Abstract
Uterine cervical incompetence (UCI) is a pregnancy complication affecting about 10% of the pregnancies in the western world. Studying the etiology of the UCI requires a specific approach adequate for this highly heterogenous syndrome. Oxidative status disorders are associated with various pathologies, including pregnancy complications. As such, general oxidative status profiling is a promising methodology to treat UCI. We aimed at assaying the closely interrelated oxidative status markers in the uterine cervical incompetence patients by means of the systems biology-oriented approach. Chemiluminescent assay, circulating thioredoxin 1 protein, uric acid, and homocysteine level measurements were used to assess the character of the oxidative status regulation in the UCI patients. We found UCI to be associated with the atypical plasma oxidative status deregulation; UCI plasma samples demonstrated lowered proneness to the pro-oxidative processes, and this was not due to the excessive antioxidant activity. There were neither signs of oxidative stress nor destructive pro-oxidant feedforward circuit locking in the UCI group. We also report increased circulating levels of uric acid in the UCI patients.
Introduction
Uterine cervical incompetence (UCI) is a type of pregnancy complication usually diagnosed in the second trimester and characterized by: passive painless cervical dilation in the absence of uterine contraction, bleeding, infection, and sometimes with the amniotic sac bulging through the partially dilated cervix. Left untreated, this condition may lead to premature pregnancy loss (according to the current Medical Subject Headings database (MeSH) [Citation2013] term). Uterine cervical incompetence affects about 10% of pregnancies in the western world [Owen and Mancuso Citation2012]. It is one of the notorious highly heterogeneous pregnancy complications [Owen and Mancuso Citation2012; Warren and Silver Citation2009].
To the authors’ knowledge, no exact pathophysiological nor etiological molecular mechanisms of the UCI development have been proposed to date. Different and versatile genetic and environmental, inflammatory, anatomical, and adaptive factors may be contributors to this obscure symptomatic complex [Owen and Mancuso Citation2012; Warren and Silver Citation2009].
One possible and yet surprisingly not very common approach to study UCI is investigating the pro- and antioxidative interrelations, i.e., oxidative status. Oxidative status may be defined as the qualitative and quantitative superposition of the pro- and antioxidants of all cellular compartments and intercellular space [Zolotukhin et al. Citation2013]. Ever-growing experimental evidence implicates disturbances in oxidative status in a broad variety of pathological states in humans. In most cases, the diseases are associated with one of the terminal variants of oxidative status - oxidative stress. Oxidative stress is defined as a disturbance in the prooxidant-antioxidant balance in favor of the former, leading to potential cellular damage (according to the current MeSH term). Oxidative stress involvement is associated with early pregnancy complications (non-developing pregnancy, spontaneous abortions) [Al-Gubory et al. Citation2010], mid (diabetes mellitus, incipient pre-eclampsia) [Ma et al. Citation2012; Rogers et al. Citation2006], and late gestation diseases (pre-eclampsia, hemolysis, elevated liver enzyme levels, and low platelet count (HELLP) syndrome, eclampsia) [Aris et al. Citation2009; Tranquilli and Landi Citation2010; Walker Citation2000; Warren and Silver Citation2009]. Considering this, the systemic assessment of the oxidative status seems to be a promising approach to study the molecular aspects of UCI. Thus, we aimed at assaying closely interrelated oxidative status component-markers in UCI patients by means of the systems biology-oriented approach.
Results
Group characteristics and the results of the chemiluminescent assay of circulating uric acid, homocysteine, and thioredoxin 1 (TXN) protein concentration measurements are provided in .
Table 1. Group characteristics and primary biochemical indicatives of the sample groups.
Assessing the analysis interfering influences
According to the data in the literature, oxidative status markers are relatively stable within the 20–39 year old population [Mutlu-Türkoğlu et al. Citation2003]. The age of the participants in the present study varied from 22 to 35 years in the UCI group and from 25 to 38 years in the control group. Mean (4 years) and median (1 year) group age differences do not interfere with the studied conditions (physiological pregnancy versus UCI) and may be ignored. As gestational age (GA) may significantly influence the oxidative status screening, we analyzed the groups using GA homogeneity. One-way analysis of variance (ANOVA) revealed no significant differences (p = 0.086) in the gestational age between the groups. Spectral analysis revealed no significant differences in the samples’ 430 nm absorbance between the groups (median/10–90 percentiles - controls: 1.296/0.950–2.672, UCI: 1.662/1.225–2.009; p = 0.23).
Biochemical and enzyme-linked immunosorbent assay (ELISA)-based analyses
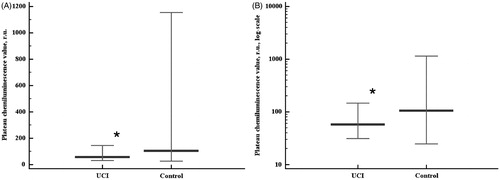
As spectral analysis in the present study revealed no significant differences in the samples’ 430 nm absorbance between the groups, there was no risk of chemiluminescent assay bias. Fast burst of chemiluminescence (Imax), sum of chemiluminescence intensities (SI), and plateau chemiluminescence value (PV) showed the same trends in the groups studied (). Surprisingly, fast burst intensities were lower in the UCI group than in the control group (p = 0.015, ). The values of the sum of intensities () and plateau chemiluminescence () were also lower in the UCI groups than in the controls (p = 0.0331 and p = 0.0454, respectively), though the differences in the data distribution between the groups were less pronounced compared to the fast burst. Rank correlation coefficients between the sum of intensities and plateau chemiluminescence values were 0.991 (p < 0.0001) in the control group and 0.925 (p < 0.0001) in the UCI group. The differences of these correlation coefficients between the studied groups were statistically significant (p = 0.0129).
Figure 1. Chemiluminescent assay results - fast burst of chemiluminescence (Imax) in linear (A) and log (B) scales. Data are represented as 5–95 percentiles and median. *:p = 0.015 - the differences are significant. r.u.: relative units.
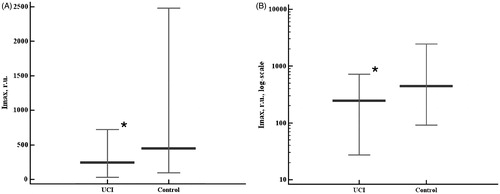
Figure 2. Chemiluminescent assay results – the sum of chemiluminescence intensities (SI) in linear (A) and log (B) scales. Data are represented as 5–95 percentiles and median. *p = 0.0331 - the differences are significant. r.u.: relative units.
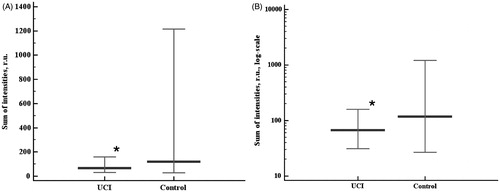
Figure 3. Chemiluminescent assay results - the plateau chemiluminescence value (PV) in linear (A) and log (B) scales. Data are represented as 5–95 percentiles and median. *p = 0.0454 - the differences are significant. r.u.: relative units.
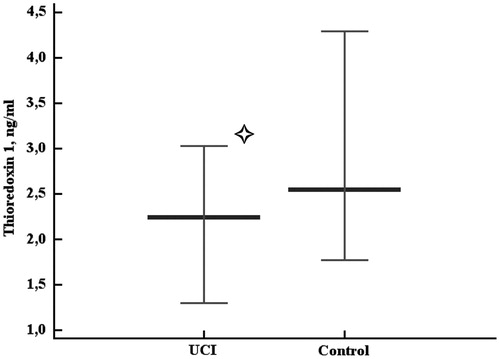
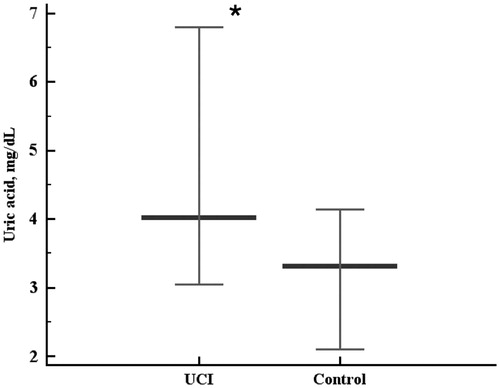
There was no ultimate conclusion on whether circulating TXN protein levels were lower in the UCI group when compared to the control group, as the statistical differences were very close to the significance level cut-off (two-tailed probability p = 0.054). The data obtained showed that the level of circulating TXN protein was not higher in the UCI patients compared to the controls (). Rank correlation coefficient between the sum of intensities and plasma TXN levels in the control group was moderate (r = 0.489, p = 0.0334). No significant rank correlation between the sum of intensities and plasma TXN levels was observed in the UCI group. Uric acid levels were higher in the UCI patients than in the controls (p = 0.0024, ). We found neither significant association of the homocysteine levels with UCI nor correlations of homocysteine with the chemiluminescent assay data in either group.
Discussion
According to the contemporary molecular and cellular biology fundamentals, cell function is inevitably dependent on the generation of the reactive intermediates. Moreover, chemically active substances, including the by-products of various metabolic systems, are evolutionally coupled to act as messengers [Newsholme et al. Citation2007; Suliman et al. Citation2007; Zhang et al. Citation2010]. Constant reactive oxygen species (ROS)/reactive nitrogen species (RNS) production is coupled with the concordant stand-by antioxidant expression, while local and (or) short-term pro-oxidant bursts activate reactive antioxidant subsystems [Jaiswal Citation2004; Moi et al. Citation1995; Kops et al. Citation2002; Suliman et al. Citation2007; Ushio-Fukai et al. Citation1998]. Together, stand-by and reactive antioxidants maintain stable oxidative status of the cell despite fluctuating pro-oxidant generation. Whatever the disturbance in this subtle tuned molecular system it may lead to significant shifts in various cellular pathways, as both pro-oxidant systems are highly regulated by feedback and feedforward circuits affecting adjacent systems. And vice versa, malfunctions of cellular metabolic systems may lead to the disturbances in oxidative status [Zolotukhin et al. Citation2013]. This feature of oxidative status makes it a good target system for revealing the character of the cellular dysfunction in versatile pathological states, including UCI along with various other pregnancy complications [Myatt Citation2010; Reyes et al. Citation2006]. Considering this, we have performed a series of experiments aimed at characterizing the cellular physiological-level oxidative status [Newsholme et al. Citation2007; Zolotukhin et al. Citation2013] in UCI patients. To our knowledge, we have performed the first attempt to reveal the higher-order pattern of oxidative status in UCI.
Based on the fast burst values in the studied groups we may conclude the UCI samples to be less prone to the pro-oxidative processes than the control samples. Although reactive oxygen and nitrogen species are harmful, the pro-oxidant system is essential for cellular signaling pathways [Poljsak et al. Citation2013]. New evidence continues to be presented in the field of the involvement of pro-oxidant signaling in adaptive cellular reactions [Wu et al. Citation2010] and etiology of oxidative-stress-associated pathologies [Lönn et al. Citation2012]. Thus, the results of the present study suggest impaired pro-oxidant function to be possibly associated with UCI, although this kind of oxidative status, as seen from literature, is rare. In comparison, fast burst analysis does not allow delineating the individual contribution of the pro- and antioxidant systems to the pro-oxidant proneness, as both decreased pro-oxidants and increased antioxidants may be the cause of the lower Imax in the UCI group. In this case, analyzing the kinetics of chemiluminescence was helpful in revealing the differences in the responses of the antioxidant system to pro-oxidant induction of the samples within the studied groups.
The sum of intensities may highlight the differences in time-dependent antioxidant system exhaustion and slow burst initiation (the second wave pro-oxidative chain reaction). The differences between the groups in the SI had the same trend as in the fast burst. The changes were significant but had lower magnitude with respect to the previously discussed changes in related index. This highlighted the different levels of the antioxidant system’s capabilities to control the intensive induced pro-oxidative processes. In order to verify this idea we analyzed the steady-state (plateau) chemiluminescence values of the sample.
Assessing the plateau chemiluminescence values allows for understanding the nature of the shifts in oxidative status revealed by the fast burst and sum of intensities analyses. As seen from and , the sum of intensities and the plateau value indicatives, demonstrate a highly similar distribution of values. In order to assess the power of the antioxidant system to maintain a system’s oxidative status, we performed the correlation analysis of the sum of intensities and the plateau value in both groups, and found significant differences between the groups. Taking into account the lowered Imax values in the UCI group and the value distributions of SI and PV, suggests the antioxidant system in the UCI group samples is not activated, as Imax value contributes less to the chemiluminescence sum in the UCI. Thus, the differences in the chemiluminescence system’s kinetics in the studied groups revealed by correlational analysis represent the slightly decreased power of the antioxidant system to handle the induced (intentionally abundant) pro-oxidative processes in the UCI group.
The chemiluminescence assay may reveal general shifts in oxidative status, but it is not sensitive to the cause and the nature of the disturbances. Additional markers are required to assess the oxidative status contributors from the pro- and antioxidant systems, and the most important contributors are locked-up feed-forward circuits of both systems. Thus, we selected circulating uric acid, homocysteine (of NOX/xanthine oxidoreductase (XOR) circuit), and TXN protein (of activator protein 1 (AP1)/NFE2L2/nuclear factor kappa B (NF-kappaB) circuits) as markers allowing, together with chemiluminescent assay, to assess the circuit locking-up. Uric acid and homocysteine were both selected as oxidative status markers, because, in conjunction with Imax, they reflect the locking up of the hazardous NOX/XOR feed-forward system [Molvarec et al. Citation2009; Myatt Citation2010; Sautin et al. Citation2007; Sipkens et al. Citation2012; Pendyala and Natarajan Citation2010; Tranquilli and Landi Citation2010].
Addressing the question of the urate/NOX/XOR and homocysteine/NOX feed-forward systems’ contribution to the pro-oxidant capacity (represented as fast burst and chemiluminescence kinetics) by means of the correlation analysis revealed no significant interrelations, thus indicating other systems to be major contributors to the pro-oxidative capacity in both groups. Considering the chemiluminescent assay data analysis results, this is a predictable result, as none of the groups generally demonstrated oxidative status to be of inconsistent antioxidant control over the pro-oxidant system.
Though we found no involvement of the uric acid levels in pro-oxidant system activation in UCI (as there was no oxidative stress in UCI), the levels of uric acid were higher in the UCI group when compared to the controls. Thus, uric acid could have been treated as contributing antioxidant in the disease case analyzed [Shkurat et al. Citation2008] be there any sign of the increased antioxidant system capacity. No correlation was observed between uric acid and PV, which would suggest the antioxidant effects of the increased uric acid levels in UCI. Instead, in the case of UCI, uric acid may rather be associated with reproductive system disorders, as uric acid was shown to compromise placental function [Bainbridge et al. Citation2009]. However, we cannot make any conclusions regarding these considerations unless more data are obtained, and the only statistically solid association in this study is that uric acid levels were associated with UCI. As the role of uric acid in reproduction is not limited by its involvement in the oxidative status, further investigations on the role of urate in the UCI development are required. For example, uric acid is converted into LDL-oxidizing [Abuja Citation1999] and enzyme-inhibiting radical; it has pro-inflammatory properties [Glantzounis et al. Citation2005] and is capable of influencing MAPK signaling pathway [Kanellis et al. Citation2003], and promoting endothelial dysfunction, inducing proliferation of vascular smooth muscle cells [Johnson et al. Citation2003], and attenuating System A amino acid transport function [Bainbridge et al. Citation2009]. All of these properties of uric acid may present in UCI, although we found no direct evidence in the literature on this topic during this preparation of the manuscript.
We found no association of homocysteine levels and UCI, as the levels of homocysteine were similar in the groups studied. Together with feed-forward system analysis, this suggests the homocysteine/NOX/XOR circuit is not an important contributor to the UCI pro-oxidant system function in the second trimester.
Among the informative antioxidant system markers, the TXN protein appears to be of most value. Thioredoxin 1 is a physiological component of the cytosol, nucleus, and plasma. It is a critical factor in the activation of several redox-sensitive transcription factors, including NF-kappaB and AP1 complexes, which are important participants regulating oxidative status [Gauss et al. Citation2007; Hirota et al. Citation1999; Manea et al. Citation2007; Nordberg and Arnér Citation2001]. Moreover, TXN gene expression is dependent on oxidative status [Kim et al. Citation2003; Levy et al. Citation2009; Um et al. Citation2011]. Thus, TXN may be used as indicative of both the cellular and the organismal antioxidant system’s activation state and the oxidative status stabilization capacity.
We found that the plasma levels of TXN were not higher in the UCI patients, when compared to the controls. Moreover, TXN levels may be lower in UCI cases than in normal pregnancies, as our groups showed that the differences were close to the significance threshold (p = 0.054). At least the induction of TXN expression was not observed in the UCI cases. This supports the idea of a non-activated antioxidant system in UCI which would account for lowered Imax, SI, and PV. Moreover, rank correlation analysis revealed significant interrelations between the kinetic oxidative status index (SI), relative antioxidant system power marker (PV), and TXN protein levels in the control group, but not in UCI patients. Further investigations are required to reveal the extent of the differences in the concentration of circulating TXN protein concentrations between physiological and UCI pregnancies.
Considering the limitations of the study, the approach used here did not allow us to determine precisely the cause of the revealed perturbations of oxidative status in UCI. We did not observe any significant changes in the level of homocysteine in the UCI group, and this might be due to statistical power limitations, thus the conclusions on homocysteine levels are limited; however, all the participants had homocysteine levels within normal range (5–15 μM). We found uric acid to be higher in the UCI group compared to the control group; however, we cannot make any conclusions on the cause-effect relationship of UCI and uric acid level changes and on the sources of the increased uric acid levels. As TXN has a complex regulation, there might possibly be the interference of several TXN expression controlling antioxidant pathways capable of increased antioxidant capacity of the UCI samples. Nevertheless, chemiluminescent assay data and correlation analyses do not suggest any antioxidant systems other than those involving TXN pathways to be activated.
The present study was the pilot attempt to assess the higher-order oxidative status in UCI and to test the locking-up of the most significant pro- and antioxidant feed-forward systems. As this approach implies there are several limitations that do not allow us to reveal the exact mechanisms of the oxidative status disturbances in UCI, further investigations are required. We believe the results of the study will facilitate the design of further experiments.
Summarizing the results of the present study, we revealed some features of the oxidative status in UCI. First, plasma oxidative status demonstrated lowered proneness to the pro-oxidative processes in UCI. Second, this atypical pattern of oxidative status in the UCI for pregnancy complications does not appear to be due to the excessively activated antioxidant system. Third, we do not observe the NOX/XOR circuit locking in either the UCI or control groups. Further investigations are required to reveal the molecular causes of oxidative status and the involvement of the pro-oxidant system dysregulation in the etiology of UCI.
Materials and Methods
Study design and participants
The research project plan and protocols were approved by the Rostov State Medical University bioethical committee. A total of 19 healthy pregnant and 11 UCI (not in labor during sampling; no sac bulging; McDonald cerclage due to cervical dilation initiation) gravid women matching in age, gestation age (mid-second trimester), and reproductive and general anamnesis were enrolled in the investigation after the primary survey of 81 potential participants (willing pregnant women being under the care of medical staff of the Rostov State Medical University during 2010–2012) and stringent anamnesis analysis. All participants were residents of Rostov-on-Don and Rostov region. All participants signed informed consent.
Blood collection
Fasting blood samples were collected only once from each patient in the morning of a doctor’s appointment (in the control group) or one to three days after the cerclage placement (in the UCI group) into the vacuum collection tubes with heparin and without anti-coagulant. Blood samples were centrifuged appropriately to obtain plasma and serum, respectively. Plasma and serum samples were aliquoted and deep frozen (−80 °C); serum samples for the chemiluminescent assay were stored at −20 °C for up to 1 w prior to the analysis.
Serum absorption spectra scanning
In order to properly assess the chemiluminescent assay data, the samples were scanned for the wave length-dependent absorption at 400–470 nm using Smart Spect Plus specrophotometer (BioRad, USA).
Chemiluminescent assay
Chemiluminescent assays were performed in the luminol-dependent hydroperoxide-induced system with automatic chemiluminometer Autolumat LB953 (Berthold technologies, Germany). Briefly, 50 μl of the serum thawed immediately prior to the analysis was added to 1.45 ml of 0.1 M TRIS-HCl-luminol buffer (pH = 6.8 at 37 °C). Blank samples contained only buffer solutions. Light intensity measurement was started after 5 min of the sample incubation in the chemiluminometer at 37 °C. Hydrogen peroxide (250 μl, 0.35 M) was injected into the tube after 5 s of the measurement. The total time of the chemiluminescence measurement was 100 s with 0.1 s increments. Sample’s maximal chemiluminescence (fast burst, or Imax) and SI were further calculated. Plateau-chemiluminescent values were the intensities of the 50th s of the induced chemiluminescence. Blank sample’s Imax and SI were treated as 100%-baselines used to normalize sample data.
Uric acid concentration determination
Serum uric acid levels were measured using the respective calorimetric biochemical kit from Olvex (Russia). Briefly, uric acid was oxidized by uricase to form hydrogen peroxide participating in the formation of the quinoneimine dye (Fosatti method). Calorimetry was performed using Smart Spect Plus spectrophotometer (BioRad).
Homocysteine level measurement
Serum concentrations of homocysteine were determined using ‘Homocysteine 96’ ELISA kit (Axis-Shield Diagnostics, Norway) and the automatic ELISA-analyzer ‘Alisei’ (Radim, Italy), following the manufacturer's protocols.
Thioredoxin 1 protein level measurement
Concentrations of circulating TXN protein were determined using ‘Human Trx1 ELISA kit’ (ABfrontier, South Korea) and the automatic ELISA-analyzer ‘Alisei’ (Radim), following the manufacturer's protocols.
Statistical analysis
All results, including significant anamnesis survey data were statistically analyzed using appropriate criteria in MedCalc 11.4. Parametric tests used were independent samples t-test (for the homocysteine, uric acid, and thioredoxin 1 measurements) and one-way ANOVA (for gestational age); non-parametric statistics used to assess the chemiluminescent data was performed using Mann-Whitney criterion. Correlation coefficients were calculated using Spearman’s method. Acceptable maximum false-positive error probability (p-level) used was 0.05. Means and standard deviations are provided in for reverse comparisons. Please note, chemiluminescent data are highly variative and cannot be assessed using parametric testing. However, the results have same trends in parametric and non-parametric calculations.
Data representation
Chemiluminescent data units are ‘relative units’ (r.u.), representing normalized values calculated as respective (Imax, plateau point, and sum of intensities) percentages of samples’ values against intra-assay series buffer. Data in the tables and figures are represented as medians and 5–95 percentiles.
Declaration of interest
The authors report no conflicts of interest. The research project was funded by the Federal targeted programme “Scientific and Academic Specialists for Innovations in Russia 2009–2013” grant number 02.740.11.0501.
Author contributions
The research project was conducted at Southern Federal University Research Institute of Biology (experimental part) and the Department of Obstetrics and Gynecology Rostov State Medical University (surveying, sampling, and follow-up of participants). Sample preparation, biochemical analyses, oxidative status systemic approach application, data analysis, manuscript writing: PZ; Sample preparation, ELISA analyses, anamnesis data analysis, biochemical/ELISA data analysis: AA; Sample preparation, biochemical analyses: AG; Study design, medical surveying, sampling, anamnesis data collection, patient follow-up: AS, AR; Study design, project guidance: TS.
| Abbreviations | ||
| ANOVA: | = | analysis of variance; |
| AP1: | = | activator protein 1; |
| ELISA: | = | enzyme-linked immunosorbent assay; |
| GA: | = | gestational age; |
| HELLP: | = | hemolysis, elevated liver enzyme levels, and low platelet count; |
| Imax: | = | fast burst of chemiluminescence; |
| MeSH: | = | Medical Subject Headings database; |
| NF-kappaB: | = | nuclear factor kappa B; |
| PV: | = | plateau chemiluminescence value; |
| RNS: | = | reactive nitrogen species; |
| ROS: | = | reactive oxygen species; |
| SI: | = | sum of chemiluminescence intensities; |
| TXN: | = | thioredoxin 1 (respective gene: TXN); |
| UCI: | = | uterine cervical incompetence; |
| XOR: | = | xanthine oxidoreductase. |
References
- Abuja, P.M. (1999) Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Lett 446:305–8
- Al-Gubory, K.H., Fowler, P.A. and Garrel, C. (2010) The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 42:1634–50
- Aris, A., Benali, S., Ouellet, A., Moutquin, J.M. and Leblanc, S. (2009) Potential biomarkers of preeclampsia: Inverse correlation between hydrogen peroxide and nitric oxide early in maternal circulation and at term in placenta of women with preeclampsia. Placenta 30:342–7
- Bainbridge, S.A., von Versen-Höynck, F. and Roberts, J.M. (2009) Uric acid inhibits placental system A amino acid uptake. Placenta 30:195–200
- Gauss, K.A., Nelson-Overton, L.K., Siemsen, D.W., Gao, Y., DeLeo, F.R. and Quinn, M.T. (2007) Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J Leukoc Biol 82:729–41
- Glantzounis, G.K., Tsimoyiannis, E.C., Kappas, A.M. and Galaris, D.A. (2005) Uric acid and oxidative stress. Curr Pharm Des 11:4145–51
- Hirota, K., Murata, M., Sachi, Y., Nakamura, H., Takeuchi, J., Mori, K., et al. (1999) Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem 274:27891–7
- Jaiswal, A.K. (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–207
- Johnson, R.J., Kang, D.H., Feig, D., Kivlighn, S., Kanellis, J., Watanabe, S., et al. (2003) Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 41:1183–90
- Kanellis, J., Watanabe, S., Li, J.H., Kang, D.H., Li, P., Nakagawa, T., et al. (2003) Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41:1287–93
- Kim, Y.C., Yamaguchi, Y., Kondo, N., Masutani, H. and Yodoi, J. (2003) Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene 22:1860–5
- Kops, G.J., Dansen, T.B., Polderman, P.E., Saarloos, I., Wirtz, K.W., Coffer, P.J., et al. (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419:316–21
- Levy, S., Jaiswal, A.K., and Forman, H.J. (2009) The role of c-Jun phosphorylation in EpRE activation of phase II genes. Free Radic Biol Med 47:1172–9
- Lönn, M.E., Dennis, J.M., and Stocker, R. (2012) Actions of “antioxidants” in the protection against atherosclerosis. Free Radic Biol Med 53:863–84
- Ma, S.G., Yu, W.N., Jin Y., Hong B. and Hu, W. (2012) Evaluation of serum ischemia-modified albumin levels in pregnant women with and without gestational diabetes mellitus. Gynecol Endocrinol 28:837–40
- Manea, A., Manea, S.A., Gafencu, A.V. and Raicu, M. (2007) Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem 113:163–72
- Medical Subject Headings database, National Center for Biotechnology Information, National Library of Medicine (Bethesda, MD), website: http://www.ncbi.nlm.hih.gov/mesh/ 19 jun 2013
- Moi, P., Chan, K., Asunis, I., Cao, A. and Kan, Y.W. (1995) Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA 91:9926–30
- Molvarec, A., Rigó. J. Jr, Lázár, L., Balogh, K., Makó, V., Cervenak, L., et al. (2009) Increased serum heat-shock protein 70 levels reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Cell Stress Chaperones 14:151–9
- Mutlu-Türkoğlu, U., Ilhan, E., Oztezcan, S., Kuru, A., Aykaç-Toker, G. and Uysal, M. (2003) Age-related increases in plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in elderly subjects. Clin Biochem 36:397–400
- Myatt, L. (2010) Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 31:S66–S9
- Newsholme, P., Haber, E.P., Hirabara, S.M., Rebelato, E.L., Procopio, J., Morgan, D., et al. (2007) Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol 583:9–24
- Nordberg, J. and Arnér, E.S. (2001) Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31:1287–312
- Owen, J. and Mancuso, M. (2012) Cervical cerclage for the prevention of preterm birth. Obstet Gynecol Clin North Am 39:25–33
- Pendyala, S. and Natarajan, V. (2010) Redox regulation of Nox proteins. Respir Physiol Neurobiol 174:265–71
- Poljsak, B., Šuput, D. and Milisav, I. (2013) Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid Med Cell Longev 2013:1--11 , 956792
- Reyes, M., Sifuentes-Alvarez, A. and Lazalde, B. (2006) Estrogens are potentially the only steroids with an antioxidant role in pregnancy: In vitro evidence. Acta Obstet Gynecol Scand 85:1090–3
- Rogers, M.S., Wang, C.C., Tam, W.H., Li, C.Y., Chu, K.O. and Chu, C.Y. (2006) Oxidative stress in midpregnancy as a predictor of gestational hypertension and pre-eclampsia. BJOG 113:1053–9
- Sautin, Y.Y., Nakagawa, T., Zharikov, S. and Johnson, R.J. (2007) Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 293:C584–96
- Shkurat, T.P., Lomteva, S.V., Aleksandrova, A.A., Azarin, K.V. and Chistyakov, V.A. (2008) The role of allantoin in the human reproduction. Valeology 4:32–7
- Sipkens, J.A., Hahn, N.E., Blom, H.J., Lougheed, S.M., Stehouwer, C.D., Rauwerda, J.A., et al. (2012) S-Adenosylhomocysteine induces apoptosis and phosphatidylserine exposure in endothelial cells independent of homocysteine. Atherosclerosis 221:48–54
- Suliman, H.B., Carraway, M.S., Tatro, L.G. and Piantadosi, C.A. (2007) A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci 120:299–308
- Tranquilli, A.L. and Landi, B. (2010) The origin of pre-eclampsia: From decidual “hyperoxia” to late hypoxia. Med Hypotheses 75:38–46
- Um, H.C., Jang, J.H., Kim, D.H., Lee, C. and Surh, Y.J. (2011) Nitric oxide activates Nrf2 through S-nitrosylation of Keap1 in PC12 cells. Nitric Oxide 25:161–8
- Ushio-Fukai, M., Alexander, R.W., Akers, M. and Griendling, K.K. (1998) p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 273:15022–9
- Walker, J.J. (2000) Pre-eclampsia. Lancet 356:1260–5
- Warren, J.E. and Silver, R.M. (2009) Genetics of the cervix in relation to preterm birth. Semin Perinatol 33:308–11
- Wu, R.F., Ma, Z., Liu, Z. and Terada, L.S. (2010) Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30:3553–68
- Zhang, Q., Pi, J., Woods, C.G. and Andersen, M.E. (2010) A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol 244:84–97
- Zolotukhin, P., Kozlova, Y., Dovzhik, A., Kovalenko, K., Kutsyn, K., Aleksandrova, A., et al. (2013) Oxidative status interactome map: Towards novel approaches in experiment planning, data analysis, diagnostics and therapy. Mol BioSyst 9:2085–96