Abstract
We successfully performed preimplantation genetic diagnosis (PGD) and simultaneous preimplantation genetic screening (PGS) using single nucleotide polymorphism (SNP) microarrays for couples with balanced chromosome rearrangements in China. A total of 428 molecular karyotypes were diagnosed from 62 couples undergoing 68 in vitro fertilization (IVF) cycles. Of these, 48.1% of the embryos were chromosomally normal without translocation errors or aneuploidy. Of the 428 total embryos, 18.0% embryos were euploid, but were imbalanced due to the transmission of single translocation chromosome derivatives. A total of 6.5% of the embryos had chromosome abnormalities involving the parental chromosome aberration and other chromosomes aneuploidies. Significantly, 27.4% of the embryos were normal/balanced for the rearranged chromosomes, but were abnormal due to aneuploidy affecting other chromosomes. When evaluated on a per IVF cycle basis, 84% of the cycles had at least one chromosomally normal embryo available for uterine transfer. The clinical pregnancy rate per IVF cycle was 54%. Diagnosing genomically balanced embryos through 24 chromosome SNP microarray PGD/PGS, rather than minimally targeted fluorescence in situ hybridization (FISH), is a promising strategy to maximize the pregnancy potential of patients with known parental chromosomal translocations. Moreover, this is the first study to report the clinical application of SNP arrays to screen all 24 chromosome pairs of blastomeres and trophectoderm cells from patients carrying reciprocal translocations in China.
Introduction
Chromosome balanced rearrangements, such as translocations and inversions, are common forms of parental structural chromosome abnormalities. Individuals who carry a balanced translocation or inversion are known to have high rates of unbalanced gametes following meiotic segregation and are at great risk of producing embryos with unbalanced chromosome complements. These chromosome imbalances can lead to high rates of miscarriage, decreased fertility, and in some cases, the birth of children affected by congenital aberrations [Escudero et al. Citation2008; Treff et al. Citation2011].
The frequency of chromosomal rearrangements is about 30-fold higher (5.34%) than found in the general population [Frynes and Van Buggenhout Citation1998]. The frequency of chromosomal translocations is relatively high, affecting about 0.6% of infertile couples. In couples suffering from recurrent pregnancy loss, the frequency of parental translocations is far higher, greater than 9% in some studies [Stern et al.Citation1999]. In couples with recurrent pregnancy loss (RPL) and a documented balanced chromosomal rearrangement in one or both parents, preimplantation genetic diagnosis (PGD) has been shown to be beneficial in improving pregnancy and live birth rates by reducing the risk of transferring chromosomally unbalanced embryos, thereby reducing recurrent spontaneous abortions and minimizing the risk of conceiving a chromosomally abnormal baby [Garrisi et al. Citation2009; Hodes-Wertz et al. Citation2012; Otani et al. Citation2006; Treff et al. Citation2011].
Traditionally, fluorescence in situ hybridization (FISH) is the most widely used method to distinguish chromosomally balanced embryos from unbalanced due to parental reciprocal translocation carriers [Munné et al. Citation2000; Verlinsky et al. Citation2005; Harper and Harton Citation2010]. This type of FISH-PGD has proved effective, resulting in thousands of healthy babies. However, the historical FISH-PGD clinical pregnancy rates are only between 33–40% [Harper et al. Citation2012]. The limitations of FISH-PGD for parental translocations are primarily due to the inability to simultaneously test for all 24-pairs of chromosomes for aneuploidy and structural chromosome imbalance.
Recently, comprehensive chromosome screening techniques, such as array comparative genomic hybridization [Alfarawati et al. Citation2011; Colls et al. Citation2012; Fiorentino et al. Citation2011] and single nucleotide polymorphism arrays [Brezina et al. Citation2011; Treff et al. Citation2010] have been introduced into PGD laboratory practices. Detecting structural chromosomal abnormalities and aneuploidy for all 24-pairs of chromosomes is thought to be of benefit to couples with a balanced translocation. While these initial results were promising and showed increased pregnancy rates as compared to FISH, the total numbers of patients evaluated in these studies were limited. Here, we report the first pregnancies in China achieved following PGD for parental chromosome rearrangements using single nucleotide polymorphism (SNP) microarrays for the simultaneous identification of translocation imbalances and aneuploidy screening for all 24-pairs of chromosomes.
Results and Discussion
We report PGD/PGS data from 475 embryos obtained from 62 couples undergoing 68 IVF cycles in which one partner had a balanced reciprocal translocation. The results are summarized in . Of the 68 cycles reviewed, 76% (52/68) of the biopsies were done at the cleavage stage and 24% (16/68) of the cycles were biopsied at the blastocyst stage. We obtained molecular karyotypes from 90.1% (428/475) of biopsied embryos. In embryos with failed DNA amplification, this strongly correlated with poor embryo quality and likely DNA fragmentation.
Figure 1. Proportion of embryos resulting from SNP array-based karyotyping. This figure shows the breakdown of various molecular karyotypes obtained from the embryos evaluated in this study. Blue indicates the percentage of euploid embryos, purple indicates the percentage of embryos with unbalanced translocation errors of maternal origin, green indicates the percentage of embryos with unbalanced translocation errors of paternal origin, and red indicates the percentage of embryos with aneuploid errors not of parental translocation origin.
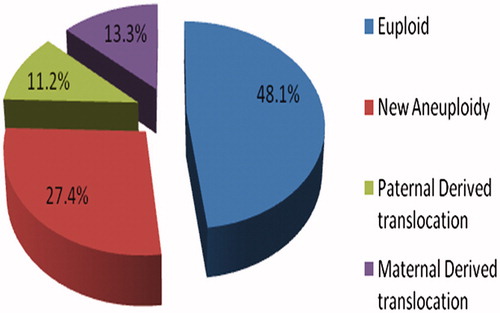
Of the 428 embryos diagnosed by SNP microarrays, 48.1% (206/428) were euploid for all chromosomes tested and without an inherited unbalanced parental translocation chromosome, 18.0% (77/428) were euploid but had a parentally derived unbalanced segregant, 27.4% (117/428) were aneuploid alone, and 6.5% (28/428) contained a parentally derived translocation imbalance and aneuploidy. Examples are shown in . Our overall results showed aneuploidy alone without the inheritance of a parental translocation chromosome imbalance in 27.4% of embryos analyzed. Traditional FISH technologies for parental translocations would have missed these abnormal embryos. As summarized in , all identified translocation errors in embryos were associated with 2:2 adjacent-1 or adjacent-2 segregation during meiosis of the parental quadrivalent chromosomes. No 3:1 segregation patterns were observed resulting in aneuploidy of the translocation chromosomes.
Figure 2. 23-chromosome SNP microarray. This figure shows a normal and an abnormal molecular karyotypic sample reading using 23-chromosome SNP microarrays. (A) shows the normal diploid diagnostic reading obtained from a blastomere for chromosome 8 which comes from 45,XY,t(14;21)(q10;q10) carrier couple. Normal AA, AB, and BB alleles and a 0 reading for the smooth log R ratio is observed. (B) demonstrates the deletion of p21.1→pter reading of chromosome 6 from a Cleavage Stage embryo which comes from 46,XX,t(4;5)(q25;p15) carrier couple. AA, AB and BB alleles are observed from p12.3 to qter of chromosome 6, however AA and BB alleles are observed without AB from p21.1 to pter of chromosome 6 represented. A significant shift in the smooth log R ratio is observed from p21.1 to pter of chromosome 6. (C) represents the normal diploid reading of chromosome 6, from a TE cell population. Normal AA, AB and BB alleles and a 0 reading for the smooth log R ratio is observed. No shifts are observed in the smooth log R ratio and B allele frequency. (D) demonstrates a monosomy reading of chromosome 14, from a TE cell population. AA and BB alleles are observed without AB alleles represented. A significant shift in the smooth log R ratio is observed, consistent with the monosomy karyotype.
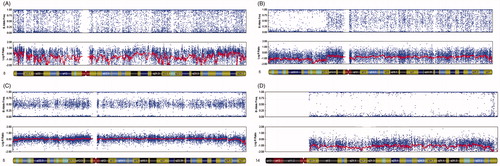
Table 1. Embryos resulting from couples undergoing preimplantation genetic diagnosis (PGD) for known parental rearrangements.
Of the 68 IVF cycles in this study, 84% of couples had at least one normal embryo that was both euploid and genetically balanced for the parentally inherited translocation chromosome and available for uterine transfer. The average number of embryos transferred was 1.3. The clinical pregnancy rates following SNP analysis was 54% per cycle and was higher if the biopsy was done on blastocysts (65.6%) versus cleavage stage embryos (52%). The average size of the chromosomal imbalance due to the transmission of unbalanced translocations was 40 megabases (Mb) for duplication errors and 35 Mb for deletion errors.
In couples with RPL and a documented reciprocal translocation PGD has been utilized in an attempt to improve pregnancy and live birth rates [Otani et al. Citation2006]. This report is the first demonstration in China of the simultaneous application of PGD and PGS in couples with documented chromosomal translocations using 23-chromosome SNP microarrays. The 23-chromosome SNP microarray platform utilized in this study is superior to FISH because it can simultaneously test for all 23-pairs of chromosomes for aneuploidy and test for the parental translocation imbalance [Brezina et al. Citation2012].
In our study, 27.4% of all embryos evaluated possessed aneuploid errors without any translocation imbalance. Fluorescence in situ hybridization-PGD focuses exclusively on the chromosomes involved in the known parental translocation. Therefore, FISH would have failed to diagnose these embryos with aneuploidy of chromosomes other than the parental translocations. These embryos could have been selected for possible transfer if only translocation PGD had been performed.
The approximate pregnancy rates obtained from FISH-PGD by our group and other laboratories in referred cases due to translocations has been approximately 33–40%. In this study, we have shown that comprehensive microarray technology is capable of substantially improving pregnancy rates beyond FISH alone (33–40% vs. >50%). This increase is most likely due to the ability to diagnose aneuploid embryos that would have been missed with the use of FISH-PGD alone.
Additionally, SNP microarrays do not require the technically demanding and complicated procedure of nuclei fixation onto slides as required using FISH technologies. The removal of this complicated step simplifies the sample preparation procedure and enables broader use of PGD/PGS in IVF programs.
Single nucleotide polymorphism arrays can also diagnose complex chromosome translocation imbalances. Such diagnoses using FISH are extremely difficult, technically demanding, and most likely unable to be successfully diagnosed. In one case during this study, the male had a complex chromosome rearrangement karyotype that was 46,XY,t(2;4)(q21;q31),t(2;5)(p23;q35), and the female was normal 46, XX.
One limitation of any modality used for PGD/PGS IVF for reciprocal translocations is the ability to accurately diagnose very small genetic imbalances. The platform used by our laboratory utilizes a dense SNP microarray that detects approximately 300,000 genetic markers. Utilizing this dense microarray platform, we were able to identify all parental translocation imbalances in embryos analyzed that the resolution of SNP microarray can detect.
Another limitation of PGD IVF using SNP microarrays is that balanced translocations cannot be differentiated from normal karyotypes. Single nucleotide polymorphism microarrays will not be able to provide assurance that the transferred embryo does not harbor a balanced translocation chromosome, only that the transferred embryo is genetically balanced.
Diagnosing viable embryos through whole genome SNP microarray PGD/PGS is a promising strategy to maximize the pregnancy potential of patients with balanced translocations. Our study reports the first pregnancies following PGD/PGS for parental reciprocal translocations by SNP arrays using both cleavage-stage and blastocyst biopsies in China.
Materials and Methods
All patients with balanced reciprocal chromosomal rearrangements, at risk of producing abnormal gametes leading to miscarriages or abnormal offspring, and desiring to undergo PGD/PGS, were assessed by geneticists and reproductive endocrinologists at our clinic. Genetic counseling involved reviewing the couple’s three-generation family history, an explanation of the PGD/PGS process as well as the accuracy and the limitations of the microarray PGD/PGS. Possible genetic outcomes, success rates, and the risks of misdiagnosis were also discussed. All women enrolled in this study were documented to have normal ovarian reserve, defined by a follicle stimulating hormone (FSH) level of less than 10 mlU/ml, and no other gynecologic abnormalities including anatomical uterine defects. A written informed consent was obtained from all patients, in which the possible risk of misdiagnosis was specified and confirmatory prenatal diagnosis for any ensuring pregnancy was recommended and Institutional Review Board (IRB) approval for this study was granted.
We conducted a retrospective review of PGD/PGS results from 475 embryos generated from 62 couples undergoing 68 IVF cycles. The mean maternal age was 32 and the mean paternal age was 34. All couples underwent standard IVF protocols using controlled ovarian hyperstimulation (COH). The COH, oocyte retrieval, fertilization, and embryo culture protocols were managed by the IVF clinicians and embryologists from their respective institutions. All embryos underwent a biopsy on either day-3 of cleavage stage development or at the blastocyst stage. One cell at the cleavage stage or multiple cells from the trophectoderm were placed in 5 µL of DNA stabilizing buffer (0.2M KOH) for SNP microarray analysis. The cell samples from each embryo then underwent cell lysis, DNA extraction, and a whole genome amplification (WGA) protocol. For SNP arrays, the cells were first lysed using an alkaline denaturation buffer (0.2 M NaOH) followed by a 4 h modified multiple displacement amplification (MDA) protocol using phi 29 polymerase to generate template DNA. Four microliters (200 ng) of each MDA amplified DNA product then underwent a 13 h WGA amplification protocol again using phi 29 polymerase. Each amplified DNA product then underwent enzymatic end-point fragmentation followed by DNA precipitation and resuspension in hybridization buffer. The fragmented, resuspended DNA samples were dispensed onto Human CytoSNP-12 DNA analysis beadchips (Illumnia, San Diego, CA, USA) and allowed to hybridize for 12 h. Following hybridization, the beadchips underwent immunostaining followed by stringency washes to remove un-hybridized and non-specifically hybridized DNA. The beadchips were dried in a desiccator using a vacuum and scanned using an Illumina iScan Bead Array Reader. Raw data analysis was accomplished using Illumina Genome Studio software and Karyostudio software. An embryonic cell normalized data set was established as the reference database before the study and the clinical data was compared to the reference data to remove SNPs with poor or incomplete genotype information. Data from the Illumina system was interpreted to establish whether each embryo was normal diploid and/or had a genomic imbalance associated with the parental reciprocal translocation chromosome or aneuploidy. Y chromosome-specific PCR was performed to distinguish the normal male karyotype from Y chromosome loss, as CytoSNP-12 beadchip is less able to detect Y chromosome.
Embryos free of any genetic imbalances due to the parental translocation and euploid were considered for transfer on D5 or D6 based upon the embryo development. Clinical pregnancy was determined by ultrasound demonstration of a gestational sac coupled with fetal heart beat at 5–6 w after embryo transfer. Prenatal diagnosis was recommended for all pregnancies.
Declaration of interest
This study was supported by the grants from key project of Henan Province (112102310103), medical tackle key problems in science and technology of Henan Province (2011020011, 2011020045), and Youth Innovation Project of the First Affiliated Hospital of Zhengzhou University. The authors have no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Author contributions
Conceived and designed the study: GL,Y-pS; Performed the study: GL, H-xJ, Z-mX, Y-cS, PRB,ATB,WGK; Analyzed the data: Y-pS, PRB; Wrote the manuscript: GL, WGK.
| Abbreviations | ||
| PGD | = | preimplantation genetic diagnosis |
| PGS | = | preimplantation genetic screening |
| SNP array | = | single nucleotide polymorphism microarray |
| IVF-ET | = | in vitro fertilization-embryo transfer CGH: comparative genomic hybridization |
| FISH | = | fluorescence in situ hybridization |
| RPL | = | recurrent pregnancy loss |
| BAF | = | B allele frequency |
References
- Alfarawati, S., Fragouli, E., Colls, P., and Wells, D. (2011) First births after preimplantation genetic diagnosis of structural chromosome abnormalities using comparative genomic hybridization and microarray analysis. Human Reprod 26:1560–74
- Brezina, P.R., Brezina, D.S., and Kearns, W.G. (2012) Preimplantation genetic testing. BMJ 18;345:e5908
- Brezina, P.R., Benner, A., Rechitsky, S., Kuliev, A., Pomerantseva, E., Pauling, D., et al. (2011) Single-gene testing combined with single nucleotide polymorphism microarray preimplantation genetic diagnosis for aneuploidy: a novel approach in optimizing pregnancy outcome. Fertil Steril 95:1786.e5–8
- Colls, P., Escudero, T., Fischer, J., Cekleniak, N.A., Ben-Ozer, S., Meyer, B., et al. (2012) Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod Biomed Online 24:621–9
- Escudero, T., Estop, A., Fischer, J., and Munne, S. (2008) Preimplantation genetic diagnosis for complex chromosome rearrangements. Am J Med Genet 146:1662–9
- Fiorentino, F., Spizzichino, L., Bono, S., Biricik, A., Kokkali, G., Rienzi, L., et al. (2011) PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Human Reprod 26:1925–35
- Fryns, J.P. and Van Buggenhout, G. (1998) Structural chromosome rearrangements in couples with recurrent fetal wastage. Eur J Obstet Gynecol Reprod Biol 81:171–6
- Garrisi, J.G., Colls, P., Ferry, K.M., Zheng, X., Garrisi, M.G., and Munné, S. (2009) Effect of infertility, maternal age, and number of previous miscarriages on the outcome of preimplantation genetic diagnosis for idiopathic recurrent pregnancy loss. Fertil Steril 92:288–95
- Harper, J.C., and Harton,G. (2010) The use of arrays in preimplantation genetic diagnosis and screening. Fertil Steril 94:1173–7
- Harper, J.C., Wilton, L., Traeger-Synodinos, J., Goossens, V., Moutou, C., SenGupta, S.B., et al. (2012) The ESHRE PGD consortium: 10 years of data collection. Hum Reprod Update 18:234–47
- Hodes-Wertz, B., Grifo, J., Ghadir, S., Kaplan, B., Laskin, C.A., Glassner, M., et al. (2012) Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertil and Steril 98:675–80
- Munné, S., Sandalinas, M., Escudero, T., Fung, J., Gianaroli, L., and Cohen, J. (2000) Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril 73:1209–18
- Otani, T., Roche, M., Mizuike, M., Colls, P., Escudero, T., and Munné, S. (2006) Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod Biomed Online 13:869–74
- Stern, C., Pertile, M., Norris, H., Hale, L., and Baker, H.W. (1999) Chromosome translocations in couples with in-vitro fertilization implantation failure. Human Reprod 14:2097–2101
- Treff, N.R., Su, J., Tao, X., Levy, B., and Scott, R.T. Jr. (2010) Accurate single cell 24 chromosome aneuploidy screening using whole genome amplication and single nucleotide polymorphism microarrays. Fertil Steril 94:2017–21
- Treff, N.R., Northrop, L.E., Kasabwala, K, Su, J., Levy, B., and Scott, R.T. Jr. (2011) Single nucleotide polymorphism microarraybased concurrent screening of 24-chromosome aneuploidy and unbalanced translocations in preimplantation human embryos. Fertil Steril 95:1606--12.e1--2
- Verlinsky, Y., Tur-Kaspa, I., Cieslak, J., Bernal, A., Morris, R., Taranissi, M., et al. (2005) Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod Biomed Online 11:219–25
