Abstract
Literature regarding the effects of sildenafil citrate on sperm function remains controversial. In the present study, we specifically wanted to determine if mitochondrial dysfunction, namely membrane potential, reactive oxygen species production, and changes in energy content, are involved in in vitro sildenafil-induced alterations of human sperm function. Sperm samples of healthy men were incubated in the presence of 0.03, 0.3, and 3 μM sildenafil citrate in a phosphate buffered saline (PBS)-based medium for 2, 3, 12, and 24 hours. Sperm motility and viability were evaluated and mitochondrial function, i.e., mitochondrial membrane potential and mitochondrial superoxide production were assessed using flow-cytometry. Additionally, adenosine triphosphate (ATP) levels were determined by high performance liquid chromatography (HPLC) analysis. Results show a decrease in sperm motility correlated with the level of mitochondria-generated superoxide, without a visible effect on mitochondrial membrane potential or viability upon exposure to sildenafil. The effect on both motility and superoxide production was higher for the intermediate concentration of sildenafil (0.3 µM) indicating that the in vitro effects of sildenafil on human sperm do not vary linearly with drug concentration. Adenosine triphosphate levels also decreased following sildenafil exposure, but this decrease was only detected after a decrease in motility was already evident. These results suggest that along with the level of ATP and mitochondrial function other factors are involved in the early sildenafil-mediated decline in sperm motility. However, the further decrease in ATP levels and increase in mitochondria-generated reactive oxygen species after 24 hours of exposure might further contribute towards declining sperm motility.
Introduction
Sildenafil Citrate (Viagra®, Pfizer) was the first oral agent approved by the FDA for the treatment of erectile dysfunction (ED), in 1998. The drug amplifies the downstream effects of nitric oxide (NO)-mediated smooth muscle relaxation by inhibiting cyclic guanosine monophosphate (cGMP) degradation by PDE5, therefore facilitating penile erection [Ramani and Park Citation2010]. Sildenafil citrate concentration in both blood and seminal plasma peaks at approximately 1.5 hours after administration [Al-Ghazawi et al. Citation2007; Aversa et al. Citation2000; Gupta et al. Citation2005; Purvis et al. Citation2002], although the compound continues to be present in blood plasma at micromolar concentrations up to 24 hours after administration [Al-Ghazawi et al. Citation2007; Gupta et al. Citation2005]. Given the predicted increase in the prevalence of ED [Aytaç et al. Citation1999], and the concomitant increase in sildenafil use that may thus ensue, it seems important to determine the potential effects of the drug on several aspects related to male fertility, particularly in terms of sperm function. Previous reports on the effects of sildenafil on sperm function, carried out both in vivo and in vitro, are controversial, ranging from no changes [Burger et al. Citation2000; Purvis et al. Citation2002] to improved sperm parameters [Cuadra et al. Citation2000; Dimitriadis et al. Citation2008; Glenn et al. Citation2009].
Interestingly, the specific target for sildenafil action, phosphodiesterase-5 (PDE5), has not yet been detected in the human sperm proteome, suggesting that it may be absent or present in very low amounts [Amaral et al. 2013a]. However, other phosphodiesterases, primarily involved in dual cyclic adenosine monophosphate (cAMP)/cGMP (PDE1, PDE10, PDE11) or cAMP (PDE8, PDE6, PDE4) signaling are present [Amaral et al. 2013a]. The specific function of these phosphodiesterases in the male gamete is not well characterized, with some published data focusing on putative roles for PDE1 [Lefièvre et al. Citation2002], PDE4 [Bajpai et al. Citation2006], or PDE11 [Wayman et al. Citation2005]. Sildenafil affinity and selectivity towards all PDEs currently known to exist in human sperm is much lower than what has been described for PDE5 [Wang et al. Citation2012]. However, some effects may be functionally relevant, notably in terms of PDE1 and PDE6, the closest to PDE5, in terms of sildenafil affinity.
Alternatively, sildenafil could act via other types of mechanisms that may not even be directly related to its inhibitory activity towards phosphodiesterases. For example, in several systems sildenafil has been recently shown to affect several aspects related to mitochondrial function, including reactive oxygen species (ROS) production [Ascah et al. Citation2011; Fernandes et al. Citation2008; Wang et al. Citation2008; Whitaker et al. Citation2013]. Therefore, keeping in mind that human sperm may be exposed to relevant concentrations of sildenafil for extended periods, and that mitochondria-based events regulate different aspects of reproductive function [Ferramosca et al. Citation2012; Ramalho-Santos et al. Citation2009; Sousa et al. Citation2011] our aim was to test if and how mitochondrial dysfunction was affected by sildenafil citrate, and if these effects are relevant for sperm homeostasis. Specifically, and in addition to basic parameters (sperm motility and viability), we evaluated mitochondrial membrane potential, adenosine triphosphate (ATP) levels, and mitochondrial superoxide production, and determined how they vary in the presence of sildenafil.
Results
Sperm motility and viability
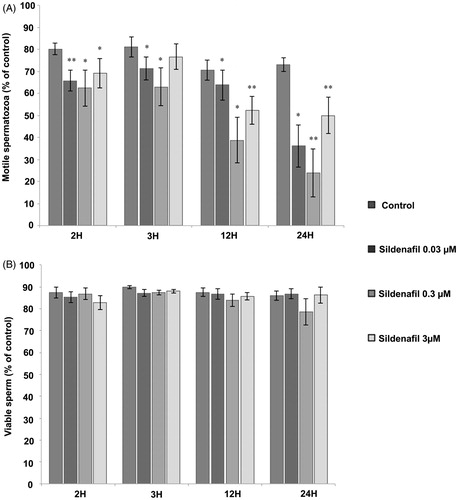
A significant decrease in sperm motility was observed after 2, 3, 12, and 24 hours of incubation without a visible effect on sperm viability, suggesting that sildenafil is not merely killing sperm, but impairing its functional activity (). Interestingly at 0.3 μM, sildenafil induced a more pronounced decrease on sperm motility than 3 μM and 0.03 μM. This data suggested that sildenafil-induced changes may involve ATP production, which we then tested.
Figure 1. Sildenafil citrate effects on sperm motility and viability. (A) Motile sperm cells. (B) Live sperm cells. Control samples were incubated in the absence of sildenafil in phosphate buffered saline (PBS)-glucose-bovine serum albumin (BSA) medium, whereas test samples were incubated for 2 hours (n = 6), 3 hours (n = 6), 12 hours (n = 8), and 24 hours (n = 6) with 0.03, 0.3, and 3 μM sildenafil citrate. Results are presented as means ± S.E.M., and are expressed as a percentage of total motile (A) and live sperm (B). *p ≤ 0.05; **p ≤ 0.01.
Sperm bioenergetical parameters
To understand if changes in motility were related to cellular energy status we evaluated possible changes in adenosine nucleotide content and energy charge for the experimental conditions in which a more drastic effect on sperm motility was observed (0.3 and 3 μM; 12 and 24 hours). As shown in , sildenafil citrate clearly decreased ATP levels and energy charge after 24 (but not 12) hours of incubation, with both 0.3 and 3 μM sildenafil citrate exerting identical effects.
Table 1. Sildenafil effect on ATP content and energy charge of human sperm exposed for 12 and 24 hours.
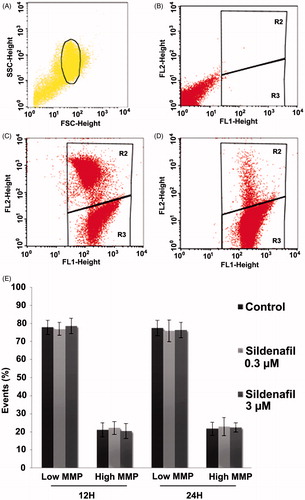
To further determine the possible contribution of mitochondria to sildenafil-induced ATP impairment, sperm mitochondrial membrane potential (MMP) was monitored using flow cytometry and the fluorescent dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1). However, no significant sildenafil-induced effects were detected ().
Figure 2. Sildenafil citrate effects on mitochondrial membrane potential (MMP). Control samples were incubated in the absence of sildenafil in phosphate buffered saline (PBS)-glucose-bovine serum albumin (BSA) medium and test samples were incubated with 0.3 and 3 µM sildenafil for 12 hours (n = 5) and 24 hours (n = 4). (A–D) Flow-cytometry dot-plot chart of a control sample. Selected sperm population (A), not labeled with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (B), labeled with JC-1 (C), and co-incubated with JC-1 and p-trifluoromethoxy carbonyl cyanide phenyl hydrazine (FCCP) (D). Red fluorescence (high MMP) was detected in the FL2 channel and the green fluorescence (low MMP) in the FL1 channel. The R2 region on the dot plots (B,C,D) represent the area with high MMP sperm and the R3 region sperm with low MMP. (E) Effects of Sildenafil on sperm mitochondrial membrane potential. MMP was determined as described in the Material and Methods section. Results are presented as mean ± S.E.M percentage of 50,000 events per sample.
Mitochondrial superoxide production
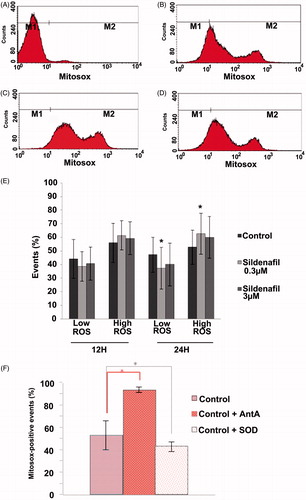
Another way mitochondria mediate cell dysfunction is via ROS production. We therefore analyzed superoxide content by flow cytometry using the fluorescent probe MitoSox-Red (MSox). No differences were observed in samples incubated with sildenafil for 12 hours. However, a significant increase of superoxide production (10%) was observed after 24 hours of incubation with 0.3 μM sildenafil, which interestingly was not observed in samples incubated with 3 μM sildenafil (). This increase in superoxide production parallels the observed effect on motility with 0.3 µM sildenafil, the concentration that most significantly decreased sperm motility.
Figure 3. Sildenafil citrate effects on mitochondrial superoxide production. Sperm was incubated with 0.3 and 3 µM sildenafil for 12 hours (n = 4) and 24 hours (n = 4) (A–D). Flow cytometry histogram of a sample not labeled with MSox (A); labeled with MitoSox-Red (MSox) (B); labeled with MSox and exposed to 0.3 µM (C) and 3 µM (D) of Sildenafil citrate. Red fluorescence was detected in the FL2 channel. The sperm population producing superoxide was included in the M2 region (MSox positive). M1 and M2 regions were defined based on appropriate controls as described in the Material and Methods section. (E) An increase in reactive oxygen species (ROS) production was observable after 24 hours of incubation with the lowest concentration of sildenafil (0.3 µM), but not with 3 µM. (F) Controls for the MSox assay, showing the typical increase upon Antimycin A (AntA) exposure, and decrease upon incubation with superoxide dismutase (SOD) of the high ROS population (see Materials and Methods for details). Results are presented as mean ± S.E.M percentage of 50 000 events per sample, *p ≤ 0.05.
Discussion
Sildenafil citrate has proven effective in the treatment of male erectile dysfunction. However the putative side effects of sildenafil on human sperm are still debated. Nanomolar range concentrations were reported to either induce an increase in sperm motility [Cuadra et al. Citation2000], or to have no effect [Burger et al. Citation2000], when samples were incubated for 3 to 4 hours. However for longer periods of incubation (24 hours), a significant decrease in motility was described [Cuadra et al. Citation2000]. In comparison, recent reports using micromolar (0.67–10 μM) concentrations of sildenafil, have been more consistent in describing an increase in sperm motility [Glenn et al. Citation2009; Mostafa Citation2007]. Conversely, the results obtained here show a significant time-dependent decrease on sperm motility, without changes in viability. Importantly, we used physiologically relevant concentrations of sildenafil found in semen, and incubation times defined according to its maximum time it could be observed in plasma.
The variability described in the literature may be related to the fact that the specific sildenafil target (PDE5) has yet to be described in human sperm, while other phosphodiesterases with lower affinity for the drug are present [Amaral et al. 2013; Wang et al. Citation2012]. Sildenafil effects on human sperm may occur via other mechanisms, and some studies have suggested sildenafil-mediated changes in cellular bioenergetics not necessarily via cGMP-related phenomena [Ascah et al. Citation2011; Fernandes et al. Citation2008; Wang et al. Citation2008; Whitaker et al. Citation2013].
Interestingly, the effects of sildenafil on sperm motility were not directly related to its concentration, since at 0.3 μM it promoted a more extensive effect on sperm motility than either 0.03 or 3 μM. These findings are corroborated by previous work in which the relationship between sildenafil concentration and its effects on sperm motility was also not linear [Mostafa Citation2007]. Presuming sildenafil is inhibiting relevant sperm phosphodiesterases, this effect might reflect changes in cyclic nucleotide-gated sperm channels resulting in non-linear changes in Ca2+ influx [Wiesner et al. Citation1998]. This would need to be demonstrated by measuring both cyclic guanosine monophosphate (cGMP)/cyclic adenosine monophosphate (cAMP) content and calcium movements in human sperm upon sildenafil exposure in the conditions described here.
As sperm motility is related to cellular bioenergetics [WHO Citation2010; Ferramosca et al. Citation2012] we analyzed sperm energy status by quantifying adenosine nucleotide content and energy charge. Our data showed that sperm ATP content and energy charge were significantly lower for sperm samples incubated with both 0.3 and 3 μM sildenafil citrate for 24 hours, and this decrease may account for the more pronounced long term effects observed in sperm motility. Surprisingly, both concentrations had the same effect. Given that motility was affected earlier than this change in energy charge, ATP production and availability may not be the only factor involved. Alternatively, sildenafil might induce changes in sperm motor machinery, in a similar manner as other drugs such as metronidazole [Mudry et al. Citation2007]. Given that a cell will use all available sources to produce ATP when stressed, it is perhaps not surprising that reduced ATP levels were only observed after the initial effects on motility.
Although changes in mitochondrial membrane potential (MMP) were clearly not involved in the effects reported here, it should be noted that mitochondria are also the main producers of ROS [Murphy Citation2009] known to be involved in a wide range of pathologies including some that involve human sperm [Tremellen Citation2008]. Keeping this in mind, we analyzed superoxide production using MSox and detected a significant increase of mitochondrial superoxide production with 0.3 μM sildenafil, but not 3 µM, somewhat mirroring changes in motility. This increase in superoxide production was observed after 24 hours, when changes in ATP levels were also evident. This is not surprising as an increase in ROS production can target mitochondria, affecting components involved in ATP production, including the ATP synthase itself [Rexroth et al. Citation2012]. Additionally ROS can damage other cellular components, such as membranes and macromolecules [Tremellen Citation2008], which might be important for motility. It might seem odd that changes in ROS production were not paralleled by changes in MMP. However, we have recently described the same pattern in human sperm after exposure to UVB irradiation [Amaral et al. 2013]. Similarly, Aitken and co-workers have reported that prolonged in vitro incubations of human spermatozoa induce a loss of motility associated with mitochondrial ROS generation without any change in MMP [Aitken et al. Citation2012]. Additionally, data on isolated rat heart mitochondria also showed that micromolar concentrations of sildenafil lead to significant changes in ROS production without altering MMP [Fernandes et al. Citation2008].
In conclusion, it seems that prolonged sildenafil citrate exposure leads to changes in sperm motility, ATP content, energy charge, and mitochondrial-generated superoxide without decreasing viability. The in vitro effects of sildenafil on human sperm are not related to changes in MMP, and do not vary linearly with relevant drug concentrations, which may account for some of the contradictory results in the literature.
Materials and Methods
Chemicals
Sildenafil citrate was obtained from Pfizer (Portugal), and the fluorescence probes SYBR-14, PI, MitoSOX-Red (M36008), and JC-1 (T3168) were acquired from Invitrogen/Molecular Probes, now Life Technologies (Eugene, OR). Sperm Preparation Medium (Origio Medicult Medium) was purchased from Origio (Mälov, Denmark). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Subjects
Human sperm samples from healthy men undergoing routine semen analysis at the Human Reproduction Service of the University Hospitals of Coimbra (Coimbra, Portugal) were used. Written informed consent was obtained from all donors involved in this study, and all biological material was processed in accordance with the Ethical and Internal Review Board (IRB) guidelines provided by the University Hospitals of Coimbra. The samples were collected by masturbation, after a 3–5 d period of sexual abstinence, and prepared by density gradient centrifugation followed by a three-hour incubation in sperm preparation medium (SPM). Standard semen analyses were carried out according to the WHO [Citation2010] guidelines, and only normozoospermic samples, without leucocytes or other round cells, were used in this study, as described previously [Alçada-Morais et al. Citation2013].
Experimental design
Sperm concentration was determined using a Neubauer hematocytometer, after sample immobilization via osmotic shock. Separate aliquots containing 107 spermatozoa/ml were then incubated in the presence of 0.03, 0.3, and 3 μM of sildenafil citrate in a PBS-based medium (PBS, 0.1 g/L CaCl2, 0.1 g/L MgCl2, 5 mM glucose, 3 g/L BSA, 1% (v/v) penicillin/streptomycin, pH 7.2–7.4), previously defined for long term sperm incubations [Amaral et al. Citation2011], supplemented with 1 mM sodium pyruvate, 10 mM sodium lactate for 2, 3, 12, and 24 h at 37°C, 5% CO2. Sildenafil concentrations were chosen given the physiological concentrations of sildenafil observed in the seminal plasma of men taking 100 mg Viagra®, as well as the concentrations used in previous studies [Aversa et al. Citation2000; Al-Ghazawi et al. Citation2007; Gupta et al. Citation2005; Purvis et al. Citation2002]. Several parameters were monitored including sperm viability and motility, adenosine nucleotide content, MMP, and ROS production, as described below.
Sperm viability and motility
Sperm viability was assessed using the LIVE/DEAD assay [Amaral and Ramalho-Santos Citation2010; Baptista et al. Citation2013]. A small volume (50 μl) of the sperm sample (107/ml) was simultaneously incubated in the medium described above with the two DNA stains SYBR-14 (100 nM) and PI (240 nM), for 20 min at 37°C. The percentages of live and dead sperm cells were determined using a Leica fluorescence microscope and a total of 100 spermatozoa were counted for each sample. Sperm motility was assessed after 2, 3, 12, and 24 h of incubation with the various concentrations of sildenafil citrate by phase contrast optical microscopy, using a Nikon Eclipse E200 microscope (20x). Results were expressed as percentage of total motile sperm (progressive + in situ), according to the WHO [Citation2010] directives.
Adenosine nucleotide content
Intracellular adenosine nucleotide content (ATP, ADP, and AMP) was determined after sperm extraction with 0.6 M perchloric acid supplemented with 25 mM sodium ethylenediaminetetraacetic acid (EDTA-Na). Sperm cells (2.5 × 107/ml) were centrifuged (14,000 rpm, 2 min at 4°C). The supernatants were neutralized with a drop wise addition of 3 M KOH in 1.5 M Tris and assayed for ATP, ADP, and AMP content by reverse-phase high performance liquid chromatography (HPLC), according to a previously described procedure [Amaral et al. Citation2006]. The HPLC system was a Beckman-System Gold, consisting of a computer-controlled 126 Binary Pump Model and 166 Variable UV detector. The detection wavelength was 254 nm, and the column was a Lichrosphere 100 RP-18 (5 mm) from Merck. An isocratic elution with 100 mM phosphate buffer (KH2PO4; pH 6.5) and 1.2% methanol was performed with a flow rate of 1 ml/min. Peak identity was determined by following the retention time of standards. Adenylate energy charge (AEC) was calculated according to the following formula: 0.5 × (ADP + 2ATP)/(ATP + ADP + AMP).
Mitochondrial membrane potential
Sperm MMP was evaluated by flow cytometry using the fluorescent dye JC-1, which can selectively enter mitochondria and shift fluorescent emission from green (low MMP) to red (high MMP). The latter represents sperm with very active mitochondria [Amaral and Ramalho-Santos Citation2010]. Primary stocks of JC-1 were prepared in dimethyl sulfoxide (DMSO) and stored at −20°C. Working stocks were prepared daily in medium, immediately prior to use. Sperm samples (2.5 × 106/ml) were incubated with JC-1 (2 μM) for 15 min at 37°C, in the dark. For each assay two controls were prepared, one where the sample was incubated in the absence of dye and another where the sample was simultaneously incubated with the dye and p-trifluoromethoxy carbonyl cyanide phenyl hydrazine (FCCP) (50 μM), an uncoupler that collapses MMP. Subsequently, sperm samples were washed (1800 rpm, 5 min) in medium and both red and green JC-1 fluorescence assessed by flow cytometry.
Mitochondrial superoxide production
The concentration of mitochondrial superoxide was determined using the MitoSOX-Red probe (MSox), which detects superoxide production, according to previously described methodology [Koppers et al. Citation2008]. A stock solution of 5 mM MSox was prepared in DMSO and then diluted and added to sperm samples (2.5 × 106) at a final concentration of 3 µM. Sperm were then incubated for 15 min at 37°C, in the dark. Appropriate controls were prepared: a positive control incubated with Antimycin A (AntA) (80 µM), known to produce a burst of superoxide (and therefore an increase in MSox fluorescence), and a negative control with superoxide dismutase (SOD) (250 U/ml), known to scavenge superoxide. Sperm suspension was then washed (1800 rpm, 5 min), ressuspended in fresh media, and MSox fluorescence assessed by flow cytometry.
Flow cytometry analysis
Flow cytometry was carried out after sperm incubation with fluorescent probes described above, and following the treatments described. The cytometer used was a Becton Dickinson FACSCalibur cell analyzer with an argon laser that operates with an excitation wavelength of 488 nm coupled with the following emission filters: 530/30 band pass (FL-1 channel/green), 585/42 band pass (FL-2 channel/red), and >620 nm long pass filter (FL-3 channel/far red). Data was collected and analyzed using Cell Quest Pro Acquisition program. Non sperm-specific events were gated out and 50 000 events were examined per assay.
Statistical analysis
Statistical analysis was performed using the SPSS software version 20.0 for Windows (SPSS Inc, Chicago, IL). All variables were tested for a normal distribution using the Shapiro-Wilk test, and the homogeneity of variances was also assessed using the Levene test. Results are presented as mean ± S.E.M. of the number of experiments indicated. Statistical significance (p) between control samples and samples exposed to each substance was accessed using the Mann–Whitney test or the t-Test according to the normality of each parameter.
Declaration of interest
The authors declare no conflict of interests. SA, RST, and CP are recipients of FCT fellowships (SFRH/BPD/63190/2009, SFRH/BD/46002/2008 and SFRH/BD/51193/2010, respectively). Center for Neuroscience and Cell Biology (CNC) funding is supported by FCT (PEst-C/SAU/LA0001/2011).
Author contributions
Conceived and designed the research study: MIS, SA, JRS; Performed the experiments: MIS, SA; Analyzed and discussed the data: MIS, SA, RST, CP, JRS; Wrote the paper: MIS, SA, JRS.
| Abbreviations | ||
| ADP | = | adenosine diphosphate |
| AEC | = | adenylate energy charge |
| AntA | = | Antimycin A |
| ATP | = | adenosine triphosphate |
| BSA | = | bovine serum albumin |
| Ca2+ | = | Calcium ion |
| CaCl2 | = | calcium chloride |
| cAMP | = | cyclic adenosine monophosphate |
| cGMP | = | cyclic guanosine monophosphate |
| DMSO | = | dimethyl sulfoxide |
| ED | = | erectile dysfunction |
| EDTA-Na | = | sodium ethylenediaminetetraacetic acid |
| FCCP | = | p-trifluoromethoxy carbonyl cyanide phenyl hydrazine |
| FDA | = | food and drug administration |
| HPLC | = | high performance liquid chromatography |
| IRB | = | internal review board |
| JC-1 | = | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide |
| KH2PO4 | = | potassium phosphate monobasic |
| KOH | = | potassium hydroxide |
| MgCl2 | = | magnesium chloride |
| MMP | = | mitochondrial membrane potential |
| Msox | = | MitoSox-Red |
| NO | = | nitric oxide |
| PBS | = | phosphate buffered saline |
| PDE5 | = | phosphodiesterase-5 |
| PI | = | propidium iodide |
| ROS | = | reactive oxygen species |
| SOD | = | superoxide dismutase |
| SPM | = | sperm preparation medium |
| WHO | = | World Health Organization. |
Acknowledgments
The authors would like to thank the Human Reproduction Service of the University Hospitals of Coimbra, under the direction of Professor Teresa Almeida Santos, particularly Ana Paula Sousa for sperm sample collection and analysis, as well as M. Baptista and B. Lourenço for technical assistance. J. Saints is acknowledged for language correction, C. Gomes and I. Almeida for statistical review, I. Nunes for assistance in flow cytometry data analysis, M.S. Santos for outstanding assistance with HPLC analysis, and all the members of the Biology of Reproduction and Stem Cell Group for useful discussions. This work was carried out, in part, in order to fulfill the requirements for a Masters in Biochemistry Degree, Department of Life Sciences, University of Coimbra (MIS).
References
- Aitken, R.J., Gibb, Z., Mitchell, L., Lambourne, S.R., Connaughton, H.S., and De Iuliis, G.N. (2012) Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod 87:1--11
- Alçada-Morais, S., Sousa, A.P., Paiva, A., Almeida-Santos, T., and Ramalho-Santos J. (2013) Anterior positioning of sex chromosomes on the head of human sperm sorted using visible wavelengths. Syst Biol Reprod Med 59:223–6
- Al-Ghazawi, M., Tutunji, M., and Aburuz, S. (2007) Simultaneous determination of sildenafil and N-desmethyl sildenafil in human plasma by high-performance liquid chromatography method using electrochemical detection with application to a pharmacokinetic study. J Pharm Biomed Anal 43:613–8
- Amaral, A., and Ramalho-Santos, J. (2010) Assessment of mitochondrial potential: implications for the correct monitoring of human sperm function. Int J Androl 33:180–6
- Amaral, S., Moreno, A.J., Santos, M.S., Seiça, R., and Ramalho-Santos, J. (2006) Effects of hyperglycemia on sperm and testicular cells of Goto-Kakizaki and streptozotocin-treated rat models for diabetes. Theriogenology 66:2056–67
- Amaral, A., Paiva, C., Baptista, M., Sousa, A.P., and Ramalho-Santos, J. (2011) Exogenous glucose improves long-standing human sperm motility, viability, and mitochondrial function. Fertil Steril 96:848–50
- Amaral, A., Castillo, J., Ramalho-Santos, J. and Oliva, R. (2013a) The combined human sperm proteome: cellular pathways and implications for basic and clinical science. Hum Reprod Update 2013 Sep 29. [Epub ahead of print]. doi: 10.1093/humupd/dmt046
- Amaral, S., Redmann, K., Sanchez, V., Mallidis, C., Ramalho-Santos, J. and Schlatt, S. (2013b) UVB irradiation as a tool to assess ROS-induced damage in human spermatozoa. Andrology 1:707--14
- Ascah, A., Khairallah, M., Daussin, F., Bourcier-Lucas, C., Godin, R., Allen, B.G., et al. (2011) Stress-induced opening of the permeability transition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am J Physiol Heart Circ Physiol 300:H144–53
- Aversa, A., Mazzilli, F., Delfino, M., Isidori, A. M., and Fabbri, A. (2000) Effects of sildenafil (Viagra™) administration on seminal parameters and post-ejaculatory refractory time in normal males. Hum Reprod 15:131–4
- Aytac, I.A., McKinlay, J.B., and Krane, R.J. (1999) The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU international 84:50–6
- Baptista, M., Publicover, S.J., and Ramalho-Santos, J. (2013) In vitro effects of cationic compounds on functional human sperm parameters. Fertil Steril 99:705–12
- Bajpai, M., Fiedler, S.E., Huang, Z., Vijayaraghavan, S., Olson, G.E., Livera, G., et al. (2006) AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol Reprod 74:109–18
- Burger, M., Sikka, S.C., Bivalacqua, T.J., Lamb, D. J., and Hellstrom, W.J. (2000) The effect of sildenafil on human sperm motion and function from normal and infertile men. Int J Impot Res 12:229–34
- Cuadra, D.L., Chan, P.J., Patton, W.C., Stewart, S.C., and King, A. (2000) Type 5 phosphodiesterase regulation of human sperm motility. Am J Obstet Gynecol 182:1013–15
- Dimitriadis, F., Giannakis, D., Pardalidis, N., Zikopoulos, K., Paraskevaidis, E., Giotitsas, N., et al. (2008) Effects of phosphodiesterase 5 inhibitors on sperm parameters and fertilizing capacity. Asian J Androl 10:115–33
- Fernandes, M.S., Vicente, J.A.F., Santos, M.S., Monteiro, P., Moreno, A.J.M., and Custódio, J.B.A. (2008) Sildenafil citrate concentrations not affecting oxidative phosphorylation depress H 2 O 2 generation by rat heart mitochondria. Mol Cell Biochem 307:77–85
- Ferramosca, A., Provenzano, S.P., Coppola, L., and Zara, V. (2012) Mitochondrial respiratory efficiency is positively correlated with human sperm motility. Urology 79:809–14
- Glenn, D.R.J., McClure, N., Cosby, S.L., Stevenson, M., and Lewis, S.E.M. (2009) Sildenafil citrate (Viagra) impairs fertilization and early embryo development in mice. Fertil Steril 91:893–9
- Gupta, M., Kovar, A., and Meibohm, B. (2005) The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J Clin Pharmacol 45:987–1003
- Koppers, A.J., De Iuliis, G.N., Finnie, J.M., McLaughlin, E.A., and Aitken, R.J. (2008) Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 93:3199–207
- Lefièvre, L., de Lamirande, E., and Gagnon C. (2002) Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod 67:423–30
- Mostafa, T. (2007) In vitro sildenafil citrate use as a sperm motility stimulant. Fertil Steril 88:994–96
- Mudry, M.D., Palermo, A.M., Merani, M.S., and Carballo, M.A. (2007) Metronidazole-induced alterations in murine spermatozoa morphology. Reprod Toxicol 23:246–52
- Murphy, M.P. (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
- Purvis, K., Muirhead, G.J., and Harness, J. (2002) The effects of sildenafil on human sperm function in healthy volunteers. Br J Clin Pharmacol 53:53S–60S
- Ramalho-Santos, J., Varum, S., Amaral, S., Mota, P.C., Sousa, A.P., and Amaral, A. (2009) Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Update 15:553–72
- Ramani, G.V., and Park, M.H. (2010) Update on the clinical utility of sildenafil in the treatment of pulmonary arterial hypertension. Drug Des Devel Ther 4:61–70
- Rexroth, S., Poetsch, A., Rögner, M., Hamann, A., Werner, A., Osiewacz, H.D., et al. (2012) Reactive oxygen species target specific tryptophan site in the mitochondrial ATP synthase. Biochim Biophys Acta 1817:381–7
- Sousa, A.P., Amaral, A., Baptista, M., Tavares, R., Caballero Campo, P., Caballero Peregrín, P., et al. (2011) Not all sperm are equal: functional mitochondria characterize a subpopulation of human sperm with better fertilization potential. PloS one 6:e18112
- Tremellen, K. (2008) Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update 14:243–58
- Wang, X., Fisher, P.W., Xi, L., and Kukreja, R.C. (2008) Essential role of mitochondrial Ca2+-activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. J Mol Cell Cardiol 44:105–13
- Wang, R., Burnett, A.L., Heller, W.H., Omori, K., Kotera, J., Kikkawa, K., et al. (2012) Selectivity of avanafil, a PDE5 inhibitor for the treatment of erectile dysfunction: implications for clinical safety and improved tolerability. J Sex Med 9:2122–9
- Wayman, C., Phillips, S., Lunny, C., Webb, T., Fawcett, L., Baxendale, R., and Burgess, G. (2005) Phosphodiesterase 11 (PDE11) regulation of spermatozoa physiology. Int J Impot Res 17:216–23
- Whitaker, R.M., Wills, LP, Stallons, L.J. and Schnellmann, R.G. (2013) cGMP-Selective Phosphodiesterase Inhibitors Stimulate Mitochondrial Biogenesis and Promote Recovery from AKI. J Pharmacol Exp Ther Sep 16 347:626--34
- Wiesner, B., Weiner, J., Middendorff, R., Hagen, V., Kaupp, U.B., and Weyand, I. (1998) Cyclic Nucleotide-gated Channels on the Flagellum Control Ca2+ Entry into Sperm. J Cell Biol 142:473–84
- World Health Organization (2010) WHO laboratory manual for the examination and processing of human semen. WHO Press: Geneva
