Abstract
This study was carried out to evaluate the protective effects of royal jelly (RJ) on taxol (TXL)-induced damage of the testis. Wistar rats were divided into control and test groups. The test group was divided into five subgroups; the first four groups along with TXL administration (7.5 mg/kg body weight (bw), weekly), received various doses of RJ (0, 50, 100, and 150 mg/kg bw). The last group received only RJ at 100 mg/kg. Royal jelly lowered the TXL-induced malondialdehyde and nitric oxide levels and enhanced the total thiol molecules in the testis. Remarkably RJ reduced the TXL-induced pathological injuries such as cellular shrinkage and seminiferous tubule depletion. Taxol-reduced sperm viability (27.5 ± 2.98 % vs. 85.0 ± 8.6% in the control group) was recovered by RJ administration as 80.5 ± 10.6% of the sperm were found alive in the group of animals which received 150 mg/kg RJ. The TXL-exposed and TXL plus RJ-administered animals showed a significant up-regulation of transcription factor E2f1 mRNA. Our data suggest that the TXL-induced histopathological and biochemical alterations could be protected by the administration of RJ. The RJ protective effects might be attributed to its antioxidant capacity and its capability in the regulation of E2f1 expression.
Introduction
Undoubtedly using chemotherapy agents during the last decades has resulted in a significant increase of cancer survivors [Thomson et al. Citation2002]. Chemotherapy agents either stop or slow the growth and proliferation of cancer cells. However, chemotherapy treatment can have severe side effects which may negatively affect the quality of life. Among the other options to lessen the severity of chemotherapy-induced side effects is the simultaneous administration of protective remedies.
Taxol (TXL) is an effective chemotherapeutic agent and mitotic inhibitor used against a wide range of solid tumors such as ovarian, breast, lung, and prostate cancers. It promotes the polymerization of tubulin and interposes with microtubule depolymerization, which is essential in cell division. Like other chemotherapy agents, TXL also has side effects such as neuropathy [Charity et al. Citation2006], endothelial dysfunction [Serizawa et al. Citation2012], cardiotoxicity, hypersensitive reactions, and gastrointestinal dysfunction which diminish its effectiveness [Zhang et al. 2010]. There are reports indicating the pathological changes in the gonads along with reduced functional activity of spermatozoa from rats that are exposed to a single injection of taxol [Borovskaya et al. Citation2009].
Royal jelly (RJ), a hypopharyngeal gland secretion of nurse bees, is an exclusive food for the queen honey bee (apis millifera) larva. Royal jelly consists of water (50% to 60%), proteins (18%), carbohydrates (15%), lipids (3% to 6%), mineral salts (1.5%), and vitamins. Additionally, RJ contains many bioactive substances such as 10-hydroxyl-2-decenoic acid that has immunomodulating properties, antibacterial proteins, fatty acids, and peptides. Royal jelly has several other pharmacological properties and activities including: antioxidant, antimicrobial, an insulin-like effect, antitumor, vasodilatotary, antihypercholesterolemic, antihypertensive, antiallergic, antifatigue, wound-healing, and a protective effect against hematopoietic dysfunction [Matsui et al. Citation2002; Kamakura et al. Citation2001].
The existing chemotherapeutic drugs cause changes in the expression of proliferation, survival, and apoptotic genes, which are expressed in normal as well as malignant cells. Among others, E2f1, a transcription factor, is capable of promoting cell cycle progression, but dysregulated E2f1 can trigger apoptosis [Wu and Levine Citation1994; Qin et al. Citation1994; De Gregori et al. Citation1997]. Regulation of E2f1 activity and stability during the cell cycle has already been reported. There are many reports of E2f1 stabilization following various DNA damaging agents and chemotherapeutic drugs such as actinomycin D and adriamycin. Due to the relatively high susceptibility of male germ cells to chemotherapy agents, we aimed to investigate the protective effects and mechanisms of RJ on the TXL-induced damage of the male reproductive system including the structural alterations in the testis and sperm quality. Early studies indicate that TXL is a unique antimicrotubule agent that disrupts the normal microtubular network of the cell and thereby forms stable microtubules, which block the cell cycle in the G2/M-phase [Rowinsky et al. Citation1988]. However, TXL-induced apoptosis may occur without a prior G2/M arrest [Dziadyk et al. Citation2004]. Overexpression of E2F1 may promote proliferation, but at the same time it may also enhance apoptosis. Therefore, expression of E2f1 testes mRNA was examined as the possible molecular mechanism of TXL-induced damage and RJ protective effect.
Results
Royal jelly reduced TXL associated body weight gain (bwg)
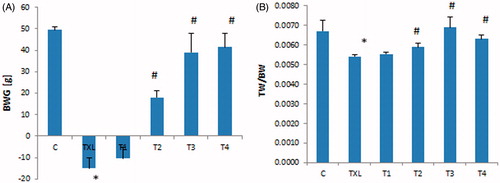
The bwg in the animals that received TXL for four weeks was reduced significantly (p < 0.01) in comparison to the control group, while those animals that were treated with RJ at 100 and 150 mg/kg dose levels showed remarkable reduction in the associated TXL bwg (). Testis to body weight ratio was significantly lower in the TXL-treated rats while RJ administration resulted in returning the ratio to the level of the control group ().
Figure 1. Effect of royal jelly on taxol (TXL)-induced changes in body weight gain (BWG) (A) and testis to body weight ratio (TW/BW) (B). Data is given as mean ± SD (n = 8). *: indicates a significant difference between the control and TXL-received groups; #: represents significant differences between the TXL-received non-treated and treated with various dose levels of RJ.
Royal jelly exerted antioxidative and anti-nitrosative effect
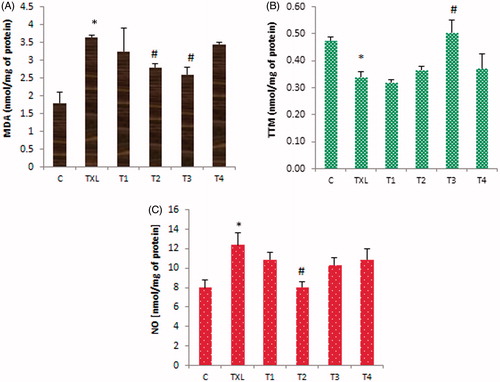
To evaluate the effect of RJ administration on the TXL-reduced antioxidant capacity, the rate of lipid peroxidation and total thiol molecules (TTM) were measured. Results showed that the malondialdehyde (MDA) content of testis was significantly (p < 0.05) elevated in the TXL-treated (i.p.) group (3.6 ± 0.5 vs. the control group1.8 ± 0.3 nmol/mg of protein), while RJ at 100 and 150 mg/kg dose levels could significantly lower the rate of lipid peroxidation (2.6 ± 0.2 nmol/mg of protein in T3 group). Administration of RJ alone also increased the level of MDA compared to the control group (). The concentration of TTM was significantly (p < 0.05) reduced in the TXL-treated animals, while RJ at 150 mg/kg prevented thiol depletion (). The nitric oxide (NO) content of testis in the TXL-treated animals was remarkably elevated (12.4 ± 1.2 vs. 8.0 ± 0.6 nmol/mg of protein in the control group). Although all three doses of RJ could reduce the TXL-induced NO, only RJ at 100 mg/kg resulted in a significant reduction of NO content in the testis ().
Figure 2. Effect of royal jelly (RJ) on taxol (TXL)-induced malondialdehyde (MDA) level (A), total thiol molecules (TTM) content (B), and nitric oxide (NO) concentration (C) in the testis. Data is given as mean ± SD (n = 8). *: indicates a significant difference between the control and TXL-received groups; #: represents significant differences between the TXL-received non-treated and treated with various dose levels of RJ.
RJ TXL associated histopathology and histomorphometry
As summarized in , histomorphometrical analyses demonstrated that in the TXL-treated animals, tubular differentiation index (TDI), repopulation index (RI), and seminiferous tubule diameter were significantly reduced. At a dose of 50 mg/kg RJ there was a significant (p < 0.05) increase in TDI and RI indices, however the slight elevation of the diameter of the seminiferous tubules was not significant (p > 0.05). Statistical analyses indicated that there are remarkable differences between the highest dose level (150 mg/kg) and the lower doses of RJ on the morphometric indices of TDI and RI. Those animals that only received RJ, showed no significant differences in indices when compared with the control group.
Table 1. Effect of royal jelly (RJ) on taxol (TXL)-induced alterations in tubular differentiation index (TDI), repopulation index (RI), and seminiferous tubules diameter.
Taxol-induced damage manifested as the reduction of germinal epithelium thickness (61 ± 16 µm vs. 106 ± 11 µm for control group), abnormalities in spermatogenesis, arrest in spermiogenesis, abundant presence of cells with typical apoptotic morphology including cell shrinkage, cytoplasmic condensation, eosinophilic cytoplasm, pyknotic nuclei, chromatin condensation around the nuclear membrane, presence of symplasts, and depletion area between Sertoli cells (-TXL). Those animals that along with TXL were treated with 50 mg/kg RJ for 28 days, did not show any remarkable improvement in the TXL-associated damage (-T1), while RJ administration at 100 and in particular 150 mg/kg dose levels resulted in a profound improvement, which presented as a reduction of apoptotic cells, limitation of the TXL-induced damage to only the first or second layer of germinal cells, and normalizing germinal epithelium thickness (-T2 and T3). The histological structure of testis in the animals which only received RJ resembled the control animals and no pathology was observed (-C and T4; ).
Figure 3. Effect of royal jelly on taxol-induced histopathological changes in the seminiferous tubules. C: control;TXL: taxol-received animals; T1, T2, T3: the TXL-treated animals which received various dose levels of RJ; T4: received RJ only; spermatozoids (black arrow), cytoplasmic residues (black arrow head), spermatids (white arrow), spermatocyte I (white arrow head), spermatogonium (blue arrow), Sertoli cell (blue arrow head), tunica albuginea (yellow arrow), pyknotic spermatocyte I (yellow arrow head), cell shrinkage (green arrow), ring from condensation of chromatin around the nuclear periphery of spermatids (green arrow head), multinucleated giant cells (red arrow), and depletion areas (red arrow head). Hematoxylin and eosin staining; original magnification -100 X and scale bars = 100 µm, and high magnification 200 X and scale bars = 25 µm.
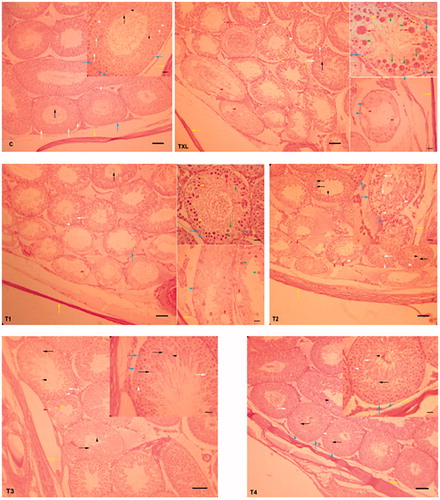
Table 2. Effect of royal jelly (RJ) on histological changes in the testis of taxol (TXL)-exposed rats.
Effect of royal jelly on sperm quality, TXL-induced DNA damage, and E2f1 expression in the testis
The sperm parameters are summarized in . The TXL-treated animals showed a significant (p < 0.05) decrease in sperm count (46.3 ± 3.8 × 106 versus 105.2 ± 9.2 × 106 in the control group), viability, and motility with an increased level of sperm DNA damage when compared to the control rats. The administration of RJ resulted in not only improvement of the sperm quality parameters but also lowered the TXL-induced DNA damage in a dose-dependent fashion. Animals that received only RJ (100 mg/kg bw) for 28 days, showed a slight increase in total sperm count, however, other sperm quality evaluating parameters including sperm viability and motility showed no significant difference (p > 0.05).
Table 3. Effect of royal jelly on the taxol (TXL)-induced negative impact on sperm count, viability, motility, and DNA double strands integrity.
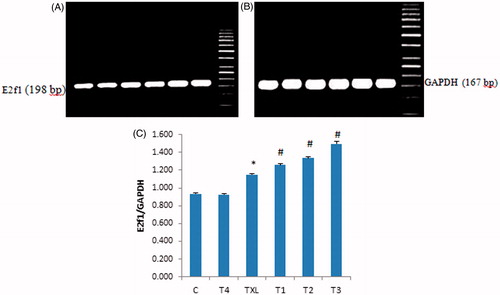
The mRNA level of testes expressed E2f1 was determined using a semi-quantitative PCR and the results were normalized against the mRNA level of GAPDH, a house-keeping gene. The TXL-treated animals showed a significant (p < 0.05) up-regulation of E2f1 mRNA. The administration of RJ enhanced the expression of E2f1 mRNA in a dose-dependent manner (). The level of testes E2f1 mRNA measured by densitometry and normalized to GAPDH mRNA expression as a function of integrated density (IDV) of E2f1 mRNA is summarized in .
Figure 4. Effect of taxol (TXL) and royal jelly on E2f1 (198 bp) (A) and GAPDH (167 bp) (B) mRNA levels in the testicular tissue. The levels of E2f1 and GAPDH mRNA were evaluated by semi-quantitative RT-PCR. (C) The density of E2f1 mRNA in the testis that were measured by densitometry and normalized to GAPDH mRNA expression level. Results were expressed as integrated density values (IDV) of E2f1 mRNA level. C: control; TXL: taxol-received animals; T1, T2, T3: The TXL-recieved animals which treated with various dose levels of RJ; T4: received RJ only. *indicates significant differences (p < 0.05) between the control (C) and TXL-treated animals; #represents remarkable difference between TXL-received non-treated and treated animals.
Discussion
This study showed that administration of TXL for a relatively long period of time (4 weeks) in rats resulted in typical chemotherapy agents side effects including body weight loss and alopecia. Moreover, the negative impact of TXL on the reproductive system of male rats was characterized by remarkable histological changes such as negative TDI and RI, depletion of seminiferous tubules (ST), and increased apoptotic cells. The TXL-induced reduction in sperm total count, sperm motility, and sperm viability was recorded. Additionally, the TXL-induced oxidative stress, histopathological changes, and sperm-related quality parameters were improved with appropriate doses of RJ. The TXL-induced damage and the RJ protective effects were associated with the up regulation of E2f1 transcription factor in the testis.
During the 4 week experiment, clinical symptoms of chemotherapy including body weight and hair loss were observed, which likely related to the TXL-induced anorexia and low food intake. At the same time, anorexia might be due to either the TXL-related central effect or TXL-related pathologic effects on gastrointestinal movements and secretion. Previous reports indicated that the peripheral neuropathy is the most common side effect of TXL [Scripture et al. Citation2006]. Although there is no direct evidence to support that the TXL-induced neuropathy could be the main reason of gastrointestinal disorders in the TXL-treated human patients or animal models, neuronal-associated gastrointestinal motility interruption might be the reason for low food intake and subsequently anorexia and ultimately body weight loss.
The negative impact of TXL on the male reproductive system was examined in the histological feature of testis and sperm quality. The negative TDI and RI indices along with seminiferous tubule depletion indicated that TXL administration resulted in pronounced dismantling of the testis structure and also sperm quality. The antiproliferative effect of TXL is based on its capacity to stimulate the assembly of abnormal microtubules from tubulin dimers, therefore the negative TDI and IR may be explained with antiproliferative effect of TXL. Disorders in the division due to the toxic effect of the drug on microtubules underlie its antiproliferative effect. Presumably, this effect of TXL explains damage to mitotically and meiotically dividing epitheliocytes [Borovskaya et al. Citation2009]. Morphological changes in the testis were paralleled with functional disorders such as lower sperm motility and count. Both sperm count and motility are directly affecting fertility rate. Additionally, we observed a considerable amount of abnormal (small size, without tail) sperms in the rats that received TXL, suggesting that TXL can affect all stages of spermatogenesis and spermiogenesis. In addition to the direct effect of TXL on microtubules, it is also possible that the postmiotic effect of TXL on mature sperm is mediated via disintegrated cell membrane, which resulted from the TXL-induced lipo-peroxidation activity [Bogush et al. Citation2001].
The present study showed that the MDA content of testis as the end product of lipid peroxidation was remarkably enhanced in the TXL-treated animals, suggestive of TXL-induced lipid peroxidation. The results of other antioxidant biomarker assays, such as the level of total thiol molecules, NO concentration also support the fact that TXL not only acts via its capacity to stimulate the abnormal microtubule formation, but it is also able to stimulate the NO production and deplete the level of reduced sulfhydryl groups as the oxidative and the nitrosative stress increases. The involvement of ROS and reactive nitrogen species in the TXL-induced apoptosis in various cell lines has been documented [Ramanathan et al. Citation2005]. Recently it has been reported that oxidative stress plays a crucial role in the development of TXL-induced peripheral neuropathy characterized by swollen and vacuolated mitochondria in the peripheral sensory nerves. Moreover, TXL-induced apoptosis in chronic myelogenous leukemia cells by inducing intracellular oxidative stress and TXL-induced endothelial dysfunction in living rats and direct effect of TXL on the free radical formation [Meshkini and Yazdanparast Citation2012; Serizawa et al. Citation2012; Varbiro et al. Citation2011] has been observed. This supports the view that TXL via several pathways may damage various organs [Fidanboylu et al. Citation2011].
The protective effect of RJ on TXL-induced damage in the testis and sperm quality was also studied. Indeed our results showed a protective effect of RJ in particular at 100 mg/kg (bw) dose level on the TXL-induced structural damage and also on functional activities of the testis. The antioxidant effect of RJ has been previously demonstrated in various studies such as the effect of cisplatin-induced damage of the testes and its scavenging ability against free radicals such as superoxide anion radical [Silici et al. Citation2009]. Taking into account the chemical composition of RJ and the physiological functions of its proteins on one hand and its remarkable antioxidant effects on the other hand, may explain its beneficial and protective effects on sperm quality [Nagai and Inoue Citation2004]. It has been reported that RJ contains free amino acids such as proline, cystine, and cysteine and their role in the synthesis of glutathione and scavenging the free radicals have been well documented [Seminotti et al. Citation2008].
Despite the dose-dependent improvement in sperm quality which was shown by sperm count, viability, and motility, we failed to show a complete restoration of the testis structure such as the full disappearance of cytoplasmic vacuolization and interstitial edema. This finding is supported by previous reports, where Qingrui and Tongsheng [2009] demonstrated that ROS is not the only promoter of the TXL-induced cytoplasmic vacuolization [Qingrui and Tongsheng Citation2009]. Therefore, as the main therapeutic effects of RJ are related to its antioxidant property, failing to recover from all structural damage caused by TXL seems the course. It appears that the TXL-induced DNA damage in rat sperm, which presented by special DNA staining of acridin orange, is also mediated via the TXL-induced oxidative stress as the RJ administration remarkably could reduce the percentage of DNA damaged sperm.
To gain insight and begin to uncover partly the molecular mechanism of RJ protective effect, we evaluated the expression of E2f1, a transcription factor. Our findings indicate that both the TXL-received non-treated and RJ-treated animals showed a remarkable up regulation of E2f1 at the mRNA level. Histopathological examination clearly suggests the presences of TXL-induced apoptosis in the testis, which was characterized by cell shrinkage, cytoplasmic condensation, eosinophilic cytoplasm, pyknotic nuclei, and chromatin condensation around the nuclear membrane (see ). On one hand, in this regard, E2f1 up regulation in the TXL-treated animals is suggestive of its role in TXL-induced apoptosis. There is increasing evidence indicating the over-expression of E2f1 in the induction of p53-dependent and p53-independent apoptosis [Ginsberg Citation2002; Hershko et al. Citation2012; Liu et al. Citation2004]. On the other hand using substances like growth factors can induce the D-type cyclins, which are expressed throughout the G1 phase of cell cycling. Subsequently phosphorylation of the activated cyclins, by pRb leads to pRb-E2F1 dissociation and release of E2F1 inhibition [Lomazzi et al. Citation2002]. Over-expression of E2F1as observed in the RJ-treated animals in this study, has been shown to induce S phase entry in quiescent cells, overcomes G1 arrest, and mediates proliferation [De Gregori Citation2002]. Histopathological examination showed that in the animals which were treated with 100 and 150 mg/kg RJ, cell proliferation was restored and total sperm count was enhanced, suggesting restoring of cell proliferation, which is likely mediated through the E2f1 overexpression. The RJ-induced changes in the D-type cyclins and further cascade of events need to be clarified with regard to this mechanism.
The results presented above showed the RJ protective effects on the TXL-induced damage as evidenced in sperm quality and testis structure. The protective effects of RJ are likely related to its antioxidant properties and may involve E2f1 in its protective effect against TXL-induced apoptosis.
Materials and Methods
Chemicals
5.5′-Dithiobis-2-nitrobenzoic acid (DTNB) and guanidine hydrochloride were purchased from Sigma-Aldrich (Germany). Thiobarbituric acid, phosphoric acid (85%), trichloracetic acid (TCA), dimethyl sulfoxide (DMSO), and ethanol were obtained from Merck (Germany). N-butanol was obtained from Carl Roth, GmbH Co. (Germany). TRIzol reagent was purchased from Applied Biosystems, by life technologies (Nieuwerkerk, The Netherlands). All other chemicals were commercial products of analytical grade. Royal jelly was collected from beehive no: 28 and 74, Sardrood, Hamedan province, Iran, during 2012 and kept at −20 °C until use. It was dissolved in distilled water and given orally.
Animals and experimental design
Forty eight adult male Wistar rats (200–220 g) were obtained from the animal resource of the Faculty of Veterinary Medicine, Urmia University. The rats were in good health. The animals were acclimatized for one week and had free access to food and water. The experimental protocols were approved by the ethical committee of Urmia University in accordance with principles of laboratory animal care.
Animals were assigned into control and test groups (n = 8). Animals in the test group subdivided to the following five groups: A) TXL group: animals in this group received TXL (7.5 mg/kg bw, intraperitoneally, each 7 days); B) T1 group: animals in this group along with TXL received RJ (50 mg/kg bw, at 11:00 am, daily); C) T2 group: animals in this group along with TXL received RJ (100 mg/kg bw, at 11:00 am, daily); D) T3 group: animals in this group along with TXL received RJ (150 mg/kg bw, at 11:00 am, daily); E) T4 group: animals in this group only received RJ (100 mg/kg bw, at 11:00 am, daily). The control group received only saline (0.9%, 5 ml/kg) during the experiment. All test groups received RJ and TXL for 4 weeks. The RJ dose levels were selected based on previous reports [Silici et al. Citation2009] and our primary pilot experiments. Before the experimental procedures, all animals were weighed individually and this procedure was repeated at the end of the study to evaluate any treatment-related changes in the bwg.
Serum preparation and tissue sample collection
On day 29, blood samples were obtained by cardiac puncture under light anesthesia, using diethyl ether. After one h at room temperature, the samples were centrifuged at 3000 × g for 10 min to obtain the serum. The serum samples then stored at −20 °C until further analyses.
The anesthetized animals were ultimately euthanized by using CO2 gas. The testis specimen were immediately removed and rinsed with chilled saline. One of the testis samples from each individual rat was snap frozen in liquid nitrogen and kept in −70 °C until further biochemical and molecular analyses and another testis was fixed in Bouin's solution for histopathological examinations.
Histopathological examinations and histomorphometric analyses
Previously fixed testicular samples in Bouin's solution (containing 10% formaldehyde, 5% acetic acid, 5% methanol and 0.5% picric acid) were given histological examinations. The samples embedded in paraffin and sections (5–6 µm) were stained with hematoxylin and eosine and were analyzed under light microscope by multiple magnifications.
To estimate the tubular differentiation index (TDI), the percentage of seminiferous tubules (STs) that were showing more than three layers of differentiated germinal cells from spermatigonia type A, 20 sections (6 µm) were prepared from one sample and the STs which showed more than three and/or four layers considered as TDI positive [Porter et al. Citation2009]. To evaluate the effect of RJ on TXL-induced damages on spermatocytogenesis, the repopulation index (RI) was determined. The repopulation index is the percentage of tubules populated with germ cells that had clearly reached the intermediate spermatogonial stage or later. The RI, as the ratio of active spermatogonia (spermatogonia type B with light nucleus) to inactive spermatogonia (spermatogonia type A with dark nucleus), in STs was calculated in 20 prepared sections as described earlier [Meistrich and Van Beek Citation1993].
To evaluate the level of damages following exposure to TXL and/or TXL plus various treatments, indices such as cell shrinkage, pyknotic cells, and depletion area in the testis were scored numerically. The evaluation criteria were as follows: zero for no detectable lesion, 1 for mild changes, 2 for moderate changes, and 3 for severe damages. For each animal in the tests and control groups at least three slides were prepared and scored.
Evaluating the epididymal sperm parameters
The epididymis was separated carefully from the testicles under a 20 × magnification provided by a stereo zoom microscope (model TL2, Olympus Co., Tokyo, Japan). The epididymis was divided into 3 segments; head, body, and tail. The epididymal tail was trimmed and the content was added to 5 mL pre-warmed Hams F10 medium. After 20 min the epididymal tissue was separated from the released spermatozoa. The sperm count was performed according to standard hemocytometric method as described previously by Pant and Srivastava [Citation2003].
Sperm motility and viability analyses
The sperm motility was examined based on WHO [Citation1999] standard method for manual examination of sperm motility. In brief, the sperm samples were diluted 1:8 in Ham’s F10 medium before examination. A 20 µl of sperm sample placed on sperm examination area and examined under 10 × magnification loop. Only the motile sperms with forward progression were counted within 10 boxes and recorded. Finally, motility was evaluated based on the following equation:
The eosin-nigrosin staining was performed for sperm viability assay [WHO Citation1999]. In brief, 50 µl of epididymal sperm was mixed with 20 µl of eosin in a sterile test tube. After 5 s 50 µl of nigrosin was added and mixed thoroughly. The mixture of stained sperm was smeared on the slide and examined under bright field microscope (1000 × magnification, Olympus, Germany). The colorless sperm were considered as live and stained sperm were marked as dead. A hundred spermatozoa from each animal were counted. The sperm viability and motility were reported in percentage.
Acridine orange (AO) staining for sperm DNA strands
The AO staining was performed in order to estimate sperm DNA fragmentation [Tejada et al. Citation1984]. In brief, air dried slides were stained for 10 min with freshly prepared AO (0.19 mg/ml), washed in distilled water, then the cover-slip was applied on the slides. The slides were evaluated on the same day using an epi-fluorescent microscope (Model GS7, Nikon co., Japan). In all preparations, at least 100 spermatozoa were evaluated at 40 × magnification. Spermatozoa with green fluorescence were considered to have native double strand DNA (DS-DNA) and the spermatozoa with yellow or red fluorescence were marked as cells with damaged DNA. The percentage of green, yellow, and red spermatozoa were assessed and compared between the groups.
Testicular malondialdehyde (MDA) analyses
To determine the lipid peroxidation rate, the MDA content of collected testis samples was measured using the thiobarbituric acid (TBA) reaction as described previously [Niehaus and Samuelsson Citation1968]. In short, 0.3–0.4 g of the testis samples were homogenized in ice-cold KCl (150 mM), and then the mixture was centrifuged at 3000 × g for 10 min. Thereafter 0.5 ml of the supernatant was mixed with 3 ml phosphoric acid (1% v/v) and then following vortex mixing, 2 ml of 6.7 g L−1 TBA was added to the samples. The samples were heated at 100 °C for 45 min and chilled on ice. Finally, 3 ml N-butanol was added and the samples were further centrifuged at 3000 × g for 10 min again. The absorbance of supernatant was measured spectrophotometerically at 532 nm and the MDA concentration calculated according to the simultaneously prepared calibration curves using MDA standards. The amount of MDA was expressed as nmol per mg protein of the samples. The protein content of the samples was measured according to the Lowry method [Lowry et al. Citation1951].
Measurement of total thiol molecules (TTM)
Total sulfhydryl level in the testes samples was measured as described previously [Hu and Dillared Citation1994]. Briefly, 0.3–0.4 g of the testes samples was homogenized in ice-cold KCl (150 mM), and the mixture was centrifuged at 3000 × g for 10 min. To 0.2 ml of the supernatant of the tissue homogenate, 0.6 ml Tris-EDTA buffer (Tris base 0.25 M ethylendiamintetraacetic acid 20 mM, pH 8.2) and thereafter 40 µl 5.5′-dithiobis-2-nitrobenzoic acid (10 mM in pure methanol) were added. The final volume of this mixture was made up to 4.0 ml by the addition of pure methanol. After 15 min incubation at room temperature, the samples were centrifuged at 3000 × g for 10 min and ultimately the absorbance of the supernatant was measured at 412 nm. The TTM capacity was expressed as nmol per mg of protein in samples. The protein content of the samples was measured according to Lowry et al. [Citation1951].
Nitric oxide measurement
The total nitrate/nitrite content of testicular tissues was measured according to the Griess reaction [Green et al. Citation1982]. In the Griess reaction nitric oxide rapidly converted into more stable nitrite, which in an acidic environment nitrite is converted to HNO2. In reaction with sulphanilamide, HNO2 forms a diazonium salt, which reacts with N-(1-naphthyl) ethylenediamine.2HCL to form an azo dye that can be detected by absorbing at 540 nm wavelength. The NO content of the testis was expressed as nmol per mg of protein in samples.
RNA isolation and TR-PCR
To evaluate the effect of RJ on the expression of E2f1 at mRNA level, total RNA was isolated from the testis samples using the standard TRIZOL method [Chomczynski and Sacchi Citation2006]. To avoid genomic DNA contamination extra care was taken when the colorless aqueous phase collected after chloroform extraction. The RNA amount was determined spectrophotometrically (260 nm and A260/280 = 1.8–2.0), and the samples were stored at −70 °C. For RT-PCR, cDNA was synthesized in a 20µl reaction mixture containing 1 µg RNA, oligo(dT) primer (1 µl), 5 × reaction buffer (4 µl), RNAse inhibitor (1 µl), 10 mM dNTP mix (2 µl) and M-MuLV Reverse Transcriptase (1 µl) according to the manufacturer’s protocol (Fermentas, GmbH, Germany). The cycling protocol for 20 µl reaction mix was 5 min at 65 °C, followed by 60 min at 42 °C, and 5 min at 70 °C to terminate the reaction.
Second strand cDNA synthesis
The products of RT-PCR were separated on 1.5 % agarose gel containing ethidium bromide and visualized using Gel Doc 2000 system (Bio-Rad). The specific primers for Ratus E2f1 and GAPDH were designed [Hariya et al. Citation1999; Wong et al. Citation2011] and manufactured by CinnaGen (CinnaGen Co. Tehran, Iran). Primers pairs for RT-PCR were as depicted in .
Table 4. Neuclotid sequences, anneling tempratures (AT), and product size for primers used in RT-PCR.
The RT-PCR reaction was carried out in a total volume of 25 µl containing PCR master mix (12.5 µl), FWD and REV specific primers (each 0.75 µl), and cDNA as a template (1 µl) and nuclease free water (10 µl). PCR conditions were run as follows: general denaturation at 95 °C for 3 min, 1 cycle, followed by 40 cycles of 95 °C for 20 s; annealing temperature (63 °C for GAPDH and 58 °C for E2f1) for 30 s, and elongation 72 °C for 1 min and 72 °C for 5 min.
Statistical analysis
For all numerical results mean and standard deviation of the measured parameters were calculated. The results of three independent experiments for each assessment were analyzed using Graph Pad Prism software (version 2.01. Graph Pad Software Inc. San Diego, California, USA). The comparisons between groups were made by analysis of variance (ANOVA) followed by Bonferroni post-hoc test. For comparing the graded degree of pathological findings between groups, the Kruskal-Wallis test was used. A P value <0.05 was considered significant.
Declaration of interest
The authors report no declarations of interest. We declare that this study was conducted with personal budget and the authors are not employed by the Government of Iran.
Author contributions
Designed and performed the experiments and wrote the manuscript: HM; Performed the experiments: FD-K; Performed the experiments: MK; Performed the experiments: HJ-A; Analyzed and discussed the data: AR-G.
| Abbreviations | ||
| AO | = | Acridine-orange |
| DMSO | = | dimethyl sulfoxide |
| MDA | = | malondialdehyde |
| NO | = | nitric oxide |
| RJ | = | royal jelly |
| TXL | = | taxol |
| TDI | = | tubular differentiation index |
| RI | = | repopulation index |
| TTM | = | total thiol molecules |
Acknowledgments
We wish to thank Dr. M. Razi (Department of Histology, Faculty of Veterinary Medicine, Urmia University) for his kind assistance.
References
- Bogush, T.A., Koldaeva, E.Y., Smirnova, G.B., Bogush, E.A., Konyaeva, O.I., and Khrustalev, S.A. (2001) Effect of mesna on lethal effect and hematological toxicity of taxol and vepeside in mice. Bull Exp Biol Med 132:301–305
- Borovskaya, T.G., Goldberg, V.E., Rumpel, O.A., Pahomova, A.V., Perova, A.V., and Goldberg, E.D. (2009) The rat spermatogenesis after injection of paclitaxel (Antitumor Agent). Bull Exp Biol Med 147:715–718
- Charity, D.S., Figg, W.D., and Sparreboom, A. (2006) Peripheral Neuropathy Induced by Paclitaxel: Recent Insights and Future Perspectives. Curr Neuropharmacol 4:165–172
- Chomczynski, P. and Sacchi, N. (2006) The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc 1:581–585
- De Gregori, J., Leone, G., Miron, A., Jakoi, L., and Nevins, J.R. (1997) Distinct roles for E2F proteins in cell growth control and apoptosis. PNAS 94:7245–7250
- De Gregori, J. (2002) The genetics of the E2F family of transcription factors: shared functions and unique roles. Biochimca Biophysica Acta 1602:131–150
- Dziadyk, J.M., Sui, M., Zhu X., and Fan, W. (2004) Paclitaxel-induced apoptosis may occur without a prior G2/M-phase arrest. Anticancer Res 24:27–36
- Fidanboylu, M., Griffiths, L.A., and Flatters, S.J.L. (2011) Global Inhibition of Reactive Oxygen Species (ROS) Inhibits Paclitaxel-Induced Painful Peripheral Neuropathy. Plos one 6:e25212
- Ginsberg, D. (2002) E2F1 pathways to apoptosis. FEBS Lett 529:122–125
- Green, L.C., Wagner, A.D., Glogowski, J., Skipper, P.L., Wishnok, J.S., and Tannenbaum, S.R. (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
- Hariya, S.M., Hatao, T., Ashikaga, M., and Ichikawa, T.H. (1999) Quantitative polymerase chain reaction using an external control mRNA for determination of gene expression in a heterogeneous cell population. Toxicolo Sci 49:290–296
- Hershko, T., Chaussepied, M., Oren, M., and Ginsberg, D. (2012) Novel link between E2F and p53: proapoptotic cofactors of p53 are transcriptionally upregulated by E2F. Cell Death Differ 12:377–383
- Hu, M. and Dillared, C.J. (1994) Plasma SH and GSH measurement. Methods Enzymol 233:385–387
- Kamakura, M., Mitani, N., Fukuda, T., and Fukushima, M. (2001) Antifatigue effect of fresh royal Jelly in mice. J Nutr Sci Vitaminol (Tokyo) 47:394–401
- Liu, K., Luo, Y., Lin, F.T., and Lin, W.C. (2004) TopBP1 recruits Brg1/Brm to repress E2F1-induced apoptosis, a novel pRb-independent and E2F1-specific control for cell survival. Genes Dev 18:673–686
- Lomazzi, M., Moroni, M.C., Jensen, M.R., Frittoli, E., and Helin, K. (2002) Suppression of the p53- or pRB-mediated G1 checkpoint is required for E2F-induced S-phase entry. Nat Genet 31:190–194
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
- Matsui, T., Yukiyoshi, A., Doi, S., Sugimoto, H., Yamada, H., and Matsumoto, K. (2002) Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J Nutr Biochem 13:80–86
- Meistrich, M.L., and Van Beek, M.E.A.B. (1993) Spermatogonial stem cells: assessing their survival and ability to produce differentiate cells. Methods Toxicol 3:106–123
- Meshkini, A. and Yazdanparast, R. (2012) Involvement of oxidative stress in taxol-induced apoptosis in chronic myelogenous leukemia K562 cells. Expe Toxicol Pathol 64:357–365
- Nagai, T. and Inoue, R. (2004) Preparation and functional properties of water extract and alkaline extract of royal jelly. Food Chem 84:181–186
- Niehaus, W.G. and Samuelsson, J.R.B. (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
- Pant, N., and Srivastava, S.P. (2003) Testicular and spermatotoxic effect of quinaphos in rats. J Appl Toxicol 23:271–274
- Porter, K.L., Shetty, G., Shuttlesworth, G.A., Weng, C.C.Y., Huhtaniemi, I., Pakarinen, P., et al. (2009) Estrogen Enhances Recovery From Radiation-Induced Spermatogonial Arrest in Rat Testes. J Androl 30:440–451
- Qin, X.Q., Livingston, D.M., Kaelin, W.G., and Adams, P.D. (1994) Deregulated transcription factor E2f1 expression leads to S-phase entry and p53-mediated apoptosis. PNAS 91:10918–10922
- Qingrui, S. and Tongsheng, C. (2009) Reactive oxygen species (ROS) is not a promotor of taxol-induced cytoplasmic vacuolization. Proc. SPIE 7178, Biophotonics and Immune Responses IV, 71780K (February 25, 2009); doi:10.1117/12.807256
- Ramanathan, B., Jan, K.Y., Chen, C.H., and Hour, T.C. (2005) Resistance to Paclitaxel Is Proportional to Cellular Total Antioxidant Capacity. Cancer Res 65:8455–8460
- Rowinsky, E.K., Donehower, R.C., Jones, R.J., and Tucker, R.W. (1988) Microtubule changes and cytotoxicity in leukemia cell lines treated with taxol. Cancer Res 48:4093–4100
- Scripture, C.D., Figg, W.D., and Sparreboom, A. (2006) Peripheral Neuropathy Induced by Paclitaxel: Recent Insights and Future Perspectives. Curr Neuropharmacol 4:165–172
- Seminotti, B., Leipnitz, G., Amaral, A.U., Fernandes, C.G., da Silva Lde, B., Tonin, A.M., et al. (2008) Lysine induces lipid and protein damage and decreases reduced glutathione concentrations in brain of young rats. Int J Dev Neurosci 26:693–698
- Serizawa, K., Yogo, K., Aizawa, K., Tashiro, Y., Takahari, Y., Sekine. K., et al. (2012) Paclitaxel-Induced Endothelial Dysfunction in Living Rats Is Prevented by Nicorandil via Reduction of Oxidative Stress. J Pharmacol Sci 119:349–358
- Silici, S., Ekmekcioglu, O., Ersalan, G., and Demirtas, S. (2009) Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology 74:545–551
- Tejada, R.I., Mitchel, J.C., and Norman, A. (1984) A test for the practical evaluation of male infertility by acridine orange (AO) fluorescence. Fertil Steril 42:87–91
- Thomson, A.B., Campbell, A.J., Irvine, D.C., Anderson, R.A., Kelnar, C.J. Wallace WH, et al. (2002) Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case-control study. Lancet 360:361–366
- Varbiro, G., Veres, B., Gallyas, F., and Sumegi, B. (2011) Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic Biol Med 31:48–358
- WHO (1999) WHO Laboratory manual for the examination of human semen and sperm cervical mucus interaction, World Health Organization/Cambridge University Press
- Wong, J.W., Yao, G., Nevins, J.R., and You, L. (2011) Viral-Mediated Noisy Gene Expression Reveals Biphasic E2f1 Response to MYC. Mol Cell 41:275–285
- Wu, X. and Levine, A.J. (1994) P53 and E2f1 cooperate to mediate apoptosis. PNAS 91:3602–3606
- Zhang, K., Heidrich, F.M., De Gray, B., Boehmerle, W., and Ehrlich, B.E. (2010) Paclitaxel accelerates spontaneous calcium oscillations in cardiomyocytes by interacting with NCS-1 and the InsP3R. J Mol Cell Cardiol 49:829–835
