Abstract
Sperm chromatin damage has been associated with male infertility, increased risk for spontaneous abortion, and poor embryo development. Available methods for detecting chromatin damage render the sperm no longer suitable for clinical use. Early apoptotic events resulting in chromatin damage are associated with increased permeability of the cell membrane to large ions. We propose the use of a large fluorescent organic cation, proprietary fluorochrome (PF-1), for fluorescence-activated cell sorting (FACS) for negative selection of sperm without chromatin damage. Sperm with chromatin damage are PF-1 positive. Performance of cell sorting by PF-1 was verified with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) after FACS on PF-1(+) and PF-1(-) subpopulations. Whereas 19.5% of PF-1 positive sperm were TUNEL positive only 1.5% sperm in the PF-1(-) fraction were TUNEL positive (p < 0.00001). TUNEL values below 1.9% were considered background fluorescence. Post-sorting motility and vitality were 49.4% (SD: 12.5) and 65.0% (SD: 14.99), respectively. Proprietary fluorochrome activated sperm sorting may decrease or most likely eliminate all of TUNEL positive sperm without adverse effects on viability, providing a new therapeutic avenue for men with a high percentage of TUNEL positive sperm. Further research is needed to determine if the reduction in TUNEL positive sperm using PF-1 will improve in vitro fertilization (IVF) outcomes.
Introduction
Sperm DNA integrity is recognized as a significant parameter of sperm quality and affects the outcome of assisted reproductive procedures. Studies have demonstrated an association between sperm chromatin integrity and male fertility potential [Zini et al. Citation2001]. There are reports of an inverse relationship between the percentage of sperm with abnormal chromatin and male fertility, especially when the proportion of aberrant cells, as measured by the sperm chromatin structure assay (SCSA), are greater than 40% [Spano et al. Citation2000]. Sperm chromatin damage may subject couples to the risk of poor embryo development or recurrent spontaneous abortions [Brahem et al. Citation2011; Kumar et al. Citation2012]. However, the interaction of abnormal sperm chromatin integrity and DNA strand breaks that normally occur during DNA compaction is poorly understood. Epigenetic alterations, Y chromosome (Ch) and autosomal Chs microdeletions, and errors in mismatch repair mechanisms contribute to compromised fertility [Tarozzi et al. Citation2007]. Beyond the immediate effects of the DNA damage, it is also not apparent how DNA integrity assays can be effectively used in clinical practice [Holt and Van Look Citation2004; Sharma et al. Citation2004].
In IVF, selection of the sperm is based on motility and morphology without insight into the DNA structure, sperm surface receptor expression, and other biological properties of sperm. In nature, biological sperm selection mechanisms end up rejecting all but very few spermatozoa that are released with ejaculation [Manning and Chamberlain Citation1994]. The existence of large numbers of spermatozoa may be seen as a compensatory mechanism for inexorable errors and defects in sperm. While the competitive nature of sperm selection is largely removed with intracytoplasmic sperm injection (ICSI), simulating the natural selection of sperm may not provide optimal IVF outcomes and prevent problems with embryo development. It is presently unclear how the events in the female reproductive tract preceding fertilization and the egg itself screen for the biological quality of sperm including DNA quality [Henkel Citation2012]. It is possible that early changes in sperm membrane are used by nature as proxy for sperm DNA integrity.
There are presently several sperm DNA evaluation procedures used with sperm in assisted reproductive therapies. None of such DNA integrity procedures permits sperm to be used clinically subsequent to analysis. Other sperm selection techniques that preserve sperm largely fail to look at DNA quality in sperm. These methods range from selective microscopic examinations to computer-assisted sperm motility analysis. These methods tend to sort the sperm on significant external properties or defects, and therefore turn a blind eye to DNA integrity and chromatin damage, which arguably may be important traits in the context of IVF.
Available methods for detecting sperm chromatin damage include sperm chromatin structure assay (SCSA), TUNEL, Halosperm, and Comet assays [Dugum et al. Citation2011]. While these assays are able to test for DNA integrity, they require permanent fixation of the sperm. Permanent fixation of the sperm renders them no longer suitable for clinical use; therefore, these assays are consumptive [Henkel Citation2012]. In this, there is no opportunity to select between sperm with varying degrees of chromatin damage and select those most suitable for immediate use with IVF or cryopreservation if necessary.
Another shortfall of these methods is that they may also be unsuitable for the detection of apoptosis, a process that has been implicated in sperm chromatin damage[Lemasters et al. 1998]. Early apoptotic events resulting in chromatin damage are associated with increased permeability of the cell membrane to large ions [Morrison et al. Citation2005]. In somatic cells, annexin-V (AV) and propidium iodide (PI) are typically used to determine cells which are apoptotic and necrotic, respectively. In normal live cells, phosphatidylserine (PS) is located on the cytoplasmic surface of the cell membrane. However, in apoptotic cells, PS is translocated from the inner to the outer leaflet of the plasma membrane, thus exposing PS to the external cellular environment. Annexin-V binds to PS and thus allows for detection of late stages of apoptosis. Our efforts concentrated on screening a large number of fluorochromes to select one which would negatively select normal sperm to avoid carry-on of a foreign molecule into the oocyte and allow for very early detection of apoptotic events. Through computational screening of fluorochrome libraries, we selected fluorochromes that were least likely to negatively affect oocyte function if carry-over events occur, are excited by the 488 nm spectral line of the argon-ion laser to avoid the damaging effect of UV, and can be used for both fluorescence microscopy and flow cytometry (FC). In the current project we used a large fluorescent organic cation, PF-1, for fluorescence-activated cell sorting (FACS) to select sperm without chromatin damage. TUNEL assay was used to evaluate the number of sperm with chromatin damage. It has previously been shown that chromatin damage and apoptosis in sperm are related; therefore, in cases of sperm chromatin damage which occurs as a result of apoptosis, our hypothesis assumed that damaged sperm can be identified and removed by sorting using very early marker of apoptosis, in pursuit of improved IVF outcomes and reduced issues with embryo development.
Results
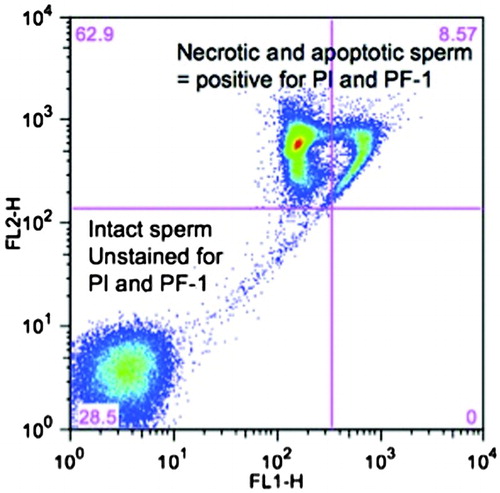
In the sorting experiment, data from all patients were combined for final analysis. Highly specific PF-1 binding allowed for clear-cut sorting as intact sperm had signal intensity less than 101 whereas PF-1 positive, damaged sperm, had PF-1 signal higher than 102 (). In the subfraction positive for PF-1 and PI, 431 x 103 of 2,167 x 103 sperm (19.5%) were TUNEL positive. In the non-staining group (PF-1(-) and PI(-)) only 33 x 103 of 2,263 x 103 (1.5%) were TUNEL positive. The difference was highly statistically significant (p <0.00001). The 1.5% value on TUNEL assay is a result of background fluorescence based on analysis of TUNEL performance characteristics. Incubation of sperm with PF-1 does not seem to impair motility as even PF(+) sperm has excellent progressive motility; we have included a video clip illustrating this as a supplement.
Figure 1. Bivariate plot of flow cytometry based sperm analysis following staining with propidium iodide (PI) and a proprietary fluorchrome (PF-1). The combination of two fluorochromes enables the separation of necrotic and apoptotic sperm (PI(+) and PF-1(+)) sperm from intact ‘normal’ sperm (PF-1(-)). Logarithmic scale transformation on the X and Y axis depicts signal intensity of PF-1 (X-axis) and PI (Y-axis). FL1-H: detection channel with 488 nm excitation and 530/30 nm filter; FL2-H: detection channel for PI.
To test that PF-1 detects small changes in the apoptotic sperm population, sperm were treated with sodium nitroprusside. Of 100,000 sperm analyzed, 0.5% before and 1.2% after induction were positive for annexin-V staining, a classic stain for apoptosis in somatic cells. In the same sample, 7.8% before and 10.7% after induction were positive for PF-1 staining. A higher number of PF-1 sperm reflects better characteristics performance in assessing early apoptotic changes in sperm using PF-1. All differences were statistically significant ().
Table 1. Change in percent of apoptotic sperm before and after apoptosis induction using annexin V and PF-1 staining.
Mean per cent TUNEL negative and positive sperm and 95% CIs were 1.02 (0.94-1.13) and 98.6% (98.5-98.8) for negative and positive controls respectively. Distribution curves fitting showed Weibull as the most appropriate model. Normal Q-Q plot and weighted percentiles analysis revealed that assay should be rejected if negative TUNEL is > 2% (p < 0.05) thus indicating that any TUNEL results less than 1.9% can be considered the result of background fluorescence as in negative controls no dye was added.
The mean motility before FACS was 53.52% (SD: 10.5) and after FACS was 49.4% (SD:12.5), t-student paired test p = 0.11, a non-statistically significant difference. There was no statistically significant difference between in mean vitality before and after FACS: 68.8 % (SD:15.3) vs. 65.0 (SD14.99), p = 0.33.
Discussion
In the current study, we have demonstrated that sperm FACS with PF-1 enables sensitive and early detection of sperm with chromatin damage while preserving PF-1 negative sperm for potential clinical use. PF-1 allows for binary selection of normal and abnormal sperm as shown by distinct clustering of intact PF-1 negative and damaged PF-1 positive sperm populations during FACS (Fig.1). A significant portion (19.5%) of the group of sperm positive for PF-1 and PI staining were also TUNEL positive. This is intuitive as PF-1 detects early changes in cell membrane occurring during the beginning of apoptosis and TUNEL detects a late event, i.e., breaks in DNA. As many processes underlying chromatin damage are gradual, it takes time for DNA breaks to occur; thus one would assume that not all sperm positive for PF-1 would be TUNEL positive immediately after sorting. However this is a desirable property of our test to detect all TUNEL positive sperm and early apoptotic events to then select the best sperm. In the PF-1 negative group, only 1.5% of sperm were TUNEL positive, which is consistent with background fluorescence based on analysis of negative and positive controls for TUNEL assay. Thus PF-1 based sperm sorting selects most if not all TUNEL positive sperm and our data suggests that this method of sorting provides an efficient and effective means of separating alive and intact normal sperm from those with abnormal chromatin. The experiments with apoptosis induction using nitroprusside sodium confirm that PF-1 detects changes in membrane during early apoptosis as compared to A-V based analysis (). This can be easily explained by physicochemical differences in a way each of the fluorochrome detects apoptosis. Annexin V is a relatively late marker of apoptosis, as binding of A-V to cell membrane occurs only after cell membrane starts to disintegrate, exposing phosphatidylserine (PS) located on the cytoplasmic (inner) surface of the cell membrane. In apoptotic cells, PS is translocated from the inner to the outer leaflet of the plasma membrane, thus exposing PS to the external cellular environment and detection by A-V. Sperm selection based on A-V would still leave a significant number of sperm which although A-V negative are apoptotic. Thus sperm sorting based on A-V would be much less clinically useful. PF-1 is sensitive and an early marker of apoptosis as it reflects subtle changes of ionic membrane potentials rather than loss of cell membrane integrity. Using PF-1 for sperm sorting we are assured that even sperm with early apoptotic events can be detected and removed from the sample.
Because the test employs large molecules that require large pores in cell membrane to enter the cell, there is little risk for residual fluorochrome to be carried-over in the isolated normal sperm. Therefore this assay may be employed as a new treatment modality for couples with male factor infertility secondary to sperm chromatin damage. This technology is significant and revolutionary in that it allows for the separation and preservation of sperm that have not undergone apoptosis and sustained chromatin damage, rather than merely identifying sperm with chromatin damage as a likely culprit for male factor infertility.
It needs to be determined to what degree selection of PF-1 negative sperm improves outcomes of IVF like fertilization, early embryo development, and pregnancy rates. In the event that sorting for chromatin damage could significantly decrease the failure rate associated with IVF and ICSI, then this technology may prove instrumental in eliciting better outcomes of ART. Given the costs and health risks that can be associated with repeated ART procedures, PF-1 based sorting has the potential to mitigate these factors in its promise to assist embryologists by providing a better tool for sperm selection than currently available.
It is our hope that if further developed, PF-1 sorting would complement current techniques that are used in the selection of optimal spermatozoa. There are numerous techniques currently employed in ART, including swim-up and density gradient centrifugation methods, as well as magnetic-activated cell sorting. While these techniques begin to provide a method of separating and grading sperm in a systematic fashion, they have deficiencies in their ability to screen for sperm on the basis of DNA integrity. The swim-up technique is dependent on the intrinsic motility of sperm, and can lose efficacy in severely asthenozoospermic samples [Aitken et al. Citation2011]. Swim up techniques also pose the risk of subjecting the sperm to oxidative stress, with exposure to free radical generating leukocytes [Baker et al. Citation1996]. Density gradients perform poorly in situations where there are few sperm in the sample or where they are poorly liquefied [Mortimer Citation1994]. While these techniques do not directly evaluate for DNA damage, recent studies have shown that most techniques selecting better sperm will also reduce the number of sperm with DNA damage; this is not surprising considering that DNA damage correlates with other parameters of sperm quality like motility [Jayaraman et al. Citation2012]. Electrophoretically isolated spermatozoa have been demonstrated to have reduced DNA damage following evaluation by TUNEL assay [Aitken et al. Citation2011] however the effects of electrophysiological fields on ionic properties of sperm is yet to be explored.
Prerequisites of new, clinically relevant, and non-consumptive techniques are demanding. They must be non-invasive, accurate in discerning between healthy and damaged sperm, and also easy and expedient to perform in the clinic [Henkel Citation2012]. Although our work has focused on sperm DNA damage it is quite possible that other yet poorly understood biological properties of sperm may be as critical in selecting optimal sperm. Existing means of evaluating sperm in a non-consumptive manner include the utilization of cumulus cells and zonae pellucida of immature oocytes to improve selection of optimal sperm [Black et al. Citation2010; Franken and Bastiaan Citation2009; Paes Almeida Ferreira de Braga et al. Citation2009]. Other studies have considered the use of Raman microspectrometry; however, the technique has not been thoroughly evaluated in assessing if it improves IVF outcomes [Huser et al. Citation2009]. It is possible that the future will encompass multistep non-consumptive sperm selection to achieve optimal outcomes based on initial evaluation of the infertile couple [Henkel Citation2012]. In so far as the outlined requisites are concerned, PF-1 based sperm sorting could prove extremely useful in assisted reproductive therapy in the clinic as it dramatically reduces one of the risks of IVF failure, i.e., sperm DNA damage. It will be essential to verify, beyond the efficacy of this sorting method in the context of IVF and ART, that there is indeed total absence of fluorochrome carry-over in sperm which are selected for IVF through this method, and that there are no other disagreeable, prohibitive, or unforeseen drawbacks. We plan to continue our work using animal models and hopefully humans to help couples with recurrent IVF failures when a high degree of sperm DNA damage in ejaculated sperm has been identified.
Materials and Methods
Overview
Spent semen samples from 18 men from an andrology laboratory were cell-sorted based on PF-1 activation into PF-1 positive and PF-1 negative samples. The use of the spent sperm samples for this study was approved by the Weill Cornell Medical College Institutional Review Board. Because otherwise to be discarded and de-identified samples were used in the study, a waiver to obtain informed consent from patients was obtained. Subsequently the number of sperm with DNA damage in each fraction was measured by TUNEL assay. Sperm sorting was performed on day of semen collection.
Preparation of semen specimens
The discarded portion of the semen analysis specimen provided at a new patient visit at an infertility practice was first purified using a Percoll gradient adapted from the WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction (WHO 2010). Percoll gradient was used to resemble the most likely future clinical scenario of using PF-1 sorting when the best sperm would be first selected for ART using swim-up or Percoll gradient followed by PF-1 sorting. Once washed, the sperm concentration was estimated by viewing 4 µL of the specimen under 400x magnification and counting the number of sperm in one field.
Induction of apoptosis
As a positive control, half of each patient specimen was incubated for one h in 10−4 M sodium nitroprusside solution to induce apoptosis. The induction protocol was adapted from Wu and colleagues [Wu et al. Citation2004].
Fluorochromes and sample incubation
Semen samples of seven men were used to optimize assay conditions for the fluorescent dyes PI, A-V, and a proprietary fluorochrome (PF-1). Dead sperm are permeable to PI, dead and apoptotic cells are permeable to PF-1. Intact cells are impermeable to both. A-V binds to phosphatidylserine (PS) on the inner side of cell membranes of apoptotic cells,both live cells undergoing apoptosis and dead cells having undergone apoptosis. Fluorochromes were obtained from Invitrogen (Carlsbad, CA, USA). Sperm were suspended in PBS 1X using 12x75 mm tubes and placed on ice. Initially 1 µL of fluorochrome was added to each sample containing about 1 x 106 cells per mL as suggested in the manufacturer's instructions for Jurkat cells. Sperm were incubated for 30 min on ice prior to sorting. Immediately after sorting the motility and vitality were measured and 5 slides for TUNEL assay from PF-1 positive and PF-1 negative populations were prepared. We optimized the staining conditions for sperm for each fluorochrome to achieve best signal intensity. Subsequent analysis and sorting was performed using 100,000 sperm per mL. In addition 5,000 and 50,000 sperm per mL were analyzed to detect limits of detection. Overall close to 200 runs and analyses was performed during this study to optimize the sperm concentration, gating, and compensation parameters, in addition to concentration and incubation parameters of fluorochromes. Forward and side scatter were used as initial gate setting to select all sperm. Considering the non-circular shape of sperm head with tail and mid-piece adding their own signal-noise, as well as the fact that most analytical algorithms use round large cell models, it is critical to establish reliable gate parameters on each of the instruments. This need is especially common with the loss of operator-dependent flexibility in the control of fluidics that accompanies the advancement of instruments. A polysterene microspheres panel was used to assist with size calibration and 6 µm spheres used in fluidics calibration (BD Calibrite 3 Beads, BD Biosciences, San Jose, CA, USA). As instruments differ in their sensitivity, design, and software we recommend using 1 µL of stock fluorchrome per 1 x 106 sperm suspended in 1 ml of PBS as initial settings to optimize the assays in other laboratories. Once assays are optimized on a specific instrument then sperm concentration can be lowered to 50,000 to 100,000 per mL. Concentration adjustment will depend on the number of TUNEL positive sperms in ejaculated samples. In men with a high number of sperm with DNA damage higher concentrations for each run are needed to achieve an adequate number of PF-1 negative (normal) sperm after sorting.
FACS
Prepared semen samples from five patients were sorted with the BD FACSVantage (BD Biosciences). Using predefined settings 488 nm excitation with green fluorescence emission and 530/30 band-pass filters were used for PF-1 based sorting. Normal spermatozoa were separated from necrotic and apoptotic cells based on PF-1 signal. To verify that proposed protocol allows for selection of intact sperm, the sorted and unsorted populations were examined microscopically for motility and viability using WHO laboratory manual 3rd edition protocol (WHO 2010). Ability of PF-1 to discriminate between DNA damaged and undamaged sperm was analyzed using TUNEL assay following a modified manufactured protocol (Roche Applied Sciences, Indianapolis, IN, USA). The percentage of TUNEL positive sperm between PF-1 positive and negative populations were compared using the chi-square test for difference in the percentage of TUNEL-positive cells.
TUNEL assay
TUNEL assay was performed using 10 µL sperm spread on each of 5 microscope slides. Two slides for negative and 2 for positive controls were analyzed and 200 sperm per slides were counted. All positive controls were incubated with 500 µL of DNAse type I (Sigma-Aldrich, St. Louis, MO, USA) for 10 min at 37°C incubator prior to adding enzyme solution and labeling solution. For negative controls only labeling solution was added. All slides were then incubated for 1 h at 37°C, counter stained with DAPI, and analyzed by the same observer. Results were reported as percentage of TUNEL positive (FITC) sperm per total number of sperm (DAPI). Performance characteristics of TUNEL to assess low limit of detection were calculated based on 125 positive and 126 negative assays scored between April of 2009 and 2012 by the same observer (AB).
Induced apoptosis and sperm chromatin damage
Semen samples of 12 men were analyzed (7 to optimize assay conditions, 5 for experimental assays) with the fluorescent dyes PI, PF-1, and A-V. Samples were analyzed with the BD LSR II flow cytometer (BD Biosciences). Half of each specimen was incubated with sodium nitroprusside for induction of apoptosis. The populations of live, apoptotic, and dead cells with and without apoptosis-induction were compared using multicolor analysis with all 3 fluorochromes.
Statistical analysis
Statistical significance of PF-1 triggered FACS in reducing number of TUNEL positive sperm was determined using the chi-square test (JMP 9 for Mac, SAS Institute, Cary, NC, USA). Difference in motility and vitality before and after flow cytometry was analyzed using t-student paired test for difference in mean assuming equal variance distribution. (Prizm 5 for Mac, GraphPad Software, Inc, La Jolla, CA, USA). Performance characteristics of TUNEL assay were measured using JMP 9 for Mac.
Declaration of interest: Cornell University has filed a U.S. patent application on technology invented by Dr. Paduch that is discussed in this paper. The remaining authors have nothing further to disclose and report no conflicts of interest. This study was partially supported by The Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust and Irena and Howard Laks.
Author Contributions: Conceived and designed the experiments: DAP, HK; Performed the experiments: HK, SM, AB, DAP; Analyzed the data: DAP, HK, MGF; Contributed reagents/ materials/ analysis tools: MG, PS, AB, SM; Wrote the manuscript: MGF, PNS, DAP.
Abbreviations
| FACS: | = | fluorescence-activated cell sorting |
| PF-1: | = | proprietary fluorochrome |
| FC: | = | flow cytometry |
| TUNEL: | = | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| IVF: | = | in vitro fertilization |
| SCSA: | = | sperm chromatin structure assay |
| Ch: | = | chromosome |
| ICSI: | = | intracytoplasmic sperm injection |
| AV: | = | annexin-V |
| PS: | = | phosphatidylserine |
| ART: | = | assisted reproductive technologies |
| PI: | = | propidium iodide |
Supplementary Material
Download MS Power Point (6.7 MB)References
- Aitken, R.J., Hanson, A.R. and Kuczera, L. (2011) Electrophoretic sperm isolation: optimization of electrophoresis conditions and impact on oxidative stress. Hum Reprod 26:1955–1964.
- Baker, H.W., Brindle, J., Irvine, D.S. and Aitken, R.J. (1996) Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil Steril 65:411–419.
- Black, M., Liu de, Y., Bourne, H. and Baker, H.W. (2010) Comparison of outcomes of conventional intracytoplasmic sperm injection and intracytoplasmic sperm injection using sperm bound to the zona pellucida of immature oocytes. Fertil Steril 93:672–674.
- Brahem, S., Mehdi, M., Landolsi, H., Mougou, S., Elghezal, H. and Saad, A. (2011) Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology 78:792–796.
- Dugum, M., Sandlow, J.I. and Brannigan, R.E. (2011) Sperm DNA damage evaluation techniques. J Androl 32:207–209.
- Franken, D.R. and Bastiaan, H.S. (2009) Can a cumulus cell complex be used to select spermatozoa for assisted reproduction? Andrologia 41:369–376.
- Henkel, R. (2012) Sperm preparation: state-of-the-art–physiological aspects and application of advanced sperm preparation methods. Asian J Androl 14:260–269.
- Holt, W.V. and Van Look, K.J. (2004) Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory tests of semen quality. Reproduction 127:527–535.
- Huser, T., Orme, C.A., Hollars, C.W., Corzett, M.H. and Balhorn, R. (2009) Raman spectroscopy of DNA packaging in individual human sperm cells distinguishes normal from abnormal cells. J Biophotonics 2:322–332.
- Jayaraman, V., Upadhya, D., Narayan, P.K. and Adiga, S.K. (2012) Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet 29:557–563.
- Kumar, K., Deka, D., Singh, A., Mitra, D.K., Vanitha, B.R. and Dada, R. (2012) Predictive value of DNA integrity analysis in idiopathic recurrent pregnancy loss following spontaneous conception. J Assist Reprod Genet 29:861–7.
- Lemasters, J. J., et al. 1998. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta, 1366, 177–96.
- Manning, J.T. and Chamberlain, A.T. (1994) Sib competition and sperm competitiveness: an answer to 'why so many sperms?' and the recombination/sperm number correlation. Proc Biol Sci 256:177–182.
- Morrison, M.L., Williamson, K., Arthur, K., Price, G.J., Hamilton, P.W. and Maxwell, P. (2005) Phenotypic changes in mitochondrial membrane potential (Delta psi(m)) during valinomycin-induced depolarisation and apoptosis. Cell Oncol 27:231–236.
- Mortimer, D. (1994) Sperm recovery techniques to maximize fertilizing capacity. Reprod Fertil Dev 6:25–31.
- Paes Almeida Ferreira de Braga, D., Iaconelli, A., Cassia Savio de Figueira, R., Madaschi, C., Semiao-Francisco, L. and Borges, E., Jr. (2009) Outcome of ICSI using zona pellucida-bound spermatozoa and conventionally selected spermatozoa. Reprod Biomed Online 19:802–807.
- Sharma, R.K., Said, T. and Agarwal, A. (2004) Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl 6:139–148.
- Spano, M., Bonde, J.P., Hjollund, H.I., Kolstad, H.A., Cordelli, E. and Leter, G. (2000) Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril 73:43–50.
- Tarozzi, N., Bizzaro, D., Flamigni, C. and Borini, A. (2007) Clinical relevance of sperm DNA damage in assisted reproduction. Reprod Biomed Online 14:746–757.
- World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 5th ed. Geneva: World Health Organization, 2010. 1–287.
- Wu, T.P., Huang, B.M., Tsai, H.C., Lui, M.C. and Liu, M.Y. (2004) Effects of nitric oxide on human spermatozoa activity, fertilization and mouse embryonic development. Arch Androl 50:173–179.
- Zini, A., Bielecki, R., Phang, D. and Zenzes, M.T. (2001) Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril 75:674–677.