Abstract
Despite extensive research carried out for optimization and commercialization of sperm cryopreservation media, percentage of motility and viability remain low following cryopreservation. These adverse effects have been partially ascribed to reactive oxygen species (ROS) production during cryopreservation. Therefore, we proposed that addition of a cell permeable antioxidant like Tempol, with superoxide dismutase (SOD) mimetic action, may overcome these effects in an optimized commercially available cryo-protective medium. Therefore, semen samples were cryopreserved in the presence or absence of Tempol. A concentration of 5 μM Tempol was defined as optimal since it significantly improved motility and viability post thawing and reduced DNA fragmented sperm. In addition, percentage of ROS positive sperm was reduced. These effects of Tempol can be attributed to cell permeability characteristic and ability to reduce superoxide production both at intra- and extra-cellular levels. Tempol may hold the potential for clinical applications.
Introduction
Cryopreservation of human semen is a routine procedure at assisted reproduction centers, andrology laboratories, and sperm banks [Oehninger et al. Citation2000]. Despite a lengthy history of semen cryopreservation, post-thaw survival rate remains limited and fails to meet ideal expectations [Li et al. Citation2010; Ozkavukcu et al. Citation2008]. This phenomenon is commonly referred to as cryo-damage which includes membrane disruption, mitochondrial dysfunction, diminished motility, and deteriorated viability [O’Connell et al. Citation2002].
Several mechanisms have been proposed for sperm cryo-damage including: (1) cellular dehydration followed by osmotic shock due to the increased concentration of salts, (2) physical damage resulting from intracellular ice crystal formation, and (3) thermal shocks [Di Santo et al. Citation2012; John Morris et al. Citation2012]. In addition, the generation of oxidative stress (OS) or reactive oxygen species (ROS) is reported to contribute to this phenomenon. Disproportionate ROS production can induce lipid peroxidation (LPO), structural alterations, chromatin dysfunction, and DNA damage [Alvarez and Storey Citation1992; Thomson et al. Citation2009].
To avoid ROS induced damage, seminal plasma contains an abundance of antioxidant enzymes (superoxide dismutase (SOD) and catalase) and non-enzymatic scavengers (albumin, taurine, urates, and ascorbate) [De Lamirande et al. Citation1997; Lewis et al. Citation1997]. These antioxidants help to protect sperm from oxidants, bacteria, and leukocytes as well as oxidants produced from abnormal sperm [Bansal and Bilaspuri Citation2010]. Therefore, a reduction in antioxidant defense capacity, including loss of SOD activity [Alvarez and Storey Citation1992; Lasso et al. Citation1994] and decreased glutathione levels, may account for reduced viability and motility following semen cryopreservation [Gadea et al. Citation2011].
Accordingly, cryo-protective media are commonly supplemented with different antioxidants to improve sperm cryo-survival during the freeze/thaw process [Li et al. Citation2010; Roca et al. Citation2005; Zribi et al. Citation2012]. The antioxidants routinely used for supplementation of cryo-protective media are non-enzymatic and predominantly have scavenger activity [Gadea et al. Citation2011; Kalthur et al. Citation2011].
Tempol (4-hydroxy2, 2, 6, 6-tetramethylpiperidine-1-oxyl) is a nitroxide compound with SOD mimetic activity. This compound has been used as an antioxidant in cell culture systems to protect cells from ROS [Samuni et al. Citation1990; Wilcox and Pearlman Citation2008]. Since Tempol is a cell permeable antioxidant, researchers have proposed that it can protect cells from intracellular ROS [Wilcox Citation2010]. Therefore, this study aimed to evaluate the role of Tempol in the cryopreservation of human semen using a commercially available cryopreservation medium.
Results
Defining the optimal concentration of Tempol
Initial results revealed that regardless of the Tempol concentration, the percent of motility and viability significantly decreased following freezing and thawing compared to the pre-freezing state. Supplementation of the sperm freeze solution (SFS) with different concentrations of Tempol (0.1 to 10 mM) did not significantly affect sperm motility and viability when compared to the control (0.0 mM Tempol). However, these parameters had a tendency to be higher and lower at 0.1 mM and 10 mM Tempol compared to the control (0.0 mM Tempol), respectively (). Therefore, the effect of a lower concentration of Tempol was evaluated. These results revealed that 0.05 mM (50 µM) Tempol marginally improved motility (31.99 ± 2.45 vs. 24.64 ± 2.03; p = 0.18) and significantly improved viability (41.23 ± 2.37 vs. 31.26 ± 1.92; p = 0.02) post-thawing compared to the control (0.0 mM Tempol; ).
Figure 1. Comparison of sperm motility and viability before and after freezing. Tempol concentration was varied (A, B and C) and the effect of freezing as a function of motility and viability was assessed. The results are presented as means ± SEM. Different letters show significant difference between groups at p < 0.05.
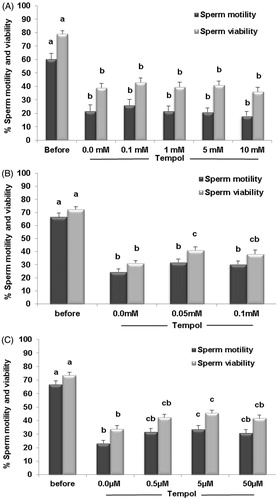
Lower concentrations of Tempol were then evaluated (0.0, 0.5, 5, and 50 µM). It was shown that at these lower concentrations, motility and viability improved and the differences were significant for 5 µM Tempol compared to the control (motility: 33.61 ± 2.66 vs. 23.10 ± 2.17; p = 0.03 and viability: 45.63 ± 1.95 vs. 33.94 ± 2.52; p = 0.00, ). Based on these results, 5 µM was considered as the optimal concentration of Tempol for supplementation of SFS.
Assessment of ROS and DNA fragmentation
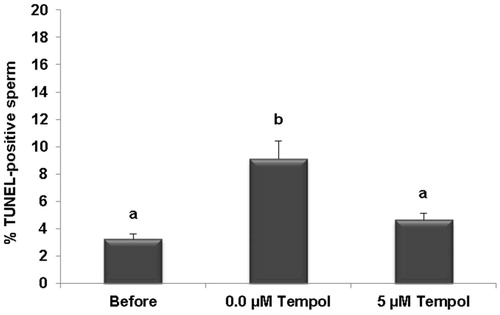
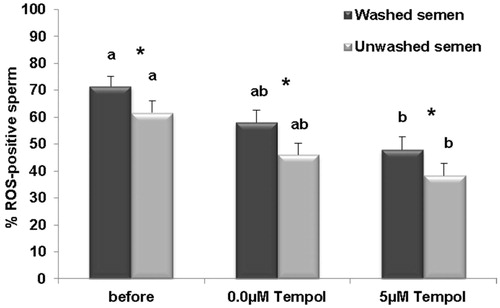
shows that the percentage of DCF or ROS-positive sperm in both washed and unwashed samples decreased following the freeze-thaw processes in both the control (0.0 µM Tempol) and Tempol treated groups (5 µM Tempol). The reduction in the percentage of ROS-positive sperm, in both washed and unwashed semen, were only significant in the Tempol group (5 µM Tempol) compared to before freezing and not significant in the control (0.0 µM Tempol) compared to before freezing. In the washed and unwashed groups, the reduction in the percentage of ROS-positive sperm was lower in the Tempol group (5 µM Tempol) compared to the control group (0.0 µM Tempol), after freezing. But this difference was insignificant. It is interesting that the percentages of ROS-positive sperm were significantly higher in the washed groups compared to the unwashed groups before freezing (71.51 ± 3.68 vs. 61.66 ± 4.40; p = 0.00) and following freezing in both the control (58.01 ± 4.65 vs. 45.99 ± 4.40; p = 0.00) and Tempol groups (47.98 ± 4.87 vs. 38.29 ± 4.68; p = 0.00). Results of the TUNEL assay revealed () that the percentage of sperm with fragmented DNA significantly increased following freezing in the control group (0.0 µM Tempol) compared to before freezing (9.11 ± 1.3 vs. 3.27 ± 0.35; p = 0.00). Tempol prevented induced DNA damage following the freeze-thaw processes compared the to control (4.66 ± 0.49 vs. 9.11 ± 1.3; p = 0.00).
Figure 2. Comparison of percentage of ROS-positive sperm, before and after freezing in the presence (5 µM) and absence (0.0 µM) of Tempol in washed and unwashed semen groups. The results are presented as means ± SEM. Different letters show significant difference between groups at p < 0.05. Stars show significant difference between washed and unwashed semen groups at p < 0.05.
Discussion
Optimization and improvement of assisted reproduction techniques (ART) profoundly influences clinical outcomes. One approach to achieve this aim is to pinpoint the underlying mechanism involved in cellular damage, in vitro [Di Santo et al. Citation2012]. It is well established that ROS production negatively influences the quality of cryopreserved materials and this effect can be partially superseded by the addition of antioxidants [Gadea et al. Citation2011; Kalthur et al. Citation2011; Li et al. Citation2010], particularly in sperm that contain minute amounts of cytoplasm with reduced antioxidant activity [Bansal and Bilaspuri Citation2010].
The basis of antioxidant supplementation to cryopreservation media relies on the work of others. This includes, antioxidant enzymes acting at the extracellular level, such as catalase [Li et al. Citation2010] and SOD [Forouzanfar et al. Citation2013; Roca et al. Citation2005]; non-enzymatic antioxidants counteracting ROS generation at cell membrane, such as vitamin E [Kalthur et al. Citation2011]; and non-enzymatic antioxidants increasing intracellular antioxidant capacity, such as glutathione (GSH) [Gadea et al. Citation2011] or genistein [Martinez-Soto et al. Citation2010].
In this study, we have assessed the role of Tempol, as a cell permeable scavenger of superoxide anions or as a ‘SOD-mimetic’ agent. Tempol meditates conversion of superoxide anion () to hydrogen peroxide (H2O2) [Acosta et al. Citation2013] and through this activity, it may reduce the formation of hydroxyl radicals via the Fenton or Haber-Weiss reactions at both extra- and intra-cellular levels [Wilcox and Pearlman Citation2008]. Hydroxyl radicals are considered as the most toxic radical. Our results have revealed that 5 μM Tempol, improved motility and viability of human sperm post-thawing in a commercial optimized SFS media (). Improved motility and viability might be partially attributed to Tempol-protected lipid peroxidation [Acosta et al. Citation2013]. In line with our results, previous studies in ram have shown that supplementation with Tempol increases sperm motility and fertility [Mara et al. Citation2005]. However, in contrast to our results and those of Mara et al. [Citation2005], a recent study by Mata-Campuzano et al. [Citation2012] showed that antioxidants like Tempol and N-acetyl-cysteine (NAC) decrease sperm motility, while in accordance with our results, Tempol reduces ROS, DNA fragmentation, and lipid peroxidation due to cryopreservation induced oxidative stress. These authors and other studies suggest that the excessive reduction of ROS level by an antioxidant to below physiological level may inhibit sperm motility [Aitken and Curry Citation2011; Mata-Campuzano et al. Citation2012]. They observed a significant reduction of motility at 1 mM Tempol and we only observed a marginal reduction in motility at 10 mM Tempol. These differences may be related to inter-species differences, type of cryoprotectant, extender, and experimental conditions implemented [Foote et al. Citation2002; Mara et al. Citation2007]. A strong point regarding our study is the fact that we added Tempol to a commercially available sperm freezing solution which is a chemically defined and animal free medium and this may facilitate comparison between studies in future.
The result of this study revealed that the percentage of ROS-positive sperm decreased upon freezing semen compared to fresh samples (). Such an observation was expected and is in agreement with previous studies [Guthrie and Welch Citation2006; Kadirvel et al. Citation2009; Wang et al. Citation1997]. After freezing, a significant percentage of sperm lose viability and their mitochondria are non-functional. Thereby, they do not produce ROS [Wang et al. Citation1997]. Interestingly, notwithstanding improved motility and viability in the 5 µM Tempol group compared to the control group following freezing, we observed a decrease in the percentage of ROS or DCF-positive sperm compared to the control following freezing. This observation implies, Tempol reduced H2O2 production [Wilcox and Pearlman Citation2008] and is expected to increase the percentage of ROS positive sperm as live sperm produce ROS.
The results of this study showed that in vitro manipulation () increases the percentage of ROS-positive sperm [Ainsworth et al. Citation2005]. A few reasons have been proposed for this increase including: physical shearing forces during centrifugation, removing antioxidants from semen plasma during the washing process [Agarwal et al. Citation1994; Alvarez et al.Citation1993; Allamaneni et al. Citation2005], altered permeability of the sperm surface to water, ions, and cryoprotectant which results in a weakening of the cell that reduces its ability to withstand future stress, and the release of proteins from the sperm from mechanical forces [Leahy and Gadella Citation2011].
One of the consequences of ROS production is DNA fragmentation. Our results show an increase in the percentage of DNA fragmentation following cryopreservation and are in agreement with previous findings [Thomson et al. Citation2009; Zribi et al. Citation2010]. This increased DNA fragmentation was significantly reduced by Tempol supplementation () and may have important consequences on assisted reproductive technology (ART) outcomes. Our results using human semen are in agreement with previous reports showing that Tempol improves animal semen quality in terms of motility, viability, and DNA damage following cryopreservation [Acosta et al. Citation2013; Donoghue and Donoghue Citation1997; Mara et al. Citation2005]. This appears to act by reducing ROS stress and thereby reducing DNA damage when commercially available cryopreservation medium is supplemented.
Materials and Methods
Semen samples
This study was approved by the Ethics Committees of the Institutional Review Board of Royan Institute and Isfahan Fertility and Infertility Center (IFIC). Semen samples were collected from men with normal sperm parameters who attended the IFIC from October 2012 to December 2013. Participants signed written informed consent. Semen samples were collected by masturbation in sterile containers after 3–4 d of sexual abstinence and were assessed according to World Health Organization (WHO) guidelines [WHO Citation2010].
Sperm cryopreservation and thawing
Sperm cryopreservation was performed using an egg yolk-free cryoprotectant medium, Sperm Freeze Solution™ (SFS) (Sperm Freeze Solution™ 10137, Vitrolife, Goteborg, Sweden). Following semen liquefaction, each sample was diluted (v/v) at room temperature with SFS that contained different concentrations of Tempol (Calbiochem, Darmstadt, Germany). The diluted semen samples were drawn into 0.5 ml French straws (Biovet, L’Agile, France) and sealed. Horizontally placed filled straws were exposed to liquid nitrogen (LN2) vapor for 30 min at 1–3 cm above the LN2 level, then plunged into LN2 and stored until their use for evaluation of sperm parameters. Straws were thawed individually in a water bath at a temperature of 35 ± 2 °C for 30 s. Immediately after thawing, one trained individual evaluated all semen samples for motility, viability, DNA fragmentation, and ROS status.
Study design
In order to obtain the optimal concentration of Tempol, 10 semen samples were cryopreserved using SFS supplemented with 0.0, 0.1, 1, 5, and 10 mM of Tempol. Motility and viability of each sample for different Tempol concentrations were assessed before and after cryopreservation using computer-aided sperm analysis (CASA) and Eosin-Nigrosin [Nasr-Esfahani et al. Citation2002; WHO Citation2010] staining, respectively. Based on these results, an additional 19 semen samples were cryopreserved with 0.0, 0.05, and 0.1 mM Tempol. The results revealed that 0.05 mM (50 µM) Tempol significantly improved viability post-thawing. Accordingly, an additional 19 semen samples were cryopreserved with 0.0, 0.5, 5, and 50 µM Tempol. Based on viability and motility results 5 µM concentration of Tempol was considered to be the optimal concentration.
After defining the optimal concentration of Tempol (5 µM), 23 semen samples were cryopreserved with 0.0 and 5 µM Tempol. We assessed ROS production by using a 2′, 7′-dichlorodihydrofluorescein diacetat (DCFH-DA, Sigma Co, USA) probe with high specificity for hydrogen peroxide (H2O2) [Kiani-Esfahani et al. [Citation2012]. Briefly, we prepared a 2.5 mM stock solution of H2DCFDA in dimethyl sulfoxide (DMSO, Merck, Darmstadt, Germany) which was stored at −70°C. A total of one million sperm before and after thawing were treated with 5 µM H2DCFDA for 30 min, after which the percentages of DCF-positive sperm were defined by flow cytometry.
To analyze the percentage of ROS-positive sperm, both fresh and frozen-thawed semen were divided into two equal portions. One portion was directly used for assessment of ROS and labeled as unwashed, while the other fraction was washed with PBS at 330 × g for 5 min, after which the levels of ROS were assessed.
DNA fragmentation
DNA fragmentation was evaluated with the aid of the terminal dUTP nick-end labeling (TUNEL) Assay Kit (Apoptosis Detection System Fluorescein, Promega, Mannheim, Germany) [Kheirollahi-Kouhestani et al. Citation2009] only for the optimal concentration (5 µM) of Tempol on 15 semen samples. Briefly, before and after freezing, 20 µl of each washed sample was fixed by 4% methanol-free formaldehyde and placed on slides for 25 min at room temperature. The percentages of TUNEL-positive spermatozoa were estimated from recording 500 randomly selected spermatozoa as visualized with an Olympus fluorescent microscope (BX51,Tokyo, Japan) with the appropriate filters (460–470 nm) at 1000× magnification.
Statistical methods
Data was evaluated using the Statistical Package for the Social Sciences software (SPSS 18; SPSS, Chicago, IL, USA). We used one-way ANOVA to compare the mean value between different groups. Differences between all groups were compared by the Tukey multiple comparison post hoc test. The paired-sample t-test was used for comparison between washed and unwashed semen. Differences with values of p < 0.05 were considered as statistically significant. The results in the text and figures are presented as means ± SEM.
Declaration of interest
The study was funded by Royan Institute and the authors received no commercial benefits from Vitrolife. The authors have no financial or commercial conflicts with this project. Royan Institute is an NGO (Nongovernmental organization) belonging to ACECR (Iranian Academic Center for Education, Culture & Research). The authors state that no authors are employed in any respect by the Government of Iran.
Author contributions
Designed the study, interpreted the results, contributed to the writing of the paper, revised the paper, contributed to the editing of the paper, and gave final approval: MHN-E. Contributed to andrology methods, performed the statistical analysis, interpreted the results, contributed to the writing of the paper, and revised the paper: LA, MT. Performed the semen analysis, prepared samples, collected data, and contributed to the writing of the paper: ZB. Supervisor of student: MF. Flow cytometry analyses: AK-E.
| Abbreviations | ||
| SOD | = | superoxide dismutase |
| ROS | = | reactive oxygen species |
| LPO | = | lipid peroxidation |
| DCFH-DA | = | 2′, 7′-dichlorodihydrofluorescein- diacetate |
| ART | = | assisted reproductive technology |
| CASA | = | computer-aided sperm analysis |
| SFS | = | sperm freeze solution |
Acknowledgments
We would like to express our appreciation and thanks to Royan Institute for their financial support (project no.855), and the Isfahan Fertility and Infertility Center for their kind co-operation. The clinical trial for sperm freezing solution for Vitrolife was undertaken by our group. SPS was gifted from Vitrolife.
References
- Acosta, A.S., Vargas, S.E., Cuya, M.V., González, J.R. and Gutiérrez, R.S. (2013) Effect of the addition of two superoxide dismutase analogues (Tempo and Tempol) to alpaca semen extender for cryopreservation. Theriogenology 79:842–6
- Agarwal, A., Ikemoto, I. and Loughlin, K.R. (1994) Effect of sperm washing on levels of reactive oxygen species in semen. Arch Androl 33:157–62
- Ainsworth, C., Nixon, B. and Aitken, R.J. (2005) Development of a novel electrophoretic system for the isolation of human spermatozoa. Hum Reprod 20:2261–70
- Aitken, R.J. and Curry, B.J. (2011) Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal 14:367–81
- Allamaneni, S.S., Agarwal, A., Nallella, K.P., Sharma, R.K., Thomas, A.J. Jr. and Sikka, S.C. (2005) Characterization of oxidative stress status by evaluation of reactive oxygen species levels in whole semen and isolated spermato zoa. Fertil Steril 83:800–3
- Alvarez, J.G. and Storey, B.T. (1992) Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 13:232–41
- Alvarez, J.G., Lasso, J.L., Blasco, L., Nuñez, R.C., Heyner, S., Caballero, P.P., et al. (1993) Centrifugation of human spermatozoa induces sublethal damage; separation of human spermatozoa from seminal plasma by a dextran swim–up procedure without centrifugation extends their motile lifetime. Hum Reprod 8:1087–92
- Bansal, A.K. and Bilaspuri, G.S. (2010) Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int 2011:1–7
- De Lamirande, E., Jiang, H., Zini, A., Kodama, H. and Gagnon, C. (1997) Reactive oxygen species and sperm physiology. Rev Reprod 2:48–54
- Di Santo, M., Tarozzi, N., Nadalini, M. and Borini, A. (2012) Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol 2012:1–12
- Donoghue, A.M. and Donoghue, D.J. (1997) Effects of water- and lipid-soluble antioxidants on turkey sperm viability, membrane integrity, and motility during liquid storage. Poult Sci 76:1440–5
- Foote, R.H., Brockett, C.C. and Kaproth, M.T. (2002) Motility and fertility of bull sperm in whole milk extender containing antioxidants. Anim Reprod Sci 71:13–23
- Forouzanfar, M., Fekri Ershad, S., Hosseini, S.M., Hajian, M., Ostad-Hosseini, S., Abid, A., et al. (2013) Can permeable super oxide dismutase mimetic agents improve the quality of frozen-thawed ram semen? Cryobiology 66:126–30
- Gadea, J., Molla, M., Selles, E., Marco, M.A., Garcia-Vazquez, F.A. and Gardon, J.C. (2011) Reduced glutathione content in human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 62:40–6
- Guthrie, H.D. and Welch, G.R. (2006) Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in viable boar sperm using fluorescence activated flow cytometry. J Anim Sci 84:2089–100
- John Morris, G., Acton, E., Murray, B.J. and Fonseca, F. (2012) Freezing injury: the special case of the sperm cell. Cryobiology 64:71–80
- Kadirvel, G., Kumar, S. and Kumaresan, A. (2009) Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Anim Reprod Sci 114:125–34
- Kalthur, G., Raj, S., Thiyagarajan, A., Kumar, S., Kumar, P. and Adiga, S.K. (2011) Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw-induced DNA damage. Fertil Steril 95:1149–51
- Kheirollahi-Kouhestani, M., Razavi, S., Tavalaee, M., Deemeh, M.R., Mardani, M., Moshtaghian, J., et al. (2009) Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum Reprod 24:2409–16
- Kiani-Esfahani, A., Tavalaee, M., Deemeh, M.R., Hamiditabar, M. and Nasr-Esfahani, M.H. (2012) DHR123: an alternative probe for assessment of ROS in human spermatozoa. Syst Biol Reprod Med 58:168–74
- Lasso, J.L., Noiles, E.E., Alvarez, J.G. and Storey, B.T. (1994) Mechanism of superoxide dismutase loss from human sperm cells during cryopreservation. J Androl 15:255–65
- Leahy, T. and Gadella, B.M. (2011) Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 142:759–78
- Lewis, S.E., Sterling, E.S., Young, I.S. and Thompson, W. (1997) Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril 67:142–7
- Li, Z., Lin, Q., Liu, R., Xiao, W. and Liu, W. (2010) Protective effects of ascorbate and catalase on human spermatozoa during cryopreservation. J Androl 31:437–44
- Mara, L., Accardo, C., Pilichi, S., Dattena, M., Chessa, F., Chessa, B., et al. (2005) Benefits of TEMPOL on ram semen motility and in vitro fertility: a preliminary study. Theriogenology 63:2243–53
- Mara, L., Dattena, M., Pilichi, S., Sanna, D., Branca, A. and Cappai, P. (2007) Effect of different diluents on goat semen fertility. Anim Reprod Sci 102:152–7
- Martinez-Soto, J.C., De diosHourcade, J., Gutiérrez-Adán, A., Landeras, J.L. and Gadea, J. (2010) Effect of genistein supplementation of thawing medium on characteristics of frozen human spermatozoa. Asian J Androl 12:431–41
- Mata-Campuzano, M., Alvarez-Rodríguez, M., Alvarez, M., Anel, L., de Paz, P., Garde, J.J., et al. (2012) Effect of several antioxidants on thawed ram spermatozoa submitted to 37°C up to four hours. Reprod Domest Anim 47:907–14
- Nasr-Esfahani, M.H., Aboutorabi, R., Esfandiari, E. and Mardani, M. (2002) Sperm MTT viability assay: a new method for evaluation of human sperm viability. J Assist Reprod Genet 19:477–82
- O’Connell, M., McClure, N. and Lewis, S.E. (2002) The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod 17:704–9
- Oehninger, S., Duru, N.K., Srisombut, C. and Morshedi, M. (2000) Assessment of sperm cryodamage and strategies to improve outcome. Mol Cell Endocrinol 169:3–10
- Ozkavukcu, S., Erdemli, E., Isik, A., Oztuna, D. and Karahuseyinoglu, S. (2008) Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet 25:403–11
- Roca, J., Rodríguez, M.J., Gil, M.A., Carvajal, G., Garcia, E.M., Cuello, C., et al. (2005) Survival and in vitro fertility of boar spermatozoa frozen in the presence of superoxide dismutase and/or catalase. J Androl 26:15–24
- Samuni, A., Krishna, C.M., Mitchell, J.B., Collins, C.R. and Russo, A. (1990) Superoxide reaction with nitroxides. Free Radic Res Commun 9:241–9
- Thomson, L.K., Fleming, S.D., Aitken, R.J., De Iuliis, G.N., Zieschang, J.A. and Clark, A.M. (2009) Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 24:2061–70
- Wang, A.W., Zhang, H., Ikemoto, I., Anderson, D.J. and Loughlin, K.R. (1997) Reactive oxygen species generation by seminal cells during cryopreservation. Urology 49:921–5
- Wilcox, C.S. and Pearlman, A.C. (2008) Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev 60:418–69
- Wilcox, C.S. (2010) Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126:119–45
- WHO. (2010) WHO laboratory manual for the examination and processing of human semen. World Health Organization, 5th ed. New York: Cambridge University Press
- Zribi, N., Feki Chakroun, N., El Euch, H., Gargouri, J., Bahloul, A. and Ammar Keskes, L. (2010) Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril 93:159–66
- Zribi, N., Chakroun, N.F., Ben Abdallah, F., Elleuch, H., Sellami, A., Gargouri, J., et al. (2012) Effect of freezing-thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 65:326–31