Abstract
The aims of the study were to evaluate the effects of chocolate and propolis-enriched diets on rabbit spermatogenesis, sperm motility, and ultrastructure following bacterial lipopolysaccharide (LPS) treatment. Thirty-two New Zealand White rabbits were divided into four groups. The LPS-Propolfenol® group received propolis (500 mg/kg/day) in their diet for 15 days, while the LPS-chocolate group was fed 70% cacao chocolate (1 g/1 kg/day) for the same period. Following the diet treatments, rabbits in the LPS-Propolfenol® and LPS-chocolate groups, and an LPS group received a single intraperitoneal dose of 50μg/kg LPS, and the control group received only saline. Kinematic sperm traits were evaluated with a computer assisted sperm analyzer (CASA) system, and ultrastructural characteristics were examined by transmission electron microscopy (TEM). Testicular and epididymal tissues were observed by light microscopy and TEM and multiplex real time reverse transcriptase-polymerase chain reaction (RT-PCR) assay was used to detect and quantify toll-like receptor-4 (TLR-4) gene expression. The values of the analyzed semen parameters of rabbits treated with LPS-Propolfenol® and LPS-chocolate did not show any variations compared with the control group, but they were lower in rabbits treated only with LPS. Alterations observed in the testicular tissue of LPS treated-rabbits were not detected in specimens from the LPS-chocolate and LPS-Propolfenol® groups, which showed normal spermatogenesis. The TLR-4 mRNA expression was similar in controls, in LPS treated, and in LPS-chocolate groups, but it was significantly (p < 0.01) decreased in LPS-Propolfenol® rabbits. In conclusion, a chocolate and propolis-enriched diet showed a protective effect on the spermatogenetic process of buck rabbits following LPS treatment.
Introduction
Recently, there has been growing interest in researching the effects of plant-derived antioxidants in food on human health. The beneficial influence of many foodstuffs and beverages, including fruits, vegetables, red wine, tea, coffee, cocoa, and chocolate, on human health has been recently recognized to originate from their antioxidant activity [Gülçin Citation2012]. Cocoa is one of the most abundant sources of phenolic antioxidants, more specifically, flavonoids and oligomer proanthocyanidins. These compounds are also present in chocolate and they are able to scavenge a reactive oxygen species (ROS). Accordingly, cocoa and chocolate consumption may stimulate changes in the redox-sensitive signaling pathways involved in gene expression and immune response. Cocoa and chocolate could protect tissues from injury and inflammation, skin from oxidative damage from ultraviolet radiation, and they could have beneficial effects on oxidative stress related disorders and autoimmune pathogenesis [Quiñones et al. Citation2011; Ramos-Romero et al. Citation2012].
Propolis, a natural substance collected by honeybees from the buds of various plants, is made by adding beeswax to salivary secretions. Consequently, the biological properties of propolis are gathered from different phyto-geographical areas, and the species of plant pollens and time periods vary greatly. Propolis contains more than 180 chemical compounds, such as polyphenolic compounds (i.e., flavonoids), cinnamic acid derivatives, various steroids, amino acids, glucose, fructose, vitamins (B1, B2, C, and E), and essential elements such as magnesium, calcium, nickel, iron, and zinc. Several studies have demonstrated that propolis exerts anti-inflammatory, anti-bacterial, anti-viral, antioxidant [Sud’ina et al. Citation1993], immunostimulatory and anti-proliferative activities, as well as anti-mutagenic and anti-tumoral effects in several tumor cell lines [El-khawaga et al. Citation2003].
Bacterial lipopolysaccharide (LPS) is an active component of the gram negative bacterial cell wall that promotes an acute inflammatory response. LPS induced animal models allow the opportunity to investigate the mechanisms of different diseases providing information to biomarkers and possible drug targets.
A previous study [Brecchia et al. Citation2010] demonstrated that the rabbit can be a useful model for a longitudinal, long-term study on the effects of sub-acute endotoxemia on sperm parameters. We have chosen the rabbit as a model organism for the intrinsic possibility to easily collect ejaculated semen for a continuous assessment of its traits without having to sacrifice the animal [Foote and Carney Citation2000]. Intra-peritoneal inoculation of LPS triggered a reversible inflammation-like status in buck rabbits which lasted three days, and subsequently reproductive activity and sperm quality were progressively restored [Brecchia et al. Citation2010]. LPS-induced inflammation, reduced sperm concentration and motility, and increased ultrastructural alterations were detected 14–30 days after treatment [Collodel et al. Citation2012]. LPS induced an inflammatory response in the host, possibly by interacting with the toll-like receptor (TLR-4) localized in cells of the immune and reproductive tracts [Palladino et al. Citation2007; Winnal and Hedger Citation2011]. TLR-4 binding LPS stimulates the release of pro-inflammatory cytokines, which in turn induces the production of ROS and nitric oxide (NO) [O’Bryan Citation2000a]. Pro-inflammatory cytokines and oxidative mediators induce structural and functional modifications of the male reproductive tract [O’Bryan et al. Citation2000b; Reddy et al. Citation2006; Liew et al. Citation2007]. LPS can affect steroidogenesis and sperm quality directly, by binding to cells of the reproductive tract, or indirectly, by stimulating immune cells [Hedger Citation2011].
The aims of the study were to evaluate the effects of chocolate and propolis-enriched diets on spermatogenesis as well as sperm motility and ultrastructure following LPS treatments in rabbits. In addition, TLR-4 gene expression in testicular and epididymal tissues was analyzed.
Results
Polyphenols
The total polyphenol content in Propolfenol® was 513.2 ± 25.0 mg/g of dried substance. The cocoa chocolate polyphenol content was 27.0 ± 1.1 mg/g and flavan-3-ols content was 14.7 ± 0.5 mg/g. The antiradical activity of 2,2-di(4-tert-octylphenyl)-1 picrylhydrazil (DPPH) test of Propolfenol® and cocoa chocolate showed an in vitro half maximal inhibitory concentration (IC50), respectively, of 8.2 ± 0.3 μg/ml and 4941.0 ± 547.6 μg/ml, confirming previous data. The viability of the cells (propidium iodide test) incubated with hydrogen peroxide alone was 0%. In the presence of Propolfenol® and cocoa chocolate, cell viability increased respectively to 61.01% ± 3.44% and 23.70% ± 1.41%.
Regarding the anti-inflammatory activity of these compounds, interleukin (IL)-6 concentrations were 4.14 pg/ml in untreated human peripheral blood mononuclear cells (PBMC), 205.51pg/ml in PBMC incubated with LPS, 121.66 pg/ml in PBMC incubated with LPS and cocoa chocolate, and 32.47 pg/ml in PBMC treated with LPS and Propolfenol®. The citotoxicity test showed similar concentrations of lactate dehydrogenase (LDH) release when PBMC were incubated with increasing concentrations of Propolfenol® (percentage of viable cells 97.5%--100%) and cocoa chocolate (97.8%–100%).
Health status
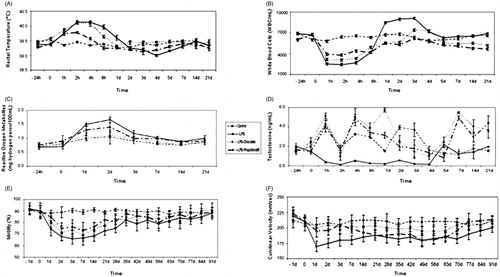
Three hours after LPS treatment, rectal temperature showed a peak (40–41°C) in the LPS and LPS-chocolate groups, that was lower in the LPS-Propolfenol® group (). One hour after LPS treatment, the numbers of white blood cells (WBCs) in the LPS-chocolate and the LPS-Propolfenol® groups were lower than those of the LPS-group (). Food intake decreased 24 hours after LPS treatment; the LPS-chocolate and LPS-Propolfenol® groups ate the regular dose after 48 hours, while the LPS-group ate the regular dose only after 72 hours.
Figure 1. Rectal temperature (A), white blood cell count (B), seminal reactive oxygen metabolites (C), blood testosterone (D), progressive sperm motility (E), and curvilinear velocity (F) in control, lipopolysaccharide (LPS), LPS-chocolate, and LPS-Propolfenol® treated rabbits (mean ± SD). The 95% confidence interval (upper and lower limits) obtained from the ANOVA model is reported.
Oxidative seminal status, testosterone (T) levels, and semen traits
The LPS-treated group showed an increase in reactive oxygen metaboilites (ROMs) that lasted around one-two days, while the control and LPS-chocolate groups were stable and lower during the analyzed period, and the LPS-Propolfenol® results were between those of the control and the LPS treated groups (). The LPS-treated group had a reduction in T levels within 1 hour post treatment (). During the experimental period, the LPS-group showed the lowest T levels with mean values of about 1.1 ng/ml, while the LPS-chocolate and LPS-Propolfenol® groups always maintained, albeit with ups and downs, a higher concentration compared to day 1. In these groups, the levels decreased more and more up to day 4, when they again increased and remained around 4 ng/ml.
The semen parameters of rabbits treated with LPS-chocolate and LPS-Propolfenol®, monitored before and after LPS treatment, did not show variation. There were no differences in sperm concentrations, linearity of curvilinear path (LIN), amplitude of lateral head displacement (ALH), or in semen volume (data not shown); on the contrary, progressive sperm motility () showed variations mainly in the LPS-group from day 1 and up to day 70 after treatment. Curvilinear velocity (VCL) showed a lower trend during the experimental design in the LPS-treated and LPS-Propolfenol® groups. The LPS-chocolate group had a trend similar to the control group ().
Sperm ultrastructure
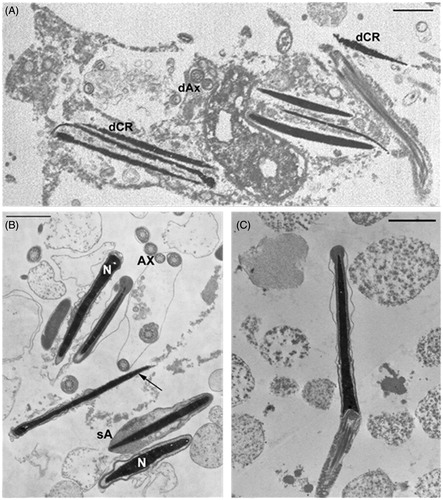
In controls, the percentages of altered sperm remained constant during the experiment (day 0: 4.0%; 3.5%–4.5%, day 91st: 5.5%; 5.0%–6.0%). In LPS-treated rabbits, the percentages of altered sperm increased during the experimental period from 4% (3.0%–4.0%) at day 0 to 25% (23.5%–32.6%) at day 28 (). In rabbits treated with LPS-chocolate and LPS-Propolfenol®, the percentages of altered sperm increased respectively to 12.0% (11.0%–12.0%) and 11.5% (10.5%–12.0%) at day 28, and then returned to baseline (). Normal sperm showed a well shaped nucleus, acrosome, and axoneme; altered sperm showed reacted/absent acrosomes, disrupted chromatin, and axonemes with altered patterns and disassembled or absent outer dense fibers (). No lymphocytes or macrophages were observed. These alterations were present in the samples obtained on days 21, 28, and 42. In the same days, some proportions of ejaculated sperm from rabbits treated with LPS-chocolate () and LPS-Propolfenol® () showed similar ultrastructural alterations to those detected in sperm from LPS-treated rabbits.
Figure 2. Percentages of altered spermatozoa (median and interquartile range, 25th and 75th percentile) monitored by transmission electron microscopy (TEM) in control, lipopolysaccharide (LPS), LPS-chocolate, and LPS-Propolfenol®-treated rabbits. LPS-treated from 4% to 25%, LPS-chocolate and LPS-Propolfenol® altered sperm increased to, respectively, 12.0% and 11.5% at day 28, and successively returned to baseline. In controls, altered sperm was 4.0% and it remained constant up to the 91st day.
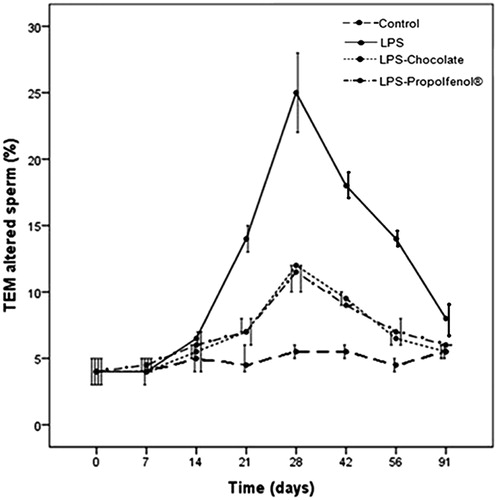
Figure 3. Micrographs of ejaculated spermatozoa (transmission electron microscopy). A) Spermatozoa from lipopolysaccharide (LPS)-treated rabbits (day 28 after LPS treatment) show altered plasma membranes, absent acrosomes, disorganized axoneme assembly (dAx) and disrupted chromatin (dCR); these alterations are typical characteristics of necrosis. B) Spermatozoa from LPS-chocolate-treated rabbits (day 28 after LPS treatment) show regular nuclei (N), acrosomes, and axonemes (Ax); spermatozoa with swollen (sA) or absent (arrow) acrosomes are also present. C) Normal spermatozoon from a LPS-Propolfenol® -treated rabbit. Bar 1 μm.
Light and electron microscopy of epididymal and testicular tissues
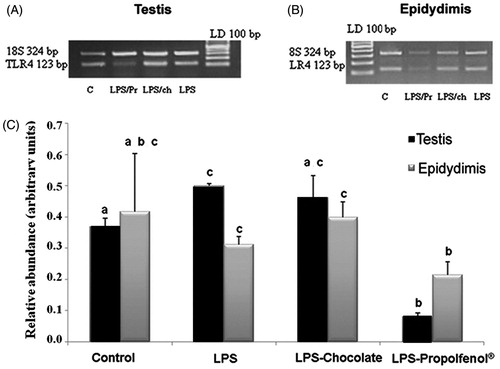
The testicular tissues from control rabbits () did not differ at any time from those of the LPS-Propolfenol® () or LPS-chocolate treated rabbits (). The seminiferous tubules showed a normal germinal epithelium, detected with light and electron microscopy. Spermatogonia, spermatocytes, and spermatids were present and they exhibited regular morphology (). Sertoli cells did not show any ultrastructural changes. In only one case (a rabbit treated with chocolate, day 7), we noted rare spermatocytes intermingled with ballooning Sertoli cells (), as frequently observed in testis from LPS-treated rabbits. At the 7th day, the testicular samples from the LPS-treated rabbits showed tubules with an evident reduction of the germinal epithelium () and Sertoli cells were increased in number and their cytoplasm showed extremely dilated cisterns of smooth reticulum and abundant dark lysosomes (). No morphological alterations, numerical variations of Leydig cells, or inflammatory cell infiltrates were observed. No structural alterations were found in the epididymis from rabbits in any of the treated groups (LPS, LPS-chocolate, LPS-Propolfenol®) or controls.TRL-4 gene expression was detected in the testis () and epididymis () of all groups. TLR-4 mRNA levels in the testis and epididymis were similar in the control, LPS, and LPS-chocolate groups, while they were significantly (p < 0.01) lower in the LPS-Propolfenol® group ().
Figure 4. Micrographs of testicular tissue examined by optical and transmission electron microscopy. Samples of control (A) and lipopolysaccharide (LPS)-Propolfenol® -treated rabbits (B, C day 7 after LPS treatment) show tubules (black arrows) with a normal germinal epithelium (white arrows). In C) spermatids (arrows) at different stages of maturation are visible. D, E) Rare spermatocytes intermingled with ballooning Sertoli cells were detected (day 7 after LPS treatment, arrow) in a rabbit treated with chocolate, detected by optical (D) and transmission electron microscopy (E). F) LPS-treated rabbits (day 7 after LPS treatment), the tubules show a conspicuous reduction in the germinal epithelium (arrows). G) the presence of an increased number of Sertoli cells with abundant dark lysosomes is indicated (arrows). A,B,C, F: bar 100 μm, D: bar 4 μm, E: bar 10 μm, G: bar 2 μm.
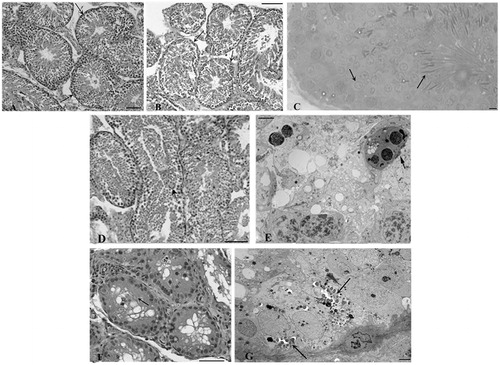
Figure 5. Relative abundance of toll-like receptor4 (TLR-4) mRNA in testis (A) and epididymis (B) harvested from control (lane C), lipopolysaccharide (LPS) treated, LPS-chocolate (LPS/ch), LPS-Propolfenol® (LPS/Pr)-treated rabbits. The upper panels show representative images of ethidium bromide stained gels showing the presence of the expected bp products yielded after reverse transcriptase polymerase chain reaction using primers for target TLR-4 (123 bp) and 18S (324 bp). Lane LD is the kb DNA marker. For each experimental group, the values (means ± SD) derived from densitometric analyses of TLR-4 reported in arbitrary units relative to 18S expression, combined with the results from two different rabbits for each experimental group (C). Different letters indicate a significantly different value (p < 0.01).
Discussion
It is self-evident that infection and inflammation in the reproductive tract can inhibit male fertility, but the observation that fertility may also be compromised by systemic inflammation is more difficult to explain [Hedger Citation2011]. Animals can provide valuable models for studying the male genital tract during systemic inflammation, which can be mimicked in vivo by the administration of LPS [Brecchia et al. Citation2010; Reddy et al. Citation2010]. LPS inoculation produces an alteration in semen quality by reducing sperm motility and plasma membrane integrity in a time dependent manner [Collodel et al. Citation2012].
Natural products, in particular the secondary metabolites of polyphenols, have been studied in great detail due to their strong antioxidant and anti-inflammatory activity. The non-specific, multi-target action of polyphenols is also responsible for abundant clinical evidence obtained by evaluating the activity of medicinal plants and polyphenols from the diet. The antioxidant and anti-inflammatory actions of dark cocoa, chocolate polyphenols and propolis have also been confirmed in in vitro and in vivo results [Rusconi and Conti Citation2010; Biagi et al. Citation2011; Sodano Citation2011]. The beneficial antioxidant effects of propolis have been reported in rabbit reproduction [Yousef et al. Citation2010] with a significant increase in serum testosterone levels, an improvement in semen characteristics, an enhancement of glutathione S-transferase, transaminases, phosphatases, and a decrease in levels of free radicals and lactate dehydrogenase [Yousef et al. Citation2010].
The aims of the study were to evaluate the effects of chocolate and propolis-enriched diets on rabbit spermatogenesis, as well as sperm motility and ultrastructure, following LPS treatment. Different mechanisms are involved in LPS-induced damages to reproductive activity and it is widely known that acute or chronic infections and inflammation can lead to disorders in steroidogenesis and spermatogenesis, as well as transitive or permanent male infertility in species other than rabbit [O’Bryan et al. Citation2000a, O’Bryan et al. Citation2000b; Reddy et al. Citation2006].
One of these mechanisms is the direct effect of LPS on TLR-4 [Zhan et al. Citation2013], that induces the production of several cytokines or mediators, such as ROS and NO, which could be responsible for structural, metabolic, and functional disorders in Sertoli and germ cells [Agarwal et al. Citation2003; Palladino et al. Citation2008], for a decrease in testosterone concentrations [Allen et al. Citation2004; Hales et al. Citation2000; O’Bryan et al. Citation2000b] and alterations in semen traits [Eggert-Kruse et al. Citation2001]. TLR-4 has been found to be expressed in rat epididymal epithelium and sperm [Cao et al. Citation2010]; recently Zhang et al. (Citation2012) determined the expression profiles of TLRs in the testis and epididymis of two groups of roosters treated with LPS or phosphate-buffered saline (PBS) injection. TLR-4 expression in the testis of the LPS-injected group was significantly increased by 6 hour postinjection and was significantly greater than the PBS-injected control group at 6 hour postinjection. However, the change in the expression in the epididymis was not statistically significant.
In this paper we have demonstrated, for the first time, TRL-4 expression in rabbit testicular and epididymal tissues and we observed that TRL-4 mRNA levels in the LPS-chocolate group were similar to those observed in the LPS-group, while they were significantly lower in the LPS-Propolfenol® group. These results seem to suggest different mechanisms of action of Propolfenol® and chocolate to counteract LPS damage on spermatogenesis. However, immunohistochemical and immunoblotting analyses are needed to more precisely detect the cells that express TLR-4 and to identify the presence of other members of the TLR family in the different tissues of the reproductive tract of the buck rabbits.
We hypothesize that there are two different mechanisms of action of these substances. Propolfenol® exerts antioxidant activity but also seems to act on genetic expression, reducing TRL-4 expression, and cocoa seems to exert a scavenger action on free radicals through its phenolic antioxidant component. An anti-inflammatory effect of a cocoa diet on an intestinal inflammation model in rats was shown because it down-regulated serum tumour necrosis factor-α, colon inducible nitric oxide synthase activity, and decreased colon cell infiltration [Perez-Berezo et al. Citation2012]. Despite the two different proposed mechanisms, the effects of Propolfenol® and cocoa on the analyzed variables were similar. Testosterone levels decreased in the LPS-treated group, but in the LPS-chocolate and LPS- Propolfenol® groups reached values similar to those observed in the control group. In the LPS-chocolate and LPS- Propolfenol® groups, sperm motility was preserved and the production of seminal ROMs was reduced compared to that detected in the LPS group; in addition, the testis did not show a decrease in spermatocytes or early spermatids nor an increase in structurally altered Sertoli cells, such as that observed after LPS treatment alone [Collodel et al. Citation2012; Liew et al. Citation2007]. It is known that one of the major effects of acute LPS-mediated inflammation is the induction of necrosis in spermatogenetic cells [Collodel et al. Citation2012; Reddy et al. Citation2010] and in mature sperm [Collodel et al. Citation2012]; in this research, ultrastructural data demonstrated that both chocolate and Propolfenol® exerted a protective action on the ejaculated sperm of rabbits, highlighted by the low percentages of necrotic cells observed in the groups treated with these compounds.
In conclusion, in our study a chocolate and propolis-enriched diet, associated with LPS treatment, showed a protective effect on spermatogenesis and on sperm characteristics of buck rabbits. Further studies are needed to better clarify the effects of these natural substances on inflammation and on the spermatogenetic process. The properties of these substances are of particular interest in order to shed light on the current debate in the field of human male infertility and inflammation.
Materials and Methods
Animals
Animals were housed at the experimental farm of the Department of Applied Biology of the University of Perugia. The rabbits were maintained under a continuous daily photoperiod of 16 h light (40 lux) and 8 h dark [Besenfelder et al. Citation2005; Theau-Clément et al.Citation1995]. Room temperature ranged from 18°C to 27°C. Animals were fed a standard diet ad libitum [De Blas and Wiseman Citation1998] and fresh water was always available. All procedures described below were approved by the Animal Ethics Monitoring Committee of the University of Perugia.
Polyphenols analysis of Propolfenol® and chocolate
Propolis extract, Propolfenol®, was kindly furnished by Erba Vita (Chiesanuova, Repubblica di San Marino). Folin-Ciocalteau reagent, gallic acid, sodium carbonate, vanillin, hydrochloridric acid (HCl, 37% v/v), ethanol (96% v/v), and (-)-epicatechin were purchased from Sigma-Aldrich (Milan, Italy). The total polyphenol concentration in Propolfenol® was determined spectrophotometrically using the Folin–Ciocalteau colorimetric method [Biagi et al. Citation2009]. One ml of sodium carbonate solution (15% w/v in water, pH = 12) was added to the reaction mixture: 0.1 ml of a water solution (1 mg/ml) of Propolfenol®, 3.0 ml of water solution and 0.5 ml of Folin–Ciocalteau reagent. The absorbance of each solution was measured at 730 nm against blank (distilled water), in a 10 mm cell. The total polyphenol concentration was calculated from a calibration curve using gallic acid as a standard (10–2000 mg/l). The same method was used to quantify polyphenols in chocolate (1 mg/ml water). The total flavan-3-ols content in chocolate was determined with the use of the vanillin-HCl assay. Briefly, 1 ml of HCl 37% v/v was added to the reaction mixture (0.5 ml of diluted solution of the sample and 0.5 ml of a 1% w/v ethanolic vanillin solution). The mixture was placed in a dark area for 20 min, then absorbance was recorded at 500 nm against blank (distilled water) in a 10 mm cell. The total flavan-3-ols content was calculated using (-)-epicatechin as a standard (1–500 mg/l).
In vitro tests of Propolfenol® and chocolate
The antiradical activities of Propolfenol® and of 70% cocoa chocolate (200 μg/ml) were monitored on HeLa cells stimulated by hydrogen peroxide (0.3 mM) and evaluated byDPPH assay [Biagi et al. Citation2011; Sodano Citation2011]. The cell membrane damage caused by hydrogen peroxide with or without Propolfenol® or cocoa chocolate was tested at 24 h by propidium iodide incorporation assay [Biagi et al. Citation2011; Sodano Citation2011].
The anti-inflammatory activities of Propolfenol® [Biagi et al. Citation2011] and of 70% cocoa chocolate [Sodano Citation2011] were investigated by enzyme linked immunosorbent assay (ELISA) evaluating the (IL)-6 released on human PBMC stimulated with LPS. To test the toxicity of cocoa chocolate and Propolfenol®, human PBMC were incubated with these compounds at concentrations comprised between 0.5 mg/ml and 5 mg/ml. The release of LDH, a marker of cell damage, was highlighted by colorimetric assay (LDH assay, Takara Inst. Tokyo, Japan), able to evaluate the percentage of cell viability [Biagi et al. Citation2011; Sodano Citation2011]. A preliminary in vivo toxicity test was also performed on Artemia salina L. with a daily dosage of 500 mg of Propolfenol® and 500 mg of 70% cocoa chocolate.
Experimental design
Blood and seminal samples from thirty-two New Zealand White buck rabbits (8 months old, body weight, bw: 4.3 kg) were analyzed to include the animals in the trial; successively they were divided into four groups. The LPS-Propolfenol® group (n = 8) received 500 mg propolis per kg of live body weight (lbw)/day in the diet for 15 d, while the LPS-chocolate group (n = 8) was fed 70% cocoa chocolate (1 g/kg lbw/d, Lindt chocolate) for the same time [Biagi et al. Citation2011]. Then, all groups including the LPS group (n = 8), received a single ip injection of 50 μg/kg bw of E. coli LPS (0127:B8, Sigma-Aldrich). Rabbits in the control group, (n = 8) received saline. During the trial, the diets of the rabbits in the LPS-Propolfenol® and LPS-chocolate groups were supplemented with propolis and chocolate, respectively.
The inflammatory response was determined by measuring rectal temperature with a thermocouple and by counting the number of WBC (hemocytometer). Measurements were taken before (time 0), and 1, 2, 4, 8, and 24 h later, as well as 2, 3, 4, 5, 7, 14, and 21 d after the LPS treatment. Feed intake was monitored every day up to 21 d after the LPS treatment. At the same time points, blood samples were drawn from the marginal ear vein into a vacutainer containing ethylene diaminetetraacetic acid (EDTA) and immediately centrifuged at 3000 × g. Plasma was stored at −20°C for the determination of T concentrations.
Semen collection was performed using an artificial vagina just before LPS treatment (time 0) and 1, 2, and 3 d after the LPS treatment, and then at weekly intervals until day 91. Seminal samples were used for the evaluation of semen parameters, oxidative status of seminal plasma, and sperm ultrastructure. Two rabbits from each group were sacrificed by an intravenous over dosage of Tanax (Hoechst, Frankfurt, Germany) at days 7, 14, and 28 after LPS treatment to analyze the ultrastructure of testicular and epididymal tissues. Testicular and epidydimal tissues of rabbits sacrificed at day 7 were also used for TLR4-gene expression analysis. For clarity: the experiment began with 32 rabbits (8 controls, 8 LPS, 8 LPS-chocolate, and 8 LPS-Propolfenol®) up to and including day 7, after the first sacrifice there were 24 rabbits (6 per group) up to and including day 14; after the second sacrifice, there were 16 rabbits (4 per group) up to and including day 28 (last sacrifice). Sperm samples on days 42–91 were obtained from 8 rabbits (2 per group).
Hormonal assays
Testosterone plasma concentrations were determined in duplicate using the Testo-RIA-CT kit (DIAsource ImmunoAsseys, Louvain-la-Neuve, Belgium). The limit of detection was 0.05 ng/ml, and the intra- and inter-assay coefficients of variation were <3.3% and <4.8%, respectively.
Oxidative status of seminal plasma
The level of ROMs in rabbit seminal plasma was assessed by a commercially available kit (d-ROMS, Diacron Grosseto, Italy). Briefly, when a sample is dissolved in an acidic buffer, the hydroperoxides react with the transition metal ions liberated from the proteins and are converted to alkoxyl and peroxyl radicals. These radicals are able to oxidize the chromogen (N,N-diethyl-para-phenylendiamine) to the corresponding radical, which was determined by spectrophotometry (Shimadzu mod. 2550 UV-VIS Kyoto, Japan) at 505 nm. The level of ROMs was expressed as mg hydrogen peroxide/100 ml.
Semen analysis
Light microscopy and computer assisted sperm analyzer (CASA) system analysis of sperm characteristics
Immediately after semen collection, semen volume and sperm concentration were evaluated according to international guidelines [Boiti et al. Citation2005]. The CASA system (ISASv1 Proiser RD, S.L, Valencia, Spain) was used to analyze the kinematic sperm characteristics after semen dilution (1:5) with a modified Tyrode Albumin Lactate Pyruvate (TALP) extender [Brecchia et al. Citation2010]. The analyzed parameters of the kinematic sperm characteristics included progressive motility (%),VCL (µm/s), LIN (%), and ALH (µm).
Transmission electron microscopy (TEM) of sperm cell ultrastructure
Ejaculated sperm was obtained from all rabbits (to include them in the trial, before administering the Propolfenol® and chocolate diets), at time 0 (before LPS treatment), and on days 7, 14, 21, 28, 42, 56, and 91 after LPS treatment. Sperm samples were fixed in cold Karnovsky fixative and maintained at 4°C for 2 h then washed in 0.1 mol/l cacodylate buffer (pH 7.2) for 12 h. Specimens were postfixed in buffered 1% osmium tetroxide for 1 h at 4°C, then dehydrated and embedded in Epon Araldite. Ultra-thin sections were stained with uranyl acetate and lead citrate, analyzed and photographed with a Philips EM208 TEM (Philips Scientifics, Eindhoven, The Netherlands). A minimum of 300 sperm sections were analyzed for each sample by trained examiners who were blind to the experiment. Sperm were considered altered when affected by anomalies related to the acrosome (absent, reacted, empty, swollen), the chromatin (disrupted), the axoneme (disorganized), the mitochondria (swollen and badly assembled), or the plasma membrane (broken).
Light and electron microscopy of testicular and epididymal tissues
Testicular and epididymal tissues, obtained from sacrificed rabbits at days 7, 14, and 28, were fixed in 10% buffered neutral formalin and embedded in paraffin for histology; 4-μm-thick sections of each specimen were stained with hematoxilin and eosin and histologically examined by an expert pathologist. Three small fragments (1 × 1 mm) were treated for conventional TEM analysis. Briefly, the fragments were fixed in 2.5% cacodylate-buffered glutaraldehyde, pH 7.2, for 2 h at 4°C, then washed overnight in the same buffer, postfixed in buffered 1% osmium tetroxide for 1 h, washed, dehydrated through a graded series of ethanol, cleared in propylene-oxide and embedded in epoxy resin (Araldite). Semithin sections (1 µm), cut with glass knives on an LKB V Ultratome and stained with toluidine blue, were examined by light microscope (Leitz Aristoplan, Leica, Wetzlar, Germany) for a general evaluation of the tissue morphology. Ultrathin sections from selected areas were cut with a diamond knife at 50 nm, retrieved onto copper grids, double-stained with uranyl acetate and lead citrate, and examined at 100 kV with a Philips EM208 S TEM (Philips Scientifics).
TLR-4 gene expression in testis and epididymis
Testicular and epididymal tissues were obtained from two rabbits from each group, sacrificed on day 7 after LPS treatment. Within a few minutes, the testis and epididymis were excised and immediately frozen at −80°C for later evaluation of gene expression.
RNA extraction and reverse transcription
Total testis and epididymis RNA were extracted, as previously reported [Boiti et al. Citation2004]. Genomic DNA contamination was prevented by treatment with deoxyribonuclease I (DNAase I Amp. Grade, Invitrogen, S. Giuliano Milanese, Milan, Italy). Five µg of total RNA were reverse transcribed in 20 µl of SuperScript III Reverse Transcriptase (Invitrogen, Life Sciences, Burlington, Ontario, Canada) cDNA synthesis mix using random hexamers. Genomic DNA contamination was checked by polymerase chain reaction (PCR) analysis.
Multiplex real-time reverse transcriptase-PCR (RT-PCR) assay
Multiplex PCR amplifications were carried out using 1 µl of luteal cDNA as a template for targets and 18S primers (QuantumRNA 18S Internal Standards, Invitrogen) [Boiti et al. Citation2004]. In each experiment, the complete set of samples was processed in parallel in a single PCR, using aliquots of the same PCR master mix. The amplified PCR-generated products (20 µl of 25 µl total reaction volume) using primers and reaction condition outined in . Product were analyzed by electrophoresis on 2% agarose gel using ethidium bromide staining [Boiti et al. Citation2004]. The amplified products, collected from agarose gel after electrophoresis, were purified with a Nucleospin Extract II kit (Macherey Nagel Inc., Bethlehem, PA, USA) and their identity was confirmed by DNA sequencing (Dasit, Sciences, Comaredo, Milan, Italy). The mRNA bands were quantified using Quantity One Software (Bio-Rad Laboratories, Milan, Italy) and expressed as arbitrary units.
Table 1. Primers for TLR4.
Statistical analysis
All statistical analyses were performed using Stata Statistical Software: Release 9.0, (StataCorp 2005, College Station, Texas, USA). Continuous variables (rectal temperature, WBC, feed intake, T, ROMs, and semen traits) were monitored by an analysis of variance linear model (ANOVA) comprising the effect of treatment, the day of collection, and the repeated effect of the collection data on the same buck (). The 95% confidence intervals (upper and lower limits) were also calculated. The ANOVA model describes the relationship between the response and the treatment (between the dependent and independent variables). The morphological sperm features data (acrosome, chromatin, axoneme, mitochondria, or plasma membrane) were expressed as median and interquartile range (IQR: 25th and 75th percentile). Gene expression data was analyzed by oneway ANOVA (effect of treatment) followed by the Student-Newman-Keuls t test. A p < 0.05 (two-tailed) was considered statistically significant.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicial to the impartiality of the present research.
Author contributions
Contribution to conception and study design, manuscript drafting, acquisition of data (TEM analysis), critical revision: GC; Acquisition of data (TEM data), contribution to conception and study design, critical revision: EM; Acquisition of data (optical microscopy): MTDV; Acquisition of data (chocolat and propolfenol®), critical revision: MB; Acquisition of data (animal care): RC; Acquisition of data (TEM analysis): LM; Acquisition of data (rabbit physiology), critical revision: GB; Acquisition of data (TLR4 analysis): MM; Acquisition of data (chocolat and propolfenol®): DM; Critical revision, statistical analysis, and interpretation of data: CC. All authors gave approval of the final version of this manuscript.
| Abbreviations | ||
| LPS | = | lipopolysaccharide |
| TLR | = | toll-like receptor |
| CASA | = | computer assisted sperm analyzer |
| PBS | = | phosphate saline buffer |
| TEM | = | transmission electron microscopy |
| RT-PCR | = | reverse transcriptase-polymerase chain reaction |
| ROS | = | reactive oxygen species |
| NO | = | nitric oxide |
| HCl | = | hydrochloridric acid |
| DPPH | = | 2,2-di(4-tert-octylphenyl)-1 picrylhydrazil |
| ELISA | = | enzyme linked immunosorbent assay |
| IL | = | interleukin |
| PBMC | = | peripheral blood mononuclear cells |
| LDH | = | lactate dehydrogenase |
| WBC | = | white blood cells |
| EDTA | = | ethylene diaminetetraacetic acid |
| T | = | testosterone |
| ROMs | = | reactive oxygen metabolites |
| TALP | = | Tyrode Albumin Lactate Pyruvate |
| VCL | = | curvilinear velocity |
| LIN | = | linearity of curvilinear path |
| ALH | = | amplitude of lateral head displacement |
References
- Agarwal, A., Saleh, R.A., and Bedaiwy, M.A. (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79:829–43
- Allen, J.A., Diemer, T., Janus, P., Hales, K.H., and Hales, D.B. (2004) Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation mitochondria. Endocrine 25:265–75
- Besenfelder, U., Theau-Clément, M., Sabbioni, E., Castellini, C., Renieri, T., Havlicek, U., et al. (2005) Effects of different light intensities on quality of spermatozoa in rabbits. World Rabbit Sci 12:227–34
- Biagi, M., Miraldi, E., Figura, N., and Giachetti, D. (2009) Antiradical Activity and in vitro inhibition of Helicobacter pylori by Italian red wines. Nat Prod Commun 4:255–60
- Biagi, M., Miraldi, E., Figa, N., Magnano, A.R., Ierardi, G., Manca, D., et al. (2011) Gastroprotective and anti-Helicobacter pylori activities of propolis. Planta Medica 77:1418--19
- Boiti, C., Guelfi, G., Zerani, M., Zampini, D., Brecchia, G., and Gobbetti, A. (2004) Expression patterns of cytokines, p53 and nitric oxide synthase isoenzymes in corpora lutea of pseudopregnant rabbits during spontaneous luteolysis. Reproduction 127:229–38
- Boiti, C., Castellini, C., Besenfelder, C., Theau-Clément, M., Liguori, L., Renieri, T., et al. (2005) Guidelines for the handing of rabbit bucks and semen. World Rabbit Sci 13:71–91
- Brecchia, G., Cardinali, R., Mourvaki, E., Collodel, G., Moretti, E., Dal Bosco, A., et al. (2010) Short- and long-term effects of lipopolysaccharide-induced inflammation on rabbit sperm quality. Anim Reprod Sci 118:310–16
- Cao, D., Li, Y.,Yang, R., Wang, Y., Zhou, Y., Diao, H., et al. (2010) Lipopolysaccharide-induced epididymitis disrupts epididymal beta-defensin expression and inhibits sperm motility in rats. Biol Reprod 83:1064–70
- Collodel, G., Castellini, C., Del Vecchio, M.T., Cardinali, R., Geminiani, M., Rossi, B., et al. (2012) Effect of a bacterial lipopolysaccharide treatment on rabbit testis and ejaculated sperm. Anim Reprod Sci 47:372–8
- De Blas, C. and Wiseman, J. (1998) The nutrition of the rabbit. Wallingford, Oxon, UK: CABI Publishing/CAB International ed. De Blas, C. and Wiseman, J. pp. 241–253
- Eggert-Kruse, W., Boit, R., Rohr, G., Aufenanger, J., Hund, M., and Strowitzki, T. (2001) Relationship of seminal plasma interleukin (IL) -8 and IL-6 with semen quality. Hum Reprod 16:517–28
- El-khawaga, O.A., Salem, T.A., and Elshal, M.F. (2003) Protective role of Egyptian propolis against tumor in mice. Clin Chim Acta 338:11–6
- Foote, R.H., and Carney, E.W. (2000) The rabbit as a model for reproductive and developmental toxicity studies. Reprod Toxicol 14:477–93
- Gülçin, I. (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86:345–91
- Hales, K.H., Diemer, T., Ginde, S., Shankar, B.K., Roberts, M., Bosmann, H.B., et al. (2000) Diametric effects of bacterial endotoxin lipopolysaccharide on adrenal and Leydig cell steroidogenic acute regulatory protein. Endocrinology 141:4000–12
- Hedger, M.P. (2011) Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation- a perspective. J Reprod Immunol 88:130–41
- Liew, S.H., Meachem, S.J., and Hedger, M.P. (2007) A stereological analysis of the response of spermatogenesis to an acute inflammatory episode in adult rats. J Androl 28:176–85
- O’Bryan, M.K., Schlatt, S., Gerdprasert, O., Phillips, D.J., de Kretser, D.M., and Hedger, M.P. (2000a) Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod 63:1285–93
- O’Bryan, M.K., Schlatt, S., Phillips, D.J., de Kretser, D.M., and Hedger, M.P. (2000b) Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology 141:238–46
- Palladino, M.A., Johnson, T.A., Gupta, R., Chapman, J.L., and Ojha, P. (2007) Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod 76:958–64
- Palladino, M.A., Savarese, M.A., Chapman, J.L., Dughi, M.K. and Plaska, D. (2008) Localization of Toll-like receptors on epididymal epithelial cells and spermatozoa. Am J Reprod Immunol 60:541–55
- Pérez-Berezo, T., Ramírez-Santana, C., Franch, A., Ramos-Romero, S., Castellote, C., Pérez-Cano, F.J., et al. (2012) Effects of a cocoa diet on an intestinal inflammation model in rats. Exp Biol Med 237:1181–8
- Quiñones, M., Muguerza, B., Miguel, M., and Aleixandre, A. (2011) Evidence that nitric oxide mediates the blood pressure lowering effect of a polyphenol-rich cocoa powder in spontaneously hypertensive rats. Pharmacol Res 64:478–81
- Ramos-Romero, S., Pérez-Cano, F.J., Pérez-Berezo, T., Castellote, C., Franch, A., and Castell, M. (2012) Effect of a cocoa flavonoid-enriched diet on experimental autoimmune arthritis. Brit J Nutr 107:523--32
- Reddy, M.M., Mahipal, S.V., Subhashini, J., Reddy, M.C., Roy, K.R., Reddy, G.V., et al. (2006) Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod Toxicol 22:493–500
- Reddy, M.M., Reddy, C.M., Reddy, P.R., and Reddanna, P. (2010) Bacterial LPS-mediated acute inflammation-induced spermatogenic failure in rats: role of stress response proteins and mitochondrial dysfunction. Inflammation 33:235–43
- Rusconi, M., and Conti, A. (2010) Theobroma cacao L., the Food of the Gods: a scientific approach beyond myths and claims. Pharmacol Res 61:5–13
- Sodano, S. (2011) Possibile impiego di Theobroma cacao nella prevenzione delle malattie cardiovascolari. Phytother Rev 45:2–7
- Sud’ina, G.F., Mirzoeva, O.K., Pushkareva, M.A., Korshunova, G.A., Sumbatyan, N.V., and Varfolomeev, S.D. (1993) Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett 329:21–4
- Theau-Clément, M., Michel, N., Bolet, G., and Esparbiè, J. (1995) Effects of artificial photoperiods on sexual behaviour and sperm output in the rabbit. Anim Sci 60:143–9
- Winnal, W.R., and Hedger, M.P. (2011) Differential responses of epithelial Sertoli cells of the rat testis to Toll-Like receptor 2 and 4 ligands: implications for studies of testicular inflammation using bacterial lipopolysaccharides. J Inn Immu 17:123–36
- Yousef M.I., Kamel, K.I., Hassan, M.S., and El-Morsy, A.S. (2010) Protective role of propolis against reproductive toxicity of triphenyltin in male rabbits. Food Chem Toxicol 48:1846–52
- Zhan, X.X., Qing, X.R., Shang, X.J., and Huang, Y.F. (2013) Lipopolysaccharide affects male reproductive function through Toll-like receptors. Zhoun Nan Ke Xue 19:163–8
- Zhang, M., Nii, T., Isobe, N., and Yoshimura, Y. (2012) Expression of Toll-like receptors and effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokine in the testis and epididymis of roosters. Poultry Sci 91:1997–2003
