function of the male pronucleus during mammalian fertilization. In The Molecular Biology of Fertilization ed. Schatten, H., Schatten, G. Academic Press, New York, pp. 91–114.