Abstract
Sexual dysfunction is one of the diabetic complications in males. The present study aimed to evaluate the antidiabetic effect of α-mangostin and its protective role in sexual dysfunction of streptozotocin (STZ) induced diabetic male rats. Male Wistar rats were divided as control, diabetic control, diabetic rats administered with 25, 50 mg/kg body weight (bw) of α-mangostin and 1 mg/kg bw of gliclazide. The α-mangostin was administered once daily for a period of 55 days. On day 55 animals were sacrificed, serum was analyzed for testosterone levels, and sperm was collected from the epididymis and sperm parameters analyzed. Testis and epididymis were examined for antioxidant enzymes like superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) levels, lipidperoxidation products, and histopathological alterations. In diabetic rats, sperm count, motile sperms, viable sperms, and hypo-osmotic swelling tail coiled sperms were significantly decreased while sperm malformations increased when compared with normal rats. Serum testosterone levels and testicular 3β and 17 β-hydroxysteroid dehydrogenase levels were significantly decreased in diabetic rats. Significant reduction in testicular and epididymal SOD, catalase, GPx levels, and elevation in lipid peroxidation products were observed. However, α-mangostin treatment showed noteworthy recovery in all parameters towards the control levels. It may therefore be suggested that α-mangostin showed a protective effect against sexual dysfunction in STZ induced diabetic rats.
Introduction
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycemia, resulting from insufficient production of insulin by the pancreas or ineffective utilization of insulin by the body [American Diabetes Association Citation2009]. Sexual dysfunction is a chronic complication of diabetes mellitus [Isidro Citation2012]. It has been estimated that about 35-75% of men with diabetes will experience at least a minimum degree of sexual impotence during their lifetime [Phé and Rouprêt Citation2012]. The possible cause of sexual dysfunction in diabetic men may be impairment of nerves, blood vessels, and muscle function. Besides this, oxidative stress induced by hyperglycemic effect also stands as a prominent cause. The surplus amounts of reactive oxygen species (ROS) affect sperm DNA, sperm function, testicular metabolite levels, and fusion events associated with fertilization, leading to infertility [Amaral et al. Citation2008; Bozhedomov et al. Citation2009]. Administration of current available drugs for the treatment of sexual dysfunction, in diabetic patients, may interact with diabetic medications and ameliorate the complications. At this juncture, the search continues for new potential leads which balance the pro-oxidants and antioxidants, so that oxidative stress is reduced. Furthermore, it is reported that intake of antioxidants protect spermatozoa from oxidative stress [Mohasseb et al. Citation2011]. However, several medicinal plants have been reported to be a rich source of antioxidants which can stabilize the testicular metabolite levels and show a protective effect against sexual dysfunction in diabetics [Shalaby and Hamowieh Citation2010; Suresh and Prakash Citation2012].
Mangosteen (Garcinia mangostana L, Guttiferae) is a tropical evergreen plant. It is extensively distributed in Malaysia, India, Thailand, Singapore, and Sri Lanka. Its fruit is known as the queen of fruits due to its delicious taste and pleasant smell. The mangosteen pericarp contains a high amount of xanthones, such as α, β, and γ mangostin and other bioactive substances including the tannins, flavonoids, proanthocyanidins, anthocyanins, and phenolic compounds [Chin and Kinghorn Citation2008]. It has been used as a traditional medicine for the treatment of diarrhea, inflammation, skin wounds, dysentery, leucorrhea, chronic ulcer, and gonorrhea [Pedraza-Chaverri et al. Citation2008]. For example, α-mangostin is a tetraoxygenated diprenylated xanthane that exhibits various bioactivities including antibacterial, antifungal, anti-inflammatory, anti-mycobacterial, antioxidant, antiplasmodial, and cytotoxic activities [Chin and Kinghorn Citation2008; Pedraza-Chaverri et al. Citation2008]. Recent reports have shown that α-mangostin is used in the treatment of myocardial infarction [Devi Sampath and Vijayaraghavan Citation2007] and neural oxidative damage [Márquez-Valadez et al. Citation2009]. Studies of Ryu et al. [2011] demonstrated that ethanolic extract of pericarp of Garcinia mangostana L, reduced the blood glucose levels in streptozotocin (STZ) induced diabetic rats. However, reports on the protective role of Garcinia mangostana L in sexual dysfunction of diabetic males are scant. Hence, the present work was carried out to evaluate the effect of α-mangostin, an isolated compound from Garcinia mangostana L, on sperm characteristics, serum testosterone levels, and oxidative damage in STZ induced diabetic rats.
Results
Phytochemical datass
The chemical structure, fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), and mass spectral data of the isolated α-mangostin compound is presented in and summarized in . Using this preparation, acute toxicity studies revealed the nontoxic nature of the α-mangostin to mice in doses of up to 2000 mg/kg bw until 48 hours post-administration. No toxic symptoms or mortality were reported at any of the doses selected till the end of the study period.
Figure 1. Bioassay guided column chromatography of the active fraction from pericarp of Garcinia mangostana L. A) Chemical structure of α-mangostin. B) Mass spectrum of α-mangostin.
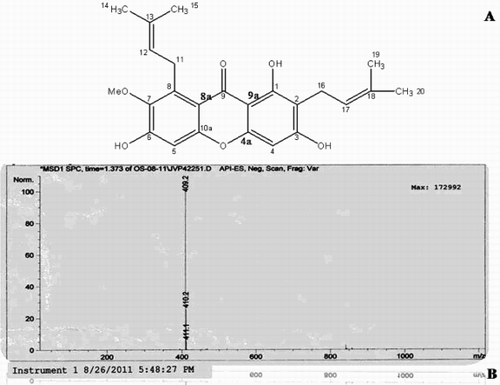
Table 1. The FTIR, NMR, and mass spectroscopic data of α-mangostin.
Changes in blood glucose levels and reproductive organ weights
summarizes the effect of α-mangostin on blood glucose levels, body weights, and tissue somatic index of STZ induced diabetic rats. On day 0, the mean blood glucose levels in control rats were 92.3 mg/dl. On day 55, the mean blood glucose levels were significantly (p < 0.01) higher in the diabetic control group when compared to that of the control rats. Whereas blood glucose levels of α-mangostin and gliclazide treated diabetic rats were significantly (p < 0.01) lower when compared to that of the diabetic control group. There was no significant difference between the groups in initial body weights. After the experimental period, the diabetic control group showed a significant (p < 0.01) decrease in body weight when compared to the control group. In addition, a significant (p < 0.01) increase in body weight was observed in 25 and 50 mg/kg bw of α-mangostin and gliclazide treated diabetic rats on the final day of the experiment when compared to that of the diabetic control group. The relative weights of testis, epididymis, seminal vesicle, and prostate were significantly (p < 0.01) reduced in the STZ induced diabetic control group. Whereas, significant (p < 0.01) improvement in their relative weights was observed in α-mangostin and gliclazide treated diabetic rats.
Table 2. Effect of α-mangostin on blood glucose levels, body weights, and tissue somatic index (W/W%) in STZ induced diabetic rats.
Sperm characteristics, seminiferous tubule diameter, epithelial layer thickness, and steroidogenic enzymes
The data on sperm characteristics (; ), showed a significant (p < 0.01) decrease in epididymal sperm count, progressive motility, sperm viability, and HOS tail coiled sperms, and increase in abnormal sperms in the diabetic control group as compared to that of the control group. In comparison, α-mangostin and gliclazide treated diabetic rats showed a significant (p < 0.01) alleviation in sperm count, motility, viability, and HOS tail coiled sperms with decreased abnormality of sperms when compared to that of the diabetic control group. Seminiferous tubule diameter and epithelial layer thickness were significantly (p < 0.01) decreased in the diabetic control group when compared to the control rats. In addition, α-mangostin and gliclazide treated diabetic rats showed a significant (p < 0.01) enlargement in diameter of seminiferous tubules and epithelial layer thickness when compared to that of the diabetic control group (). The diabetic control group showed significant (p < 0.01) decrease in testicular 3β-HSD and 17 β-HSD enzyme activity levels and serum testosterone levels when compared to the control group. Whereas, α-mangostin and gliclazide treated diabetic rats showed a significant (p < 0.01) increase in testicular 3β-HSD and 17β-HSD enzyme activity levels and serum testosterone levels when compared to the diabetic control group ().
Figure 2. Phase-contrast micrographs of spermatozoa in epididymis with morphological abnormalities including: A) normal sperm, B) banana shaped hook less, C) hook less and elongated head, D) pin head, E) macro cephalous, and F) thin elongated head. Stained with eosin-nigrosin, 40 × magnification.
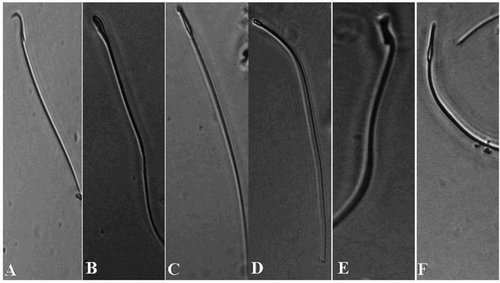
Table 3. Effect of α-mangostin on sperm parameters, diameter of seminiferous tubules, and epithelial layer thickness in STZ induced diabetic rats.
Table 4. Effect of α-mangostin on testicular 3 β- HSD and 17 β- HSD activity levels and serum testosterone in STZ induced diabetic rats.
Lipid peroxidation and antioxidant enzymes
The products of lipid peroxidation significantly (p < 0.01) increased in testis and epididymis of the diabetic control group when compared to that of the normal rats. However, α-mangostin and gliclazide treated diabetic rats showed a significant (p < 0.01) decrease in lipid peroxidation products in both testis and epididymis when compared to the diabetic control group. The antioxidant levels of enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidise (GPx) were significantly (p < 0.01) reduced in both testis and epididymis of the diabetic control group. Furthermore, α-mangostin and gliclazide treated diabetic rats showed significant (p < 0.01) increase in SOD, catalase, GPx levels in both testis and epididymis when compared to the diabetic control group ().
Table 5. Effect of α-mangostin on lipid peroxidation, superoxide dismutase, catalase, and glutathione peroxidase levels in testis and epididymis of STZ induced diabetic rats.
Histopathological findings
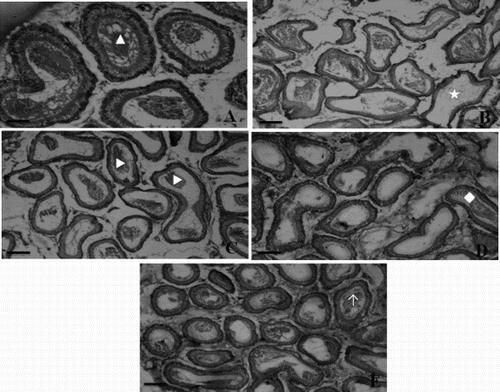
Transverse section of testis of the control rats showed well-organized seminiferous tubules with different stages of spermatogenic cells and normal interstitial connective tissue. Each seminiferous tubule contained an outer most basement membrane with spermatogonia and Sertoli cells resting on the membrane (A). However, the testicular sections of diabetic rats showed degenerative changes of the majority of the seminiferous tubules including irregular, thin basement membrane and incomplete spermatogenesis. Moreover, the seminiferous tubules were almost devoid of spermatozoa in their lumen of diabetic rats (B). Testis of α-mangostin and gliclazide treated diabetic rats showed marked improvement of spermatogenesis, evidenced by the presence of an improved number of spermatozoa in the seminiferous tubules compared to that of the diabetic rats (C, D, and E). Histopathological sections of the control rat epididymis showed the normal histological structure of regular epididymal tubules. Each lumen showed an increased number of sperms (A). The diabetic rat epididymis transverse section showed a lower number of sperms with absence of mature sperms (B). Remarkable enhancement in sperm density and epididymal structural integrity was observed in α-mangostin and gliclazide treated diabetic rats (C, D, and E).
Figure 3. Microphotographs of hematoxylin-eosin stained sections of rat testis (scale bar: 50µm). A) Testicular sections of control rat showing well organized seminiferous tubules (→) with normal spermatogenesis. B) Testicular sections of diabetic control rat showing severe degenerations in seminiferous tubules (*) with disorganized, irregular, and thin basement membrane. C) Testicular sections of 25 mg α-mangostin treated diabetic rat showing normal epithelium with a regular arrangement of germinal cells (⋄) in seminiferous tubule. D) Testicular sections of 50 mg α-mangostin treated diabetic rat showing the normal seminiferous epithelium with a remarkable increase in the number of spermatogenic cells (Δ). E) Testicular sections of gliclazide treated diabetic rat showing matured spermatozoa in seminiferous tubule (□).
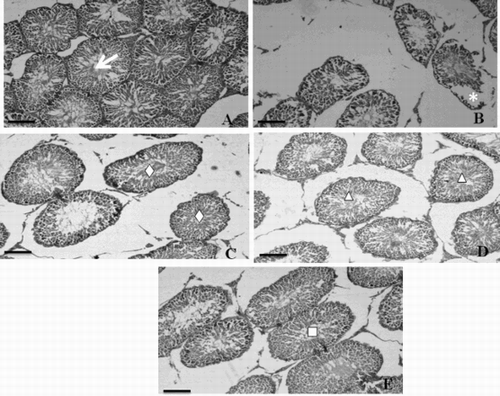
Figure 4. Representative photographs of the epididymis sections from the control, diabetic, and α-mangostin treated diabetic rats stained with hematoxylin and eosin (scale bar: 50µm). A) Epididymis of control rat showing normal epithelium and plenty of intact spermatozoa (▴) in lumen of epididymal tubule. B) Epididymis of diabetic rat showing damaged epithelial cells of tubules and loss of sperms (★). C) Epididymis of 25 mg/kg bw of α-mangostin treated diabetic rats showing improvement in sperm density (▶) and structural integrity. D) Epididymis of 50 mg/kg bw of α-mangostin treated diabetic rats showing nearly normal structure and sperm density. E) Epididymis of gliclazide treated diabetic rats showing normal structure of tubules filled with mature sperm (↑).
Discussion
Diabetes mellitus is a chronic metabolic disorder, affecting a large worldwide population. It is usually associated with serious sexual complications like erectile dysfunction, retrograde ejaculation, and changes in testosterone levels [Mallidis et al. Citation2011]. The present study addresses the antidiabetic effect of α-mangostin and its protective role in sexual dysfunction of STZ induced diabetic male rats as evidenced by a remarkable reduction of elevated blood glucose, restoration of testis weight and accessory sex organs, improvement in sperm quality and quantity, and reduction in testicular and epididymal lipid peroxidation products with increased levels of SOD, catalase, and GPx.
From the acute toxicity studies, it was found that α-mangostin was highly safe and non-toxic. In a previous study, mangosteen produced no toxic effects in rats after a single oral administration up to a dose of 5 g/kg body weight [Jujun et al. Citation2008]. Injection of STZ selectively destroyed the pancreatic β cells leading to diabetes mellitus [Szkudelski Citation2001]. Administration of α-mangostin resulted in the significant reduction of elevated blood glucose and this further strengthens its anti-hyperglycemic activity as reported by others [Ryu et al. Citation2011]. However, severe loss of body weight was observed in STZ induced diabetic rats probably due to excessive utilization of protein, indicating the marked reduction of carbohydrates available to the cells [Sathishsekar and Subramanian Citation2005]. Additionally, in our study the relative weights of the testis and epididymis, seminal vesicle, and prostate gland were also reduced significantly in STZ induced diabetic rats, as evidenced in earlier reports [Bal et al. Citation2011]. Moreover, a significant decline in the weights of the reproductive tissues may be due to low serum testosterone level, since testosterone plays a major role in regulating the normal growth and function of these organs [Klinefelter and Hess Citation1998]. Treatment of diabetic rats with α-mangostin, regained their body weights to some extent, overcoming the muscle wastage and also recovered the weights of reproductive organs towards control level.
Our observation on the sperm end points, such as epididymal sperm count, sperm motility, sperm viability, sperm morphology, and number of tail coiled sperms under hypo-osmotic swelling medium (HOS test) are consistent with those of previous reports on the reproductive disorders in diabetic males [Rabbani et al. Citation2010; Bal et al. Citation2011]. Reduction in epididymal sperm concentration, sperm motility, higher rate of sperm abnormalities, and tail coiled sperms may be due to the combined effect of decreased Leydig cell function and oxidative stress [La Vignera et al. Citation2012]. A decline in Leydig cell function can be evidenced by low testosterone levels in the serum/testis. In addition, damage from oxidative stress by free radicals deteriorates the sperm motility and viability. Our findings are further supported by the reports of a study which revealed that oxidative damage to sperm can lead to impaired membrane function and motility, diminished fertilization capacity, and DNA damage [Aitken and Roman Citation2008]. In the current study, it was found that administration of α-mangostin to diabetic male rats resulted in remarkable recoveries in the epididymal sperm count, sperm motility, sperm viability, sperm integrity, and sperm morphology. From these findings, it can be stated that α-mangostin treatment ameliorates testicular antioxidant capacity, thereby, deminishing diabetes induced oxidative damage.
It is well known that testosterone plays a critical role in sperm production and maturation. In the present study, the marked decrease in the level of serum testosterone may be associated with the reduction in activities of androgenic key enzymes, 3β-hydroxysteroid dehydrogenase (3β HSD), and 17β-hydroxysteroid dehydrogenase (17β HSD), which take part in biosynthesis of testosterone [Haider Citation2007]. The results are consistent with earlier reports by Mallick et al. [2007] who demonstrated that 3β and 17 β HSD and serum testosterone levels were decreased in diabetic rats. The reduced levels of testosterone may also be due to diminution of the level of serum insulin in STZ induced diabetic rats as evidenced by the studies of Hurtado de Catalfo et al. [1998]. Moreover, α-mangostin contributed to the normalization of 3β HSD, 17β HSD, and serum testosterone levels.
Sperm production, maturation, and morphology are very sensitive to pro-oxidant/antioxidant balance. From the demonstration of Shrilata and Muralidhara [2007] it was found that spermatozoa were susceptible to oxidative stress in diabetic rats. In the current study, deterioration of SOD, catalase, GPx enzyme activities with an elevation of lipid peroxidation products was a striking finding for diabetic rats. Increased levels of ROS in testis, may contribute to this finding. However, we observed a significant elevation of SOD, catalase, and GPx enzyme activities with a decline in lipid peroxidation products in diabetic rats treated with α-mangostin. This suggests the protective role of α-mangostin as an antioxidant, which prevents the oxidation of tissue poly unsaturated fatty acids (PUFAs) by diminishing the susceptibility of these tissue PUFAs to lipid peroxidation. The present findings are in agreement with reports of Devi Sampath and Vijayaraghavan [2007] on α-mangostin treated isoproterenol induced myocardial necrotic rats.
Consistent with the results of sperm parameters, alterations in testicular and epididymal architecture were observed in diabetic males, including loss of compactness, disorganized epithelium, lumen devoid of sperms, in addition to ruptured seminiferous tubules. The histopathological alterations in testis are in consonance with the decline in Leydig cell number and impairment of Leydig cell function. This may be associated with a lack of insulin, which down regulates the insulin mediated stimulation of androgen biosynthesis and cell proliferation in the testis of diabetic rats. Furthermore, α-mangostin administration to diabetic rats ameliorated the histopathological changes towards control level.
In conclusion, our study reveals that α-mangostin plays a pivotal role in preventing sexual dysfunction induced by diabetes in rats by its direct effect on reproductive organs and their functions, and by improving antioxidant levels. Further endocrine and molecular mechanisms which attribute to the protective role of α-mangostin in diabetes are to be explored.
Material and Methods
Chemicals and reagents
Streptozotocin, NADPH, NAD, dehydroepiandrosterone, and androstenedione were purchased from Sigma Chemicals (St. Louis, MO, USA). Thiobarbituric acid, epinephrine, and reduced glutathione were obtained from Merck (Darmstadt, Germany). All other chemicals used in the present study were of analytical grade and of the highest purity.
Isolation of α-mangostin from the pericarp of Garcinia mangostana
The fruit of mangosteen was purchased in August 2011 from the Chennai local market, India. The fruit was confirmed by Dr. K. Madhava Chetty, Botanist, Sri Venkateswara University, Tirupati, India (Voucher no. GML/SVU/005/2011). The dried Garcinia mangostana L, pericarp (2.79 kg) was macerated in hexane for 24 h to remove non-polar substances, resulting marc was macerated in benzene (4 L) at room temperature for 3 d. After filtration, the solvent was concentrated on a rotary evaporator (Rotavapor® R-210) at 40-50°C under reduced pressure. Then crude organic extract obtained (180.15 g), was subjected to column chromatography over a silica gel (Merck 100-200 mesh), and eluted with hexane-ethyl acetate (4:1) to give 5 primary fractions based on the thin layer chromatography profile. The yellow solid fraction (35.6 g) of α-mangostin was collected and further identified by comparison with spectral data [Ji et al. Citation2007] and melting point recorded on a melting point apparatus (SUNBIM) in open capillary tubes. Fourier transform infrared spectroscopy spectra of the isolated α- mangostin were recorded in an FTIR spectrometer (Bruker, Model- Alpha). The molecular weight of isolated α-mangostin was determined by Agilent 1100 Series LC/MSD. Structural identification of isolated α-mangostin was performed by 1H NMR (400 MHz) spectra on a Varian Gemini 400 MHz NMR spectrometer (Laila Impex R and D Centre, Vijayawada, India).
Animals and diet
Male albino Wistar rats (170-200 g) and Swiss albino mice (18-20 g) were obtained from Sri Venkateswara traders, Bangalore, India and were maintained under a constant 12 h light and dark cycle at 21-23°C. The animals were fed with commercial pellet diet (Godjet Agrovet Ltd, Mumbai, India) and tap water was provided ad libitum throughout the experimental period. The experiments were carried out in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India. This study was approved by the Institutional Animal Ethics Committee (Regd. No. 516/01/A/CPCSEA).
Acute toxicity study
Healthy adult male mice were starved overnight and divided into four groups (n = 6). The α- mangostin was dissolved in 40% ethanol and the mice were fed with α-mangostin orally in graded doses of 100, 500, 1,000, and 2,000 mg/kg . The animals were observed continuously for 2 h to know their behavioral, neurological, and autonomic profiles. The number of mice that died in each group after 48 h was recorded. Acute toxicity study was performed as per OECD [1998] guidelines (guideline 425).
Induction of diabetes mellitus
After overnight fasting, diabetes was induced by a single intraperitoneal injection of STZ dissolved in ice cold citrate buffer (0.1M, pH 4.5) at a dose of 55 mg/kg [Gireesh et al. Citation2009]. Control rats received vehicle only. The rats were allowed to drink 5% sucrose solution overnight after injection, to overcome the drug-induced hypoglycemia. After one w of STZ injection, animals with above 250 mg/dl of fasting blood glucose were considered as diabetic rats and used for the experiment.
Experimental design
The rats were divided into five groups; each group contains 6 animals as follows: Group 1: Normal control rats; Group 2: Diabetic control rats; Group 3: Diabetic rats treated with 25 mg/kg of α-mangostin; Group 4: Diabetic rats treated with 50 mg/kg of α-mangostin; and Group 5: Diabetic rats treated with 1 mg/kg of gliclazide.
The α-mangostin was dissolved in a few drops of 40% ethanol and dilutions were made with distilled water as per requirement of the dosage gliclazide was dissolved in 0.1N NaOH in warm water and administered orally using intragastric tube for 55 d as the rats need a period of 48-52 days for the completion of the spermatogenic cycle which includes spermatocytogenesis, meiosis, and spermiogenesis [Bennett and Vickery Citation1970]. The dosage of α-mangostin was selected on the basis of acute toxicity studies in normal mice.
Determination of blood glucose levels
Blood was collected from the retro-orbital plexus and serum was separated by centrifugation at 2000 x g for 15 min and stored at -20°C for biochemical analysis and hormonal analysis. The fasting serum glucose levels were determined on day 0 and day 55 using a semi-auto analyzer (Screen master-3000) by glucose oxidase (GOD) and peroxidase (POD) endpoint assay method using commercially available kits (Span diagnostics LTD, Surat, India).
Tissue somatic index
Initial and final body weights of rats were recorded and subsequently weight changes were calculated. After the experimental period, the rats were deprived of food overnight and then sacrificed by decapitation. Testis, epididymis, prostate gland, vas deferens, and seminal vesicle were dissected out, and attached tissue was removed and weighed on an electronic balance (Shimadzu, model number BL-220H). The tissue somatic index was determined by using the following formula: weight of the tissue in grams/weights of the body in grams x 100.
Histopathology
A small portion of testis and epididymis were kept overnight in 10% neutral formalin solution, dehydrated with an ascending alcohol series. These specimens were then cleaned with xylene, embedded in paraffin, sectioned at 4-6 micron thickness, stained with hematoxylin and eosin (H & E), and then examined under phase-contrast microscope (Nikon phase contrast 0.9 dry, Japan, NIS- Elements D). The seminiferous tubule diameters and epithelial layer thickness were randomly examined per section and measured using a computer-assisted image analyzer system consisting of a phase-contrast microscope (Nikaon H600L, Nikon DS camera control Unit DS-U2, Version 4.4).
Sperm analysis
The cauda epididymis was examined for sperm analysis. The epididymis was finely minced in 5.0 ml of isotonic saline in a petri dish. The sperms were counted by using Neubauer Chamber (Deep 1/10 mm, LABART, Darmstadt, Germany) as described by Belsey et al. [1980]. The percentage of progressively motile sperm was evaluated microscopically within 5 min following their isolation from the cauda epididymis at 37°C [Belsey et al. Citation1980]. The ratio of live to dead sperms was determined using 1% trypan blue as described in the method of Talbot and Chacon [1981]. The HOS test was performed by exposing 0.1 ml of sperm sample to 1.0 ml hypo-osmotic solution and observed for coiled tails under the phase contrast microscope, and the coiling was estimated, with the help of the method described by Jeyendran et al. [1992]. To determine morphologically abnormal sperms, the sperm samples were stained with eosin-nigrosin (1.67% eosin, 10% nigrosin and 0.1 M sodium citrate) and head, tail, middle piece abnormalities were counted [Bjorndahl et al. Citation2004]. A total of 300 sperms per rat (1,800 sperms in each group) were viewed under a microscope at 400X magnification. The data is expressed as millions/ml for sperm count and for other sperm parameters the data is expressed as percentage of total sperm.
Preparation of sub-cellular fractions
Sub-cellular fractions were prepared from the testis and epididymis by differential centrifugation [Latchoumycandane and Mathur Citation2002]. Briefly, a 10% homogenate was prepared with glass-teflon homogenizer (Remi RQ-127A, Remi Motors, Mumbai, India) in ice-cold Tris buffer (2 mM, pH 7.4, contains 0.25 M sucrose) followed by centrifugation at 600 x g for 10 min at 4°C. The mitochondrial pellet was obtained by centrifugation of the post-nuclear supernatant at 10,000 x g for 15 min at 4°C to obtain the nuclear pellet. The microsomal fractions were prepared by using the calcium chloride (CaCl2) sedimentation method of Walawalkar et al. [2006].
Assay of testicular steroidogenic enzymes
The 3β-hydroxysteroid dehydrogenase (3β-HSD) (EC 1.1.1.51) and 17β-hydroxysteroid dehydrogenase (17β-HSD) (EC 1.1.1.61) were estimated in the testicular microsomal fractions according to the method demonstrated by Bergmayer [1974]. The enzyme assays were performed under the conditions following zero order kinetics after preliminary standardization regarding linearity with respect to time of incubation and enzyme concentration. The enzyme activity was expressed as µmol of NAD converted to NADH/mg protein/min (3β-HSD) or µmol of NADPH converted to NADP/mg protein/min (17β-HSD).
Determination of serum testosterone levels
The serum testosterone levels were determined by an ELISA method using a DRG testosterone ELISA kit (ELISA EIA-1559, 96 wells kit, DRG instrument, GmbH, Marburg, Germany). The assay was performed according to the user manual. The sensitivity, intra- and inter assay variation coefficients of kit were 0.083 ng/ml, 3.34-4.16 % and 4.73- 9.94 % respectively.
Determination of lipid peroxidation and antioxidant enzymes in the testis and epididymis
Lipid peroxidation (LPx) was estimated in terms of malondialdehyde (MDA) content and determined by using the thiobarbituric acid (TBA) by the method of Hiroshi et al. [1979]. The activity was expressed as µmol of malondialdehyde formed/g wet weight of tissue. Superoxide dismutase (SOD) (EC 1.15.1.1) was assayed for its ability to inhibit the auto-oxidation of epinephrine in alkaline medium [Misra and Fridovich Citation1972]. The SOD activity levels were expressed in units per mg protein per min. Catalase (EC 1.11.1.6) was assayed by the method of Maehly and Chance [1954] by determining the decrease in the concentration of hydrogen peroxide (H2O2). The activity of the enzyme was expressed in µmol of hydrogen peroxide (H2O2) metabolized/mg protein/min. Glutathione peroxidase was assayed by the method of Rotruck et al. [1973]. The activity was expressed as μMol of GSH consumed/mg protein/min. The protein concentration was determined by using bovine serum albumin as the standard [Lowry et al. Citation1951].
Statistical analysis
The experimental data is expressed as mean ± S.D. The statistical significance was evaluated by one-way analysis of variance (ANOVA) using SPSS version 13.5 (SPSS Inc, Chicago, IL, USA) and the individual comparisons were obtained by Duncan's multiple range test. Differences were considered to be significant at p < 0.01.
Abbreviations
| 17β-HSD: | = | 17β-hydroxysteroid dehydrogenase |
| 3β-HSD: | = | 3β-hydroxysteroid dehydrogenase |
| ANOVA: | = | analysis of variance |
| bw: | = | body weight |
| DNA: | = | deoxyribo nucleic acid |
| ELISA: | = | enzyme linked immunosorbent assay |
| FTIR: | = | fourier transform infrared spectroscopy |
| GOD/ POD: | = | glucose oxidase and peroxidise |
| GPx: | = | glutathione peroxidise |
| H & E: | = | hematoxylin and eosin |
| H2O2: | = | hydrogen peroxide |
| HOS: | = | hypo-osmotic swelling |
| LPx: | = | lipid peroxidation |
| MDA: | = | malondialdehyde |
| NAD: | = | nicotinamide adenine dinucleotide |
| NADP: | = | nicotinamide adenine dinucleotide phosphate |
| NADPH: | = | nicotinamide aenine dinucleotide phosphate hydrogen |
| NMR: | = | nuclear magnetic resonance |
| OECD: | = | Organisation for Economic Co-operation and Development |
| PUFA: | = | poly unsaturated fatty acid |
| ROS: | = | reactive oxygen species |
| SOD: | = | superoxide dismutase |
| SPSS: | = | Statistical Package for Social Sciences |
| STZ: | = | streptozotocin |
| TBA: | = | thiobarbituric acid |
Acknowledgements
The authors are grateful to the Principal, A.U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam and management and staff of the Sankar Foundation Research Institute for providing necessary facilities to carry out the work.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The work was financed by the Sankar Foundation Research Institute, Visakhapatnam, India.
Author contributions: Designed the experiments: GBN, ASK, EKK; Performed the experiments and analyzed the data: GBN; Wrote the manuscript: GBN, EKK.
References
- Aitken, R.J. and Roman, S.D. (2008) Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol 636:154–171.
- Amaral, S., Oliveira, P.J. and Ramalho-Santos, J. (2008) Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 4:46–54.
- American Diabetes Association (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care 32:S62–S67.
- Bal, R., Türk, G., Tuzcu, M., Yilmaz, O., Ozercan, I., Kuloglu, T., (2011) Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in Aitken, R.J. and Roman, S.D. (2008) Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol 636:154–171.
- Belsey, M.A., Eliasson, R., Gallegos, A.J., Moghissi, K.S., Paulson, C.A. and Prasad, M.R.N. (1980) World Health Organization: Laboratory Manual for the Examination of Human Semen and Semen‐Cervical Mucus Interaction. Press Concern, MNR Prasad, Singapore.
- Bennett, J.P. and Vickery, B.H. (1970) Rats and mice. In Reproduction and breeding techniques for laboratory animals ed. E.S.E. Hafez, Lea and Febiger, Philadelphia, 299–315.
- Bergmayer, H.U. (1974) b-hydroxysteroid dehydrogenase. In Methods of Enzymatic Analysis, ed. H.U. Bergmayer, Academic Press Ltd., New York, 447–489.
- Bjorndahl, L., Soderlund, I., Johansson, S., Mohammad, M., Pourian, M. and Kvist, U. (2004) Why the WHO recommendations for eosin-nigrosin staining techniques for human sperm viability assessment must change. J Androl 25:671–677.
- Bozhedomov, V.A., Gromenko, D.S., Ushakova, I.V., Toroptseva, M.V., Galimov, S.H.N., Alekcandrova, L.A., (2009) Oxidative stress of spermatozoa in pathogenesis of male infertility. Urologiia 2:51–56.
- Chin, Y.W. and Kinghorn, A.D.. (2008) Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev Org Chem 5:355–364.
- Devi Sampath, P. and Vijayaraghavan, K. (2007) Cardioprotective effect of alpha-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol 21:336–339.
- Gireesh, G., Kumar, T.P., Mathew, J. and Paulose, C. (2009) Enhanced muscarinc M1 receptor gene expression in the corpus striatum of streptozotocin-induced diabetic rat. J Biomed Sci 16:38.
- Haider, S.G. (2007) Leydig cell steroidogenesis: unmasking the functional importance of mitochondria. Endocrinology 148:2581–2582.
- Hiroshi, O., Habuko, D. and Yagi, K. (1979) Assay for lipid peroxidation in animal tissues by thiobarbituri acid reaction. Anal Biochem 95:351–358.
- Hurtado de Catalfo, G., Nelva, I. and De Gómez Dumm, T. (1998) Lipid dismetabolism in Leydig and Sertoli cells isolated from streptozotocin-diabetic rats. Int J Biochem Cell Biol 30:1001–1010.
- Isidro, M.L. (2012) Sexual dysfunction in men with type 2 diabetes. Postgrad Med J 88:152–159.
- Jeyendran, R.S., Van Der Ven, H.H. and Zaneveld, L.J.D. (1992) The hypoosmotic swelling test: An update. Arch Androl 29:105–116.
- Ji, X., Avula, B. and Khan, I.A. (2007) Quantitative and qualitative determination of six xanthones in Garcinia mangostana L. by LC-PDA and LC-ESIMS. J Pharm Biomed Anal 43:1270–1276.
- Jujun, P., Pootakham, K., Pongpaibul, Y., Duangrat, C. and Tharavichitkul, P. (2008) Acute and repeated dose 28-day oral toxicity study of Garcinia mangostana Linn. rind extract. CMU J Nat Sci 7:199–208.
- Klinefelter, G.R. and Hess, R.A. (1998) Toxicology of the male excurrent ducts and accessory sex glands in the male. In Reproductive and Development Toxicology, ed. K.S. Korach, Basel Ltd., New York, 553–591.
- La Vignera, S., Condorelli, R., Vicari, E., D'Agata, R. and Calogero, A.E. (2012) Diabetes mellitus and sperm parameters. J Androl 33:145–153.
- Latchoumycandane, C. and Mathur, P.P. (2002) Effect of methoxychlor on the antioxidant system in mitochondrial and microsome-rich fractions of rat testis. Toxicology 176:67–75.
- Lowry, H., Rosebrough, N.I., Far, A.L. and Ranall, R.J. (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275.
- Maehly, A.C. and Chance, B. (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424.
- Mallick, C., Mandal, S., Barik, B., Bhattacharya, A. and Ghosh, D. (2007) Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol Pharm Bull 30:84–90.
- Mallidis, C., Agbaje, I., McClure, N. and Kliesch, S. (2011) The influence of diabetes mellitus on male reproductive function: a poorly investigated aspect of male infertility. Urologe A, 50:33–37.
- Márquez-Valadez, B., Lugo-Huitrón, R., Valdivia-Cerda, V., Miranda-Ramírez, L.R., Pérez-De La Cruz, V., González-Cuahutencos, O., (2009) The natural xanthone alpha-mangostin reduces oxidative damage in rat brain tissue. Nutr Neurosci 12:35–42.
- Misra, H.P. and Fridovich, I. (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175.
- Mohasseb, M., Ebied, S., Yehia, M.A. and Hussein, N. (2011) Testicular oxidative damage and role of combined antioxidant supplement action in experimental diabetic rats. J. Physiol. Biochem. 67:185–194.
- OECD (Organisation for Economic Co-operation and Development) (1998) OECD Guidelines for the Testing of Chemicals. Guideline 425 Acute Oral Toxicity-Up-and-Down Procedure. Paris: OECD.
- Pedraza-Chaverri, J., Cárdenas-Rodríguez, N., Orozco-Ibarra, M. and Pérez-Rojas, J.M. (2008) Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol 46:3227–3239.
- Phé, V. and Rouprêt, M. (2012) Erectile dysfunction and diabetes: a review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab 38:1–13.
- Rabbani, S.I., Devi, K. and Khanam, S. (2010) Pioglitazone, a PPAR-gamma ligand inhibited the nicotinamide-streptozotocin induced sperm abnormalities in type-2 diabetic Wistar rats. Pak J Pharm Sci 23:326–331.
- Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.C. and Hoekstra, W.G. (1973) Selenium: biochemical roles as a component of glutathione peroxidase. Science 179:588–590.
- Ryu, H.W., Cho, J.K., Curtis-Long, M.J., Yuk, H.J., Kim, Y.S., Jung, S., (2011) α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry 72:2148–2154.
- Sathishsekar, D. and Subramanian, S. (2005) Beneficial effects of Momordica charantia seeds in the treatment of STZ-induced diabetes in experimental rats. Biol Pharm Bull 28: 978–983.
- Shalaby, M.A. and Hamowieh, A.R. (2010) Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol 48:2920–2924.
- Shrilatha, B. and Muralidhara. (2007) Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol 23:578–587.
- Suresh, S. and Prakash, S. (2012) Effect of Mucuna pruriens (Linn.) on sexual behavior and sperm parameters in streptozotocin-induced diabetic male rat. J Sex Med 9: 3066–3078.
- Szkudelski, T. (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537–546.
- Talbot, P. and Chacon, R.S. (1981) A triple stain technique for evaluating normal acrosome reaction of human sperm. J Exp Zool 215:201–208.
- Walawalkar, P.S., Serai, P.S. and Iyer, K.R. (2006) Isolation and catalytic competence of different animal liver microsomal fractions prepared by calcium-aggregation method. Indian J Pharm Sci 68:262–265.