Abstract
In the present study the occurrence of two heavy metals, arsenic and cadmium, have been reported in the drinking water and seminal plasma of infertile male patients as compared to a control group. The study originated from a survey of geogenic groundwater contamination with the heavy metals arsenic and cadmium in Southern Assam, India as an increase in the incidence of male infertility was being reported from these areas. According to WHO protocol, patients with sperm concentration < 20x106/ml were selected as cases (oligozoospermic and azoospermic), and those with > 20x106/ml, without any extreme pathological disorders and having fathered a child within 1-2 years of marriage were the control (normozoospermic) group. The study reports an inverse relationship between total sperm count and heavy metal content in drinking water as well as seminal plasma of the subjects. Moreover, a high correlation between altered semenological parameters and lower expression of accessory sex gland markers like fructose, acid phosphatase, and neutral α-glucosidase in the seminal plasma of patients is reported. The study also highlights significant differences of the sperm function parameters like hypo-osmotic swelling, acrosome reaction, and nuclear chromatin decondensation in the patient group as compared to controls. These findings are significant as they address a likely association between heavy metal stress and altered sperm function as well as seminal enzyme inhibition.
Introduction
Every day human beings are exposed to various chemicals and metals such as arsenic, cadmium, lead, food additives, and pesticides. Accumulation of the heavy metals and chemicals leads to toxicity in the tissues and organs thereby causing nutritional deficiencies, hormonal imbalances, system dysfunctions, and infertility. Recent evaluation of infertile couples has revealed that male infertility is responsible for 50% of the cases of infertility reported. Studies confirm that male sperm counts are declining and environmental factors, such as, pesticides, exogenous agents, and heavy metals may negatively impact spermatogenesis [Sheweita et al. Citation2005]. The heavy metals that bear particular significance in studies on infertility are lead, mercury, and cadmium [Roychowdhury and Gautam Citation1995].
Arsenic (As) exposure has been documented to adversely affect the reproductive system and development of rat offspring [Zhang et al. Citation2000]. Arsenic-induced male infertility is characterized by abnormal sperms, decreased sperm counts, and decreased sperm motility in human as well as animal models [Pant et al. Citation2004; Sarkar et al. Citation2003; Centeno et al. Citation2002]. Decrease in sperm viability and impaired reproduction was observed in rats after exposure to arsenic [Jeyandran et al. Citation1992]. Arsenic intoxication in experimental animals has been found to be associated with inhibition of testicular steroidogenic function [Sarkar et al. Citation1991] and spermatogenesis [Sukla and Pandey Citation1984]. Chronic arsenic toxicity due to drinking arsenic contaminated ground water is also a major environmental health hazard throughout the world [Mazumder Citation2008]. Chronic arsenic poisoning results from drinking contaminated ground water over a long period of time.
Results from previous studies are consistent with the hypothesis that environmental cadmium (Cd) exposure may contribute significantly in reducing sperm concentration and sperm motility [Kasperczyk et al. Citation2002; Benoff et al. Citation2009]. Prostatic acid phosphatase activity also decreased significantly in the prostate glands of animals receiving 50 mg/kg cadmium chloride [Monsefi et al. Citation2010]. Epidemiological studies provided equivocal results concerning the effect of cadmium on hormone concentration, male infertility, and sperm parameters [Benoff et al. Citation2000]. Cadmium has been recognized as an endocrine disruptor because of adverse effects on the reproductive system leading to the inhibition of steroidogenesis and spermatogenesis and its ability to bind to androgen and estrogen receptors [Benoff et al. Citation2009; Yeung et al. Citation2011]. Several studies have illustrated that the testis is exceedingly sensitive to cadmium toxicity, likely due to disruption of the blood–testis barrier via specific signal transduction pathways and signalling molecules [Siu et al. Citation2009]. Exposure to cadmium has been reported to induce testicular and epididymal damage [De Souza Predes et al. Citation2010; Toman et al. Citation2011] and may contribute to male infertility by reducing sperm quality [Benoff et al. Citation2009; Xu et al. Citation2001].
The Indo-Gangetic Plain of India, Bangladesh as well as the North-Eastern States of India have water bodies and ground water geogenically contaminated with heavy metals [Frisbie et al. Citation2002; Bhattacharya et al. 2002]. A previous literature survey has revealed that although there are reports [Cullen et al. Citation1984] related to dysfunction of the male reproductive system in man and its interactivity with certain heavy metals, no such study has been undertaken in this region.
The present work is significant because two different approaches have been taken to elucidate the association between ground water heavy metals and the incidence of male infertility. Ground water samples from eleven zones of Southern Assam were analyzed for arsenic and cadmium content by atomic absorption spectrometer (AAS) following which a study of the presence of the two heavy metals, arsenic and cadmium, in the drinking water of the subjects, as well as the heavy metal load present in their seminal plasma was performed. Based on an epidemiological (case-control) model, correlation was also seen between heavy metal stress (as determined from presence of heavy metals in seminal plasma) and the occurrence of male sterility in this region. However, such correlation studies can only report a strong association between heavy metal stress and male reproductive dysfunction and not direct causation. Moreover, the study also targets to determine a causal relationship between reduced sperm count, seminal plasma components based on the biochemical markers of accessory sex glands, and sperm function parameters to elucidate the underlying mechanism of male sterility.
Results
The fertility clinics in Southern Assam under study reported that infertile males came from distinct geographic areas. This motivated us to conduct a study based on an epidemiological model to investigate these apparent pockets of male infertility. On analyzing ground water samples from the eleven zones of Southern Assam, shown in , these pockets were found to have significantly higher content of arsenic and cadmium ().
Table 1. Table showing the geographic zones under study listed with the arsenic and cadmium content.
The ground water heavy metal load was corroborated with the heavy metal content in the drinking water and seminal plasma samples of the subjects studied. Interestingly, most of the patients in this study were found to use ground water as a source of drinking water. The control group mostly consumed supply water from the Public Health Engineering (PHE) department. Two additional groups were also included in the study that were comprised of subjects who were fertile but drinking ground water and those who were infertile but drinking PHE water ().
Table 2. Odds Ratio and χ2 value for the demographic characteristics of normozoospermic and oligozoospermic/azoospermic individuals.
Based on the demographic characteristics (drinking water source, smoking habit, alcohol consumption, tobacco chewing, dietary habits) (), odds ratio (OR) and χ2 test for consistency were calculated. In our study, both the tests are consistent with each other and the values of OR and χ2 test () reveal that the study groups (fertile normozoospermic individuals; infertile oligozoospermic and azoospermic individuals) are similar regarding the lifestyle factors, they differ only regarding the source of drinking water.
It may also be inferred from the regression analysis that, individuals who consumed water from PHE had significantly higher sperm count compared to individuals who consumed ground water (B). Age had an insignificant influence on sperm count for the studied subjects (A). The coefficient of the urban dummy variable was found to be positive but insignificant (B). Number of months elapsed since marriage had a negative influence on sperm count but the coefficient was statistically insignificant (B). The coefficient of the service dummy variable was found to be negative but was statistically significant at 20% (B). Sixty-seven per cent of the total variation in sperm count was explained by the linear regression line (R2 value = 0.67). Thus, the overall regression was highly significant which was evident from the high F-value in the ANOVA table (A).
Table 3. ANOVA table of regression analysis of other demographic characteristics (age, habitat-urban, source of drinking water-PHE, time since marriage, and occupation-service (A) and the impact of demographic characteristics on semen parameters (B).
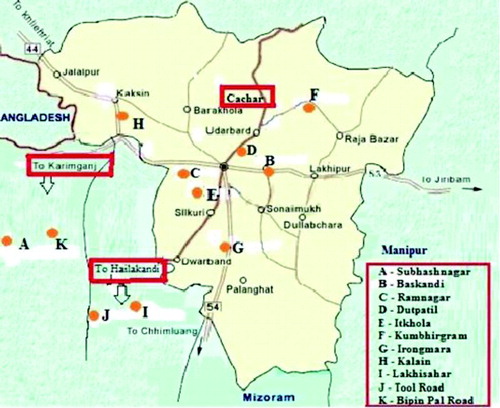
From the results it was observed that the mean drinking water arsenic and cadmium content (A and B) as well as the mean seminal plasma arsenic and cadmium content (C and D) among the oligozoospermic and azoospermic cases were significantly higher than that of the normozoospermic controls (). Our results also confirm that the correlation co-efficient of arsenic (r = 0.867) and cadmium (r = 0.892) in drinking water of the subjects studied and the heavy metal load in their respective seminal plasma indicate strong positive correlation between the variables (E and F). Thus, it is evident from the correlation coefficients that mean seminal plasma heavy metal load is manifold of the mean drinking water heavy metal content which indicates marked bioaccumulation and biomagnification of the heavy metals arsenic and cadmium in the subjects studied.
Table 4. A summary table presenting the mean sperm parameters with the arsenic and cadmium levels in drinking water and seminal plasma of oligozoospermic and azoospermic as compared to normozoospermic individuals.
Figure 2. Arsenic and cadmium content. A) The arsenic content (ppm) in drinking water from volunteers was assayed by atomic absorption spectrophotometer. Arsenic content in the drinking water of the normozoospermic was found to be significantly lower (P < 0.05) than that of the azoospermic and oligozoospermic subjects. B) The cadmium content (ppm) in drinking water from volunteers was assayed by atomic absorption spectrophotometer. Cadmium content in the drinking water of the normozoospermic was found to be significantly lower (P < 0.05) than that of the azoospermic and oligozoospermic subjects. C) The arsenic content (ppm) in the seminal plasma from volunteers was assayed by atomic absorption spectrophotometer. Significantly higher (P < 0.05) arsenic content in the seminal plasma was observed in the azoospermic and oligozoospermic subjects than that of the normozoospermic. D) The cadmium content (ppm) in the seminal plasma from volunteers was assayed by atomic absorption spectrophotometer. Significantly higher (P < 0.05) cadmium content in the seminal plasma was observed in the azoospermic and oligozoospermic subjects than that of the normozoospermic. A-D) Results are expressed in Mean ± Standard Deviation. E) The arsenic content (ppm) in drinking water was plotted against the seminal plasma of the studied subjects. The correlation co-efficient of arsenic (r = 0.867) in drinking water and the heavy metal load in their respective seminal plasma indicate strong positive correlation between the variables. That is, the higher the arsenic level in drinking water, the higher is the arsenic level in seminal plasma. F) The cadmium content (ppm) in drinking water was plotted against the seminal plasma of the studied subjects. The correlation co-efficient of cadmium (r = 0.892) in drinking water and the heavy metal load in their respective seminal plasma indicate strong positive correlation between the variables. That is, the higher the cadmium level in drinking water, the higher is the cadmium level in seminal plasma.
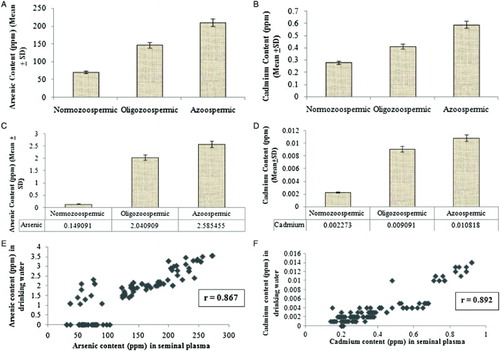
Subjects from areas of high arsenic and cadmium content have been found to be oligozoospermic or azoospermic. From the sample observations it can be stated that arsenic and cadmium content in drinking water and total sperm count are inversely related as shown in panels A and B. The correlation co-efficients (r = -0.91 and r = -0.82, respectively) indicate that presence of the two heavy metals in drinking water has a deleterious effect on total sperm count and thus, on male reproductive capacity.
Figure 3. Scatter plots of sperm counts as function of arsenic and cadmium content in the drinking water. A) The arsenic content (mg/l) in drinking water was plotted against the total sperm count of the subjects (million/ml). From the scatter diagram, it is observed that arsenic content in drinking water and total sperm count are inversely related. That is, the higher the arsenic level in drinking water, the lower the sperm count. Results are expressed as r = - 0.91. The correlation co-efficient is negative and significant at 5% level which establishes that the presence of arsenic in drinking water has a severely harmful (reducing) effect on total sperm count and thus on male reproductive capacity. B) The cadmium content (mg/l) in drinking water was plotted against the total sperm count of the subjects (million/ml). From the scatter diagram, it is observed that cadmium content in drinking water and total sperm count are inversely related. That is, the higher the cadmium level in drinking water, the lower the sperm count. Results are expressed as r = - 0.82. The correlation co-efficient is negative and significant at 5% level which establishes that the presence of cadmium in drinking water has a severely harmful (reducing) effect on total sperm count and thus on male reproductive capacity. Azoo: azoospermic, Oligo: oligozoospermic, Normo: normozoospermic
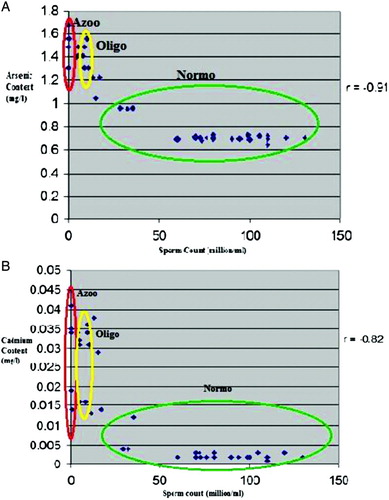
The results clearly suggest that there has been a significant decrease in mean sperm count (in million/ml), sperm motility (percent), sperm morphology (percent), and seminal plasma components in the oligozoospermic and azoospermic cases exposed to arsenic and cadmium when compared to the normozoospermic controls as shown in . Decreased sperm motility in infertile subjects likely indicates that heavy metal exposure may inhibit sperm motion and direction. Non-motile sperms positively correlate with sperms with abnormal morphology. As shown in , it can be suggested that heavy metal intoxication may somehow have changed sperm morphology, cytoskeletal structure and thereby, sperm motility. The correlation coefficient (r = 0.956) is indicative of a strong positive correlation between morphology and motility of sperm (B).
Figure 4. Morphology of affected spermatozoa. A) Human spermatozoa were stained with Diff-Quik stain to observe the morphological defects that occur in sperm organelles. The sperm with normal morphology is marked with an arrow head and those with abnormal morphology are marked with arrows. B) The sperm morphology (%) was plotted against the sperm motility (%) of the studied subjects. The value of ordinary correlation coefficient (r = 0.956) is indicative of a strong positive correlation between morphology and motility of sperms, i.e., the higher the normal morphology of sperms, the higher their motility.
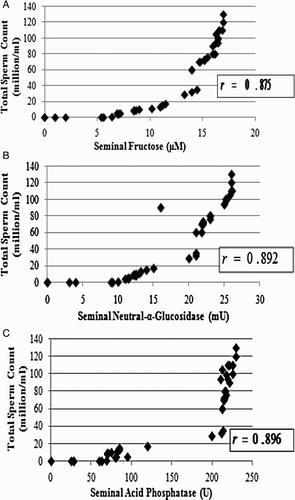
Apart from the routine semen analysis, function and obstruction of accessory sex glands can be assessed by fructose, α-glucosidase, and acid phosphatase assays. Subjects with a low sperm count were also found to have low seminal fructose, α-glucosidase, and acid phosphatase. Mean total sperm count as well as mean seminal fructose, seminal neutral α-glucosidase, and seminal acid phosphatase among the normozoospermic controls is significantly higher than that of the oligozoospermic and azoospermic cases.
The correlation coefficient (r = 0.875) is indicative of a strong positive association between total sperm count and seminal fructose (A). Distal ductal obstruction shows significantly decreased α-glucosidase values. The correlation coefficients (r = 0.892 and r = 0.896) are indicative of a strong positive association between total sperm count and seminal neutral α-glucosidase and between total sperm count and seminal acid phosphatase, respectively (B and C).
Figure 5. Association of sperm count with seminal paramaters. A) The total sperm count (million/ml) was plotted against the seminal fructose (µM) of the subjects. The scatter diagram approximately follows an exponential path (non-linear relation). Results are expressed as r = 0.875. The value of ordinary correlation coefficient is indicative of a strong positive association between total sperm count and seminal fructose, i.e., the higher the total sperm count, the higher the seminal fructose. B) The total sperm count (million/ml) was plotted against the seminal neutral-α-glucosidase (mU) of the subjects. The scatter diagram approximately follows an exponential path (non-linear relation). Results are expressed as r = 0.892. The value of ordinary correlation coefficient is indicative of a strong positive association between total sperm count and seminal neutral-α-glucosidase, i.e., the higher the total sperm count, the higher the seminal neutral-α-glucosidase. C) The total sperm count (million/ml) was plotted against the seminal acid phosphatase (U) of the subjects. The scatter diagram approximately follows an exponential path (non-linear relation). Results are expressed as r = 0.896. The value of ordinary correlation coefficient is indicative of a strong positive association between total sperm count and seminal acid phosphatise, i.e., the higher the total sperm count, the higher the seminal acid phosphatase.
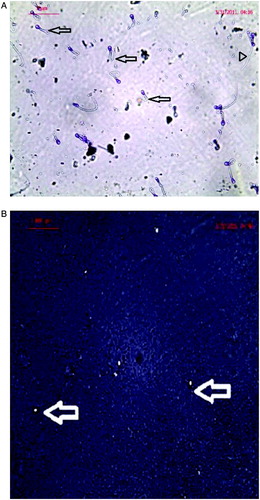
Normozoospermic healthy men with normal semen parameters are almost consistently found to have swollen sperms with coiled tail, whereas oligozoospermic and azoospermic men with abnormal semen parameters characterized by decreased sperm concentration, sperm motility, and abnormal sperm morphology with a fraction of sperms having no coiling of tail. The swollen sperm after incubation under hypo-osmotic conditions reflects the spermatozoa with intact membranes (A). Normal acrosin activity indices are also observed in the normozoospermic controls, whereas the halo diameters and halo formation rates were smaller in most cases of oligozoospermic and azoospermic cases (B).
Figure 6. Micrographs of sperm following structural assays. A) Sperm function test of human subject showing positive response for hypo-osmotic swelling test in the form of tail coiling. The sperms with coiled tail (normal) are indicated by arrow head and those without coiled tail (abnormal) are indicated with arrows. B) Sperm function test of human subject showing positive response for acrosomal reaction test with the formation of halo surrounding sperm head. The sperms with halo head are pointed with arrow (normal) and sperms showing negative response for the test are not visible.
summarizes the findings of this study. High concentrations of arsenic and cadmium can alter sperm count, motility and morphology, seminal fructose, neutral α-glucosidase, and acid phosphatase as well as functional capacity of sperm as tested by hypo-osmotic swelling test and acrosome status and function test.
Discussion
Arsenic is a potent carcinogen and is known to cause blood disorders and cardiovascular diseases. A very high exposure to arsenic has been shown to cause infertility in both males and females. Groundwater with elevated concentrations of arsenic has been recognized as a problem of global concern [Bhattacharya et al. 1997; Bhattacharya et al. 2002]. The major route of human exposure for inorganic arsenic is through consumption of contaminated drinking water, especially in some regions such as India, Bangladesh, Taiwan, and China [Ahsan et al. Citation2000; Chen et al. Citation2005; Mazumder et al. Citation1998; Xia and Liu Citation2004]. Cadmium is a heavy metal that comes from both natural and man-made sources. Drinking water is also a well-recognized pathway of exposure to this metal.
The present study addresses an association between the presence of heavy metals in geogenically contaminated ground water and the incidence of male infertility. It does not signify causation but gives evidence that the heavy metals in drinking water are highly correlated with the increased incidence of male infertility in the population.
Analyzing ground water samples from different zones, showed that zone K had the highest arsenic content (3.54 ppm) while zone G had the lowest arsenic content (1.07 ppm) in comparison to all zones (see for zone map). The cadmium content was highest in zone B (0.014 ppm) while zones A and G had no cadmium content in the ground water samples. WHO [2003] has recommended a limit of 0.01 mg/l of arsenic and 0.005 mg/l of cadmium in drinking water, respectively. From our results, we find that the drinking water of the region under study has exceeded the permissible limit of the above mentioned metals. It is a matter of great concern that the concentration of these metals in the groundwater has crossed the WHO recommended value and maximum permissible limit. The concentration of arsenic in other affected areas was found to be several times higher than the maximum contamination level (MCL) (10 µg/l) as reported by Singh et al. [2007]. The World Health Organization has also reviewed arsenic guidelines in drinking water and established a provisional guideline of 10 µg/l after concluding that inorganic arsenic is a human carcinogen and that the main route of exposure is through drinking water and food [WHO 2003].
Naturally occurring geologic deposits of arsenic and cadmium can easily dissolve into groundwater, potentially resulting in unsafe levels of these heavy metals in drinking water supplies of an area. Chronic poisoning from arsenic and cadmium results from drinking contaminated water over a long period of time. Therefore, the people living in these areas are prone to develop various ill effects of these heavy metals on long term exposure.
Since heavy metals are stable elements (they cannot be metabolized by the body) and are bio-accumulative, over time they can reach toxic levels. It has long been established that agents such as arsenic and cadmium which are known reproductive toxicants are found to accumulate in human semen [Gagnon Citation1988]. Synergistic combined effects or additive joint actions of these metals have also been documented [Enserink et al. Citation1991; Palaniappan and Karthikeyan Citation2009]. That the arsenic and cadmium load in the seminal plasma is manifold of the arsenic and cadmium content of drinking water of most oligozoospermic and azoospermic cases, indicates bioaccumulation and biomagnification of these metals in the seminal plasma.
Analysis of the demographic parameters specifically identified the drinking water as a potential source of causation of infertility in an iterative way. Apart from geogenically contaminated ground water, a potent source of cadmium toxicity is from smoking or chewing tobacco. Cadmium competitively inhibits zinc metalloenzymes as well as enzymes activated by zinc. Similarly, a vegetarian diet is associated with zinc depletion [Freeland-Graves et al. Citation1980] which impairs sperm production, produces malformed and defective sperm, and degeneration of testosterone [Moriyama et al. Citation1987]. A non-vegetarian diet can also potentially cause a fall in sperm function as well as sperm motility and viability by inducing oxidative stress [Anilakumar et al. Citation2002]. In the present study, we found no significant association of demographic characteristics such as smoking habit, alcohol consumption, chewing tobacco, nature of diet, with the incidence of male infertility as evident from the computed ORs and χ2 test for consistency. However, in the case of ground water intake, the OR and χ2 test for consistency were found to be greater than 1, thus showing significant associations between heavy metal content in ground water and incidence of male infertility. Therefore, the consistency in computed ORs and χ2 test values reveals that the incidence of male infertility in the studied group were likely to have been due to ground level drinking water and not due to other attributes. Moreover, age, type of occupation, type of habitat, and time elapsed since marriage had no significant influence on the sperm count of the population under study as validated by the reported regression analysis.
Subjects from high arsenic and cadmium content zones have been found to be oligozoospermic or azoospermic. This indicates that the presence of these two heavy metals in drinking water has a deleterious effect on male reproductive capacity. Our study has shown that arsenic and cadmium have an adverse effect on sperm count and retard the activity of live sperms.
Chromatin and flagellum in mammalian spermatozoa contain a large amount of thiol rich protamine disulfides which are involved in the maintenance of sperm stability and motility [Pant et al. Citation2004]. The decrease in sperm motility observed in the present study might be ascribed to the binding of the heavy metals to sulfydryl or thiol groups on sperm proteins or the inhibition of enzymes involved in sperm motility [Wang et al. Citation2007; Pant et al. Citation2004].
Sperm motility is one of the important factors necessary for natural pregnancy to occur and is governed by motor and cytoskeleton proteins. These proteins are regulated by a group of small G proteins called Rho GTPases (Ras homologue) (RhoA-B and Rac). These proteins control actin or tubulin cytoskeleton assembly and vesicle transport [Tapon and Hall Citation1997]. Defects in these motor or cytoskeleton proteins and their regulators lead to morphological abnormalities (teratozoospermia) and poor motility (asthenozoospermia). Defects in the tail region in these proteins might be associated with sperm immotility while head region specific RhoGTPase could be linked to failure of sperm to undergo hyperactivation (capacitation), acrosome reaction (AR) and sperm oocyte fusion. This may be because of improper sperm head structural organization as a result of abnormal cytoskeleton network proteins or their regulators. Thus, it is evident from our study that reduction in fertility potential is largely due to the changed sperm morphology and thereby sperm motility.
Biochemical analysis of seminal plasma provides insights into the function of the accessory sex glands. Chemicals that are secreted primarily by each of the accessory sex glands are typically selected to serve as a marker for each respective gland. The present work also revealed that male reproductive dysfunction can also be associated with adverse affect of arsenic and cadmium on proper functioning of seminal vesicle, epididymis, and prostate.
A low level of fructose in the seminal plasma indicates an associated dysgenesis of the seminal vesicles [Schill and Henkel Citation1999] since fructose is the biochemical marker of seminal vesicles. The disturbances at the level of the epididymis can be assessed by the neutral α-glucosidase assay [Schill and Henkel Citation1999]. Similarly, a decreased level of acid phosphatase in seminal plasma is an indicator of abnormal prostate function [Schill and Henkel Citation1999]. Our results in the present study highlight that arsenic and cadmium being sulphydryl group modifiers, can inhibit the enzymes acid phosphatase (prostrate function marker) and neutral α-glucosidase (epididymal marker) in the oligozoospermic and azoospermic cases, repress their expression at the gene level, as well as adversely affect fructose (seminal vesicle marker) metabolism. The results also showed that fructose, neutral α-glucosidase, and acid phosphatase level were significantly lower in samples from the oligozoospermic and azoospermic cases as compared to the normozoospermic controls.
Another important aspect of our present study highlights a decrease in sperm coiling and halo head sperms in the oligozoospermic and azoospermic cases as compared to the normozoospermic controls. Specialized sperm function tests like hypo-osmotic swelling (HOS) test and AR test are better predictors of fertilizing potential than traditional semen parameters assessed by standard semen analysis [El-Ghobashy and West Citation2003; Menkveld et al. Citation2003].
Some physiological processes during fertilization (capacitation, acrosomal reaction, fusion of sperm and ovum) demand the presence of an active membrane, and it is impossible to have fertilization with membranes that are physically inactive [Jeyendran et al. 1984]. The HOS test has been used to evaluate the functional integrity of plasma membranes of the spermatozoa. It can indicate whether the sperm membrane is biochemically active or not [Vazquez et al. Citation1997]. The HOS test is based on the ability of functioning sperm to swell after being exposed to the hypo-osmotic solution [Cabrita et al. Citation1999]. In the hypo-osmolar solution, fluid is transferred into the cell through the plasma membrane of spermatozoa. Trying to achieve a balance between intracellular and extracellular spaces, functionally intact membranes begin to swell starting at the tail of the spermatozoa. Such spermatozoa are denoted as swelled or HOS reactive (HOS+) signifying functionally intact membranes. Spermatozoa with functionally defective membranes do not swell and their tails do not invaginate [Jeyendran et al. 1984]. The plasma membranes of such spermatozoa may be physically intact but functionally challenged. Lack of chemically functional membranes may impair the ability of spermatozoa to undergo capacitation, AR, and binding to the zona pellucida.
The AR is an exocytotic process of spermatozoa and an absolute requirement for fertilization. It leads to the release of a variety of hydrolytic and proteolytic enzymes, mainly acrosin and hyaluronidase, which are essential for sperm penetration through the oocyte envelopes [Yanagimachi Citation1994]. The AR also results in the modification of some plasma membrane proteins at the acrosomal equatorial segment and post acrosomal level necessary for the fusion with the oocyte membrane. Several studies have found that sperm responsiveness to acrosome reaction inducers was reduced in infertile patients [Krausz et al. Citation1995; Krausz et al. Citation1996]. The results of the present investigation also showed that HOS test and AR test scores were significantly lower in samples from the oligozoospermic and azoospermic cases as compared to the normozoospermic controls.
In conclusion, our study clearly suggests that there is a direct correlation between presence of arsenic and cadmium in drinking water (and thus in the seminal plasma) and incidence of male infertility. Moreover, we also report a direct bioaccumulation and biomagnification of the heavy metals in the seminal plasma from the drinking water sources. The study also highlights a significant fall or abnormality in semen parameters in the oligozoospermic and azoospermic cases when compared with the normozoospermic controls. Thus, the results in this study provide a baseline for subsequent studies on the impact of certain heavy metals on semen quality and quantity, and sperm function as well.
Materials and Methods
Individuals were randomly chosen from the male partners of couples attending the andrology laboratory for male infertility-related problems. Based on an epidemiological survey model, a questionnaire was prepared on age, occupation, food habits, smoking habits, alcohol intake, family history, medical history, and source of drinking water of the oligozoospermic and azoospermic (cases) and normozoospermic (controls) studied subjects,. Further, a survey was done on occurrence of heavy metals in the three districts: Cachar, Karimganj, and Hailakandi of Southern Assam, India. These three districts of Southern Assam were divided into eleven arbitrary zones designated A - K (). Water samples were randomly drawn from deep tube wells and bore wells and were analyzed for the presence of arsenic and cadmium by an atomic absorption spectrophotometer (AAS).
In the second part of the study, the drinking water and seminal plasma were obtained from the case-control studied subjects. A total of 100 men (age range, 22-45 y) constituted the study population of which thirty-two were proven fertile healthy volunteers (normozoospermic, those with sperm concentration > 20x106/ml) and sixty-eight individuals were reported to be infertile. Of the infertile individuals, thirty five were found to be oligozoospermic (those with sperm concentration < 20x106/ml) and thirty-three azoospermic (those with no spermatozoa in their ejaculate). Individuals with sperm concentration < 20x106/ml (reference value set by [WHO 1999]) were selected as cases (oligozoospermic and azoospermic), those with > 20x106/ml, without any extreme pathological disorders and having fathered a child within 1-2 y of marriage were the control (normozoospermic). Controls had sperm concentration above the reference value while the cases had sperm concentration below the reference value.
Study consent
A written consent of each subject was taken after explaining the aims and objectives of the study. The study was carried out according to the guidelines and approval of the ethics committee of Assam University, Assam, India. After obtaining the informed consent, semen samples were collected from the subjects.
Water and seminal plasma analysis
The ground water, drinking water, and seminal plasma of the subjects were analyzed in an AAS for estimating the concentrations of arsenic and cadmium. Seminal plasma was separated by centrifuging the semen at 1,500 × g for 10 min. To ensure the removal of organic impurities from the samples and thus prevent interference in analysis, the samples were digested with concentrated nitric acid (HNO3). A volume of 10 ml HNO3 was added to 50 ml water in a 250 ml conical flask. The mixture was evaporated to half of its volume on a hot plate after which it was allowed to cool and then filtered [Momodu and Anyakora Citation2010]. The digested samples were then analyzed for estimating the concentrations of arsenic and cadmium in an AAS (Perkin Elmer 3110, Model: Graphite Furnace Vario 6 with detection limits, arsenic- 0.05 µg/L and cadmium- 0.002 µg/L). The method proved very accurate showing a recovery of 96% for arsenic and 97.50% for cadmium.
Semen analysis
Each subject produced a semen sample by masturbation into a sterile wide-mouthed plastic specimen container. The men were instructed to abstain from ejaculation for 2-5 d before producing the semen. The sample was allowed to liquefy at 370C for 20 min before analysis. Measurement of both sperm concentration and motility was done within 60 min of semen collection in a pre-warmed (370C) Makler counting chamber (Sefi Medical Instruments, Haifa, Israel).
Semen analysis was performed following the procedures as set out in WHO [1999] that included assessment of sperm concentration, percentage motility (rapid forward progressive, sluggish forward progressive, and immotile sperms), and morphology (normal and abnormal sperms). To measure both sperm concentration and motility, a minimum of 200 sperm cells from at least four different fields were analyzed from each specimen. ‘Motile sperm’ were defined according to the WHO grade as ‘a’ grade sperm (rapidly progressive with a velocity ≥ 25 mm/s at 370C) and ‘b’ grade sperm (sluggish progressive with a velocity ≥ 5 mm/s but, < 25 mm/s). Sperm concentration and motility was performed at 200× with a Nikon microscope. Concerning sperm morphology, at least two slides were prepared for each fresh semen sample. The resulting thin smear was allowed to air dry before staining with the Diff-Quik staining kit (Dade Behring AG, Dudingen, Switzerland). Morphological assessment was performed with a Nikon microscope using an oil immersion 1,000× (Nikon Company, Tokyo, Japan). A minimum of 200 sperm cells were counted from two slides prepared for each specimen.
Semen biochemistry
Determination of fructose in seminal plasma
After diluting 20 µl seminal plasma with 220 µl distilled water, 50 µl ZnSO4 and 50 µl NaOH were added. The test tube was allowed to stand for 15 min and centrifuged at 2,500 rpm for 15 min. To 200 µl of the clear supernatant, 200 µl indole reagent and 2 ml HCl (32%) were added. This mixture was then incubated against assay blanks to determine fructose levels at 600C for 20 min, cooled in ice water, and then analyzed in a spectrophotometer at 470 nm [Karvonen and Malm Citation1995].
Assay of neutral alpha-glucosidase activity in seminal plasma
A volume of 10 µl seminal plasma was vortexed with 100 µl p-nitrophenol glucopyranoside (PNPG) in microfuge tubes. Next 5 µl castanospermine was added to each tube and incubated for 2 h in a water bath at 370C. Incubation was stopped by adding 1ml 0.1M Na2CO3 to each tube and the absorbance was read at 405 nm against the assay blanks [Copper et al.Citation1990; Paquin et al. Citation1984].
Measurement of acid phosphatase in seminal plasma
A volume of 20 µl seminal plasma was diluted with 20 µl NaHSO4 solution in order to stabilize the acid phosphatase if not assayed immediately. Then 10 µl of the diluted sample was mixed with 0.5 ml citrate buffer. A 0.1 ml P-nitrophenol phosphate solution was put in each assay tube and warmed at 370C for 5 min. A volume of 10 µl of the diluted sample was added to the pre-warmed tubes and incubated at 370C for 30 min. The enzyme reaction was stopped by adding 1ml NaOH solution and the absorbance was read at 405 nm against the assay blanks.
Sperm function tests
Hypo-osmotic swelling (Kit method)
A volume of 500 µl of HOS solution and 50-100 µl of liquefied semen sample were mixed gently and incubated at room temperature for 5 min. At the end of the incubation time, 50 µl color stop solution was added to the mixture. One drop of the mixture was taken on a clean glass slide and observed under microscope at 400× to count percentage of spermatozoa with coiling and swelling.
Acrosome status and function (Kit method)
A volume of 500 µl of acrosome solution and 50-100 µl of liquefied semen sample were mixed gently and incubated at room temperature for 5 min. A smooth smear was made on a gelatin coated slide and incubated at 500C in a moist humid pre-warmed chamber for 30 min. Air dried slides were observed under microscope at 400× to count percentage of spermatozoa with halos surrounding their head.
Statistical analysis
Odds ratio and χ2 test for consistency and regression analysis were computed for the demographic characteristics of the study subjects. Correlation co-efficients were computed and one-tailed Student's t tests were also performed to compare the mean values of the normozoospermic controls as compared to the oligozoospermic and azoospermic cases. The results were expressed as mean ± standard error of mean.
Abbreviations
| IVF: | = | in vitro fertilization |
| AAS: | = | atomic absorption spectrophotometer |
| As: | = | arsenic |
| Cd: | = | cadmium |
| HNO3: | = | nitric acid |
| WHO: | = | World Health Organization |
| PHE: | = | Public Health Engineering |
| OR: | = | odds ratio |
| ZnSO4: | = | zinc sulphate |
| NaOH: | = | sodium hydroxide |
| HCl: | = | hydrochloric acid |
| PNPG: | = | p-nitrophenol glucopyranoside |
| Na2CO3: | = | sodium carbonate |
| NaHSO4: | = | sodium bisulphate |
| HOS: | = | hypo-osmotic swelling |
| AR: | = | acrosome reaction. |
Acknowledgments
The authors thank SAIF, North Eastern Hill University, Shillong, India for providing the facility of the AAS; the NIHFW, New Delhi, India for making a generous gift of the sperm function kits; Dr. Jayashree Rout, Professor, Department of Ecology and Environmental Science, Assam University, Silchar, India for providing the facility for microscopic photography, and Dr. Ritwik Mazumder, Assistant Professor, Department of Economics, Assam University, Silchar, India for assistance in statistical analysis.
Declaration of interests: The authors declare that they have no conflict of interests.
Author contributions: Conceived and designed the experiments: MS; Performed the experiments: ID; Analyzed the data: ID; Contributed reagents/materials/analysis tools: MS, GDS; Contributed clinical samples and medical history of patients with their informed consent: KKK; Wrote the manuscript: MS, ID.
References
- Ahsan, H., Perrin, M., Rahman, A., Parvez, F., Stute, M. and Zheng, Y. (2000) Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med 42:1195–1201.
- Anilakumar, K.R., Khanum, F., Krishna, K.R. and Viswanathan, K.R. (2002) Effects of dried fish on antioxidant levels in rat liver. Indian J Exp Biol 40(8):914–917.
- Benoff, S., Hauser, R., Marmar, J.L., Hurley, I.R., Napolitano, B. and Centola, G.M. (2009) Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers). Mol Med 15(7-8):248–262.
- Benoff, S., Jacob, A. and Hurley, I.R. (2000) Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update 6:107–121.
- Bhattacharya, P., Chatterjee, D. and Jacks, G. (1997) Occurrence of As-contaminated groundwater in alluvial aquifers from the Delta Plains, eastern India: Option for safe drinking water supply. Int J Water Res Dev 13:79–92.
- Bhattacharya, P., Jacks, G., Frisbie, S.H., Smith, E., Naidu, R. and Sarkar, B. (2002) Arsenic in the environment: a global perspective. In Heavy Metals in the Environment ed. Bibudhendra, S. Marcel Dekker, Inc. New York, 147–215.
- Bhattacharya, P., Jacks, G.M. and Khan, A.A. (2002) Arsenic in groundwater of the Bengal delta plain aquifers in Bangladesh. Bull Environ Cont Toxicol 69:538–545.
- Cabrita, E., Alvarez, R., Anel, E. and Herraez, M.P. (1999) The hypoosmotic swelling test performed with coulter counter: a method to assay functional integrity of sperm membrane in rainbow trout. Anim Reprod Sci 55:279–287.
- Centeno, J.A., Mullick, F.G., Martinez, L., Page, N.P., Gibb, H., Longfellow, D. (2002) Pathology related to chronic arsenic exposure. Environ Health Perspect 110:883–886.
- Chen, C.J., Hsu, L.I., Wang, C.H., Shih, W.L., Hsu, Y.H. and Tseng, M.P. (2005) Biomarkers of exposure, effect, and susceptibility of arsenic-induced health hazards in Taiwan. Toxicol Appl Pharmacol 206:198–206.
- Copper, T.G., Yeung, C.H., Nashan, D., Jockenhovel, F. and Nieschlang, E. (1990) Improvement in the assessment of human epididymal function by the use of inhibitors in the assay of alpha-glucosidase in seminal plasma. Int J Androl 13:297–305.
- Cullen, M.R., Kayne, R.D. and Robins, J.M. (1984) Endocrine and reproductive dysfunction in men associated with occupational inorganic lead intoxication. Arch Environ Health 39(6):431–440.
- De Souza Predes, F., Diamante, M.A. and Dolder, H. (2010) Testis response to low doses of cadmium in Wistar rats. Inter J Experim Pathol 91:125–131.
- El-Ghobashy, A.A. and West, C.R. (2003) The human sperm head: a key for successful fertilization. J Androl 24: 232–238.
- Enserink, E.L., Maas-Diepeveen J.L. and Van Leeuwen C.J. (1991) Combined effects of metals; an ecotoxicological evaluation. Water Res 25(6):679–687.
- Freeland-Graves J.H., Bodzy, P.W. and Epright, M.A. (1980) Zinc status of vegetarians. J Am Diet Assoc 77:655–661.
- Frisbie, S.H., Ortega, R., Maynard, D.M. and Sarkar, B. (2002) The Concentrations of Arsenic and Other Toxic Elements in Bangladesh's Drinking Water. Environ Health Perspect 110:1147–1153.
- Gagnon, C. (1988) The role of environmental toxins in unexplained male infertility. Semin Reprod Endocrinol 6:369–376.
- Jeyandran, R.S., Vander Ven, H.H., Perez-Pelaez, M., Crabo, B.G. and Zaneveld, L.I.D. (1984) Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 70:219–228.
- Jeyandran, R.S., Vander Ven, H.H. and Zaneveld, L.I.D. (1992) The hypo-osmotic swelling test: an update. Arch Androl 29:105–116.
- Karvonen, M.J. and Malm, M. (1995) Colorimetric determination of fructose with indole. Scand J Clin Lab Invest 7:305–307.
- Kasperczyk, A., Kasperczyk, S., Dziwisz, M., Birkner, E., Walecko, C., Winiarska, H. (2002) Lead and cadmium concentration in human semen. Ginekol Pol 73(5):449–453.
- Krausz, C., Bonaccorsi, L., Luconi, M., Fuzzi, B., Criscuoli, L., Pellegrini, S. (1995) Intracellular calcium increase and acrosome reaction in response to progesterone in human spermatozoa are correlated with in-vitro fertilization. Hum Reprod 10:120–124.
- Krausz, C., Bonaccorsi, L., Maggio, P., Luconi, M., Criscuoli, L., Fuzzi, B. (1996) Two functional assays of sperm responsiveness to progesterone and their predictive values in in-vitro fertilization. Hum Reprod 11:1661–1667.
- Mazumder, D.N.G. (2008) Chronic arsenic toxicity and human health. Indian J Med Res 128:436–447.
- Mazumder, D.N., Das Gupta, J., Santra, A., Pal, A., Ghose, A. and Sarkar, S. (1998) Chronic arsenic toxicity in West Bengal—the worst calamity in the world. J Indian Med Assoc 96(1):4–7, 18.
- Menkveld, R., El-Garem, Y., Schill, W.B. and Henkel, R. (2003) Relationship between human sperm morphology and acrosomal function. J Assist Reprod Genet 20: 432–438.
- Momodu, M.A. and Anyakora, C.A. (2010) Heavy Metal Contamination of Ground Water: The Surulere Case Study. Research Journal Environmental and Earth Sciences 2(1):39–43.
- Monsefi, M., Alaee, S., Moradshahi, A. and Rohani, L. (2010) Cadmium-induced infertility in male mice. Environ Toxicol 25(1):94–102.
- Moriyama, H., Nakamura, K., Sanda, N., Fujiwara, E., Seko, S., Yamazaki, A. ,. (1987) Studies on the Usefulness of a Long-term, High-dose Treatment of Methylcobalamin in Patients with Oligozoospermia. Hinyokika Kiya 33(1):151–156.
- Palaniappan, P.L. and Karthikeyan, S. (2009) Bioaccumulation and depuration of chromium in the selected organs and whole body tissues of freshwater fish Cirrhinus Mrigala individually and in binary solutions with nickel. J Environ Sci 21:229–236.
- Pant, N., Murthy, R.C. and Srivastava, S.P. (2004) Male reproductive toxicity of sodium arsenite in mice. Hum Exp Toxicol 23:399–403.
- Paquin, R., Chapdelaine, P., Dube, J.Y. and Tremblay, R.R. (1984) Similar biochemical properties of human seminal plasma and epididymal alpha-1, 4-glucosidase. J Androl 5:277–282.
- Roychowdhury, A. and Gautam, A.K. (1995) Alteration of human sperm and other seminal constituents after lead exposure. Ind J Physiol Allied Sci 49:58–73.
- Sarkar, M., Biswas, N.M. and Ghosh, D. (1991) Effect of sodium arsenite on testicular 5-3,17-HSD activities in albino rats: Dose and duration dependent responses. Medical Science and Research 19:789–790.
- Sarkar, M., Chaudhuri, G.R., Chattopadhyay, A. and Biswas, N.M. (2003) Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl 5:27–31.
- Schill, W.B. and Henkel, R. (1999) Advancement in biochemical assays in andrology. Asian J Androl 1:45–51.
- Sheweita, S.A., Tilmisany, A.M. and Al-Sawaf, H. (2005) Mechanisms of male infertility: role of antioxidants. Curr Drug Metab 6(5):495–501
- Singh, N., Kumar, D. and Sahu, A.P. (2007) Arsenic in the environment: effects on human health and possible prevention. J Environ Biol 28(2 Suppl):359–365.
- Siu, E.R., Mruk, D.D., Porto, C.S. and Yan Cheng, C. (2009) Cadmium-induced testicular injury. Toxicol Appl Pharmacol 3:240–249.
- Sukla, J.P. and Pandey, K. (1984) Impaired spermatogenesis in arsenic-treated fresh water fish Colisa fasciatus (Bl & Sch). Toxicol Lett 21:191–195.
- Tapon, N. and Hall, A. (1997) Rho, Rac, and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol 9:86–92.
- Toman, R., Adamkovicova, M., Hluchy, S., Cabaj, M. and Golian, J. (2011) Quantitative analysis of the rat testes after an acute cadmium and diazinon administration. Scientific Papers: Animal Science and Biotechnologies 44:188–191.
- Vazquez, J.M., Martinez, E.A., Martinez, P., Garcia-Artiga, C. and Roca, J. (1997) Hypoosmotic swelling of boar spermatozoa compared to other methods for analysing the sperm membrane. Theriogenol 47:913–922.
- Wang, T.C., Jan, K.Y., Wang, A.S. and Gurr, J.R. (2007) Trivalent arsenicals induce lipid peroxidation, protein carbonylation, and oxidative DNA damage in human urothelial cells. Mutat Res 615: 75–86.
- World Health Organization. (1999) WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. Cambridge University Press, Cambridge, UK.
- World Health Organization. (2003) Guidelines for Drinking Water Quality, (3rd edition). World Health Organization, Geneva, Switzerland.
- Xia, Y. and Liu, J. (2004) An overview on chronic arsenism via drinking water in PR China. Toxicology 198:25–29.
- Xu, L.C., Wang, S.Y., Yang, X.F. and Wang, X.R. (2001) Effects of cadmium on rat sperm motility evaluated with computer assisted sperm analysis. Biomed Environ Sci 14:312–317.
- Yanagimachi, R. (1994) Mammalian fertilization. In The physiology of reproduction, Vol. 2. ed. Knobil, E., Neill, J.D. Raven Press, NewYork, 189–317.
- Yeung, B.H., Wan, H.T., Law, A.Y. and Wong, C.K. (2011) Endocrine disrupting chemicals, multiple effects on testicular signaling and spermatogenesis. Spermatogenesis 1:231–239.
- Zhang, C., Ling, B., Liu, J. and Wang, G. (2000) Toxic effect of fluoride-arsenic on the reproduction and development of rats. Wei Sheng Yan Jiu 29(3):138–140.