Abstract
Fluoxetine is a selective serotonin reuptake inhibitor used to treat depression in pregnant and nursing women. However, recent studies have shown adverse effects in the male reproductive system after fluoxetine treatment. Aiming to analyze the extent of damage caused by fluoxetine in the testicle and safe doses for treatment during the perinatal period, the present study analyzed the effects of in utero exposure and exposure during lactation to fluoxetine in spermatogenesis of male rat offspring in adulthood. Wistar rat dams were orally treated with fluoxetine (5, 10, and 20 mg/kg) from 13 days of gestation to lactation day 21 and their offspring were analyzed at 90 days old. Results showed a reduction in the weight of testes (16%), epididymis (28%), and seminal glands (18%) in animals exposed to fluoxetine 20 mg/kg compared to the control. Seminal gland weight was also reduced 25% and 30% in animals exposed to 5 mg/kg and 10 mg/kg fluoxetine, respectively. Body weight of animals exposed to 20 mg/kg fluoxetine was reduced from post-natal day 9 to 36 compared to controls but from the post-natal day 9 to 36 there was no statistical difference. The volume of seminiferous epithelium reduced 17% and the total volume of Leydig cells reduced 30% in the group exposed to fluoxetine at 20 mg/kg. Furthermore, Leydig cells volume reduced 29% in the 5 mg/kg group. The length of the seminiferous tubules reduced 17% and daily sperm production per testicle also reduced 18% in animals exposed to the highest dose of fluoxetine compared to controls. The individual area of Leydig cells increased 7% and plasma testosterone increased 49% in animals exposed to fluoxetine at 20 mg/kg. In conclusion, exposure to 20 mg/kg fluoxetine via the placenta and during lactation may change testosterone and testicular parameters important for sperm production and male fertility in adulthood.
Introduction
Clinical depression occurs during pregnancy in 10 to 15% of women, while postpartum depression occurs between 10 to 22% of women; this condition can be dangerous to both mother and fetus. In addition, studies showed strong evidence of adverse effects of maternal postnatal depression on cognitive, motor, and emotional development of the newborn [Kim et al. Citation2006]. Depression in pregnancy may reduce the mother’s capacity for self-care which may compromise the woman's physical and mental health and may reduce fetal monitoring or restrict the growth and development of the fetus. The consequences of postnatal depression on child development in early infancy, later infancy, and early childhood have been the focus of several studies; many of these studies showed changes on cognitive, emotional, and social development in children [Leigh and Milgrom Citation2008].
Depressive disorders are common during both the prenatal period and in the early months after birth. Cooper et al. [Citation2007] showed that 8.7% of pregnant women had exposure to antidepressant drugs and 6.2% had exposure to a selective serotonin reuptake inhibitor (SSRI). Studies showed adverse effects in offspring of human mothers treated with SSRI [Mulder et al. Citation2011; Oberlander et al. Citation2006; Olivier et al. Citation2011], including adverse effects on the male reproductive system. Exposure to SSRI induced changes in the hypothalamic-pituitary-adrenal system in adolescent rats [Pawluski et al. Citation2012] and lower serum levels of LH, FSH, and testosterone in adult humans [Safarinejad Citation2008]. SSRI exposure may induce maternal and fetal toxicity and reduce male sexual behavior in adult rats [Harris et al. Citation2012; Müller et al. Citation2013], as well as impair sexual motivation in adult male mice [Gouvêa et al. Citation2008]. Also, SSRI exposure via placenta and lactation may inhibit the testicular development in prepubertal rats and decrease the number of spermatozoa in adult rats [Oliveira et al. Citation2013; Vieira et al. Citation2012]. Adult male rats treated with SSRI also showed changes in spermatogenesis and Sertoli cell population [Aggarwal et al. Citation2012; Silva Junior et al. Citation2008; Silva Junior et al. Citation2013]. Furthermore, the blockage of the serotoninergic system in prepubertal rats inhibits spermatogenesis development [Aragón et al. Citation2005].
Fluoxetine is a SSRI widely used to treat depression during pregnancy and lactation. Fluoxetine is metabolized to norfluoxetine, which in humans has a plasma half-life of approximately 15 days and action similar to fluoxetine [Kristensen et al. Citation1999]. Fluoxetine and norfluoxetine are highly soluble in lipids, crossing the placenta and being secreted in breast milk [Heikkinen et al. Citation2003]. In pregnant rats, fluoxetine and norfluoxetine can cross the placenta and reach the embryo and fetus during and after organogenesis [Pohland et al. Citation1989]. The transference of fluoxetine and norfluoxetine in the maternal milk of rats also has been used to study the effects of SSRI in offspring [Pereira et al. Citation2007].
The male reproductive system develops during the fetal period; furthermore, testicular development in rats occurs from 13 days of gestation to perinatal period, this period is critical to determine the population of Sertoli cells, which is directly related to the size of the testis and sperm production [França et al. Citation2000; Orth Citation1984]. Therefore, exposure to drugs during the testicular development period may be harmful for the offspring and may induce changes in the spermatogenic epithelium of testis and male reproductive organs. These changes in the embryonic period and neonatal period may affect spermatogenesis in adulthood.
The present study aimed to analyze the effects of fluoxetine in the spermatogenesis of adult male rats exposed during the testicular organogenesis and post-organogenesis periods via placenta and lactation.
Results
Animals used in this study in all experimental groups and controls showed no signs of toxicity or changes in health. At birth, body weight of control animals and those exposed to fluoxetine did not show a significant difference. However, from post-natal day 9 to 36 the body weight of animals exposed to 20 mg/kg fluoxetine was reduced compared to the controls; between 36 and 90 days of life there was no statistical difference of body weight related to the different doses of fluoxetine (). At 90 days old, there was a reduction (16%) in testicular weight in the animals exposed to 20 mg/kg fluoxetine with borderline statistical significance (p = 0.056). This weight reduction was not significant in relation to groups exposed to 5 and 10 mg/kg fluoxetine. The epididymal weight (28%) and seminal gland weight (30%) was reduced in animals exposed to fluoxetine at 20 mg/kg. Seminal gland weight was also reduced 25% and 30% in animals exposed to 5 mg/kg and 10 mg/kg of fluoxetine, respectively. Moreover, the prostate weight and gonadosomatic index did not change after fluoxetine exposure via placenta and lactation ().
Figure 1. Growth rate over 90 postnatal days in rats exposed to fluoxetine 5, 10, and 20 mg/kg from 13 days of gestation to 21 postnatal days.
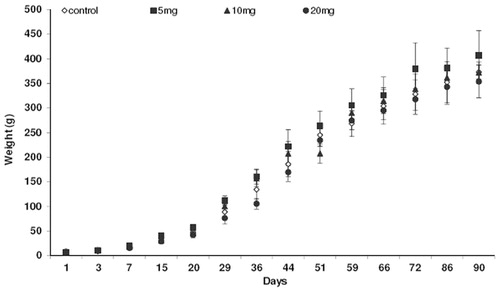
Table 1. Reproductive biometric parameters in adult rats (90 days old) exposed to 5, 10, and 20 mg/kg of fluoxetine from 13 days of gestation to 21 postnatal days.
shows the volume of testicular parenchyma constituents in the control group and rats exposed to 5, 10, or 20 mg/kg fluoxetine. The volume of seminiferous epithelium was reduced by 17% in the group exposed to the highest dose of fluoxetine. The other components of the seminiferous tubule, the lumen and tunica propria, did not change after exposure to fluoxetine. The volume of Leydig cells was reduced by 29% and 30% in the intertubular tissue of animals exposed to 5 mg/kg and 20 mg/kg fluoxetine, respectively. Conjunctive cells, blood vessel, and lymphatic space did not change between experimental groups.
Table 2. Volume of testicular components (μL) in adult rats (90 days old) exposed to 5, 10, and 20 mg/kg of fluoxetine from 13 days of gestation to 21 postnatal days.
Despite the changes in the volume of testicular components, the number of germ cells per tubule cross-section in stage VII of the seminiferous epithelium cycle did not change after exposure to 10 or 20 mg/kg fluoxetine 5 (). The number of spermatocyte I in pre-leptotene, spermatocyte I in pachytene, round spermatids, and spermatogonia, as well as Sertoli cell count did not change after fluoxetine exposure.
Table 3. Germ cell and Sertoli cells counts per cross-section of seminiferous tubule in stage VII of the seminiferous epithelium cycle in adult rats (90 days old) exposed to 5, 10, and 20 mg/kg of fluoxetine from 13 days of gestation to 21 postnatal days.
Testicular parameters in adult rats exposed to 5, 10, and 20 mg/kg fluoxetine from 13 days of gestation to postnatal day 21 are shown in . The daily sperm production per testicle reduced 18% and seminiferous tubule length reduced 17% in animals exposed to 20 mg/kg fluoxetine. Despite the reduction in daily sperm production, spermatogenesis efficiency (DSP/g) did not change after fluoxetine exposure. Tubular diameter, epithelium height, Sertoli cell index, and Sertoli cell population also showed no statistically significant changes.
Table 4. Testicular parameters in adult rats (90 days old) exposed to 5, 10, and 20 mg/kg of fluoxetine from 13 days of gestation to 21 postnatal days.
It is noteworthy that the testes of all experimental groups (controls and exposed to fluoxetine) did not show any pathological changes in optical microscopy (). shows an increase in the plasma testosterone concentration and Leydig cell area in adult rats exposed to 20 mg/kg fluoxetine via placenta and lactation.
Figure 2. Photomicrographs of the testicular parenchyma in adult control rats and rats exposed to fluoxetine via placenta and lactation from 13 days of gestation to 21 postnatal days. (A) Seminiferous tubule cross section (ST) and lymphatic space (arrow) in testis of a control rat. (B) Seminiferous tubule cross section at stage VII in detail (control); note round spermatids (black arrow), spermatocyte I pre-leptotene (white arrow), spermatocyte I pachytene (large arrow), and Sertoli cell (arrow head). (C) Seminiferous tubule cross section (ST) in testicular parenchyma of a rat exposed to fluoxetine 5 mg/kg. (D) A higher magnification of 2 seminiferous tubules in stage VI and stage VIII in an animal exposed to fluoxetine 5 mg/kg, intertubular space (ITT) and Leydig cell (arrow). (E) The lumen (L) and epithelium (E) in a seminiferous tubule cross section of a rat exposed to fluoxetine 10 mg/kg. (F) A higher magnification of the seminiferous tubule in stage VIII in a rat exposed to fluoxetine 20 mg/kg. Note the tunica propria (large arrow) and Leydig cell (arrow) in the intertubular tissue (ITT).
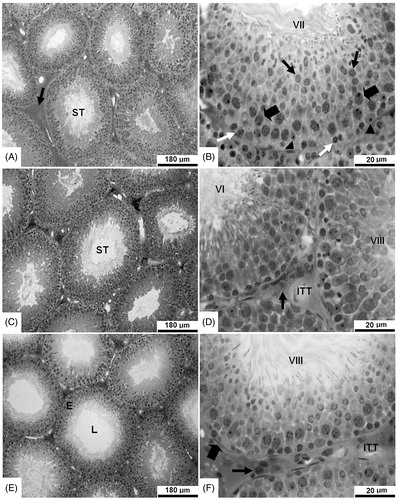
Table 5. Plasma testosterone (ng/mL) and Leydig cell area (µm2) in adult rats (90 days old) exposed to 5, 10, and 20 mg/kg of fluoxetine from 13 days of gestation to 21 postnatal days.
Discussion
In recent years several studies have been emphasizing an urgent need for further research to better understand the fetal consequences of exposure to antidepressants, including SSRI [Cooper et al. Citation2007]. The information about adverse effects of SSRI use during pregnancy or lactation is too limited to conclude whether SSRI should be discontinued [Homberg et al. Citation2010]. Nevertheless, the effects of fluoxetine in the perinatal period still needs to be better understood, to establish safe doses for this drug in this period.
According to Mendes-da-Silva et al. [Citation2002], Silva Junior et al. [Citation2008], and Oliveira et al. [Citation2013], the exposure to fluoxetine reduced the body weight of 21 day postnatal (pre-puberty) rat. It is interesting to note that Silva Junior et al. [Citation2013] showed no change in body weight in adult animals. Results in this study showed a change in body weight from postnatal days 9 to 36 and corroborate the studies cited above. This indicates that upon stopping exposure to fluoxetine in young animals, the body weight may return to normal in adulthood. A previous study using citalopram (a SSRI) administered subcutaneously in rats up to postnatal day 21 also reduced weight gain [Deiró et al. Citation2004]. Furthermore, serotonin induces an inhibitory action on food intake [Simansky Citation1996] and administration of SSRI reduced the height of intestinal villus, which may interfere in nutrient absorption [Morrison et al. Citation2005]. However, it is important to note that Vieira et al. [Citation2012] did not observe changes in body weight of rats at 21 and 100 days of life after exposure to 7.5 mg/kg fluoxetine during pregnancy and lactation.
The reduction in testicular weight shown in this study may be an important consequence for fertility because the weight of testis has a positive relationship with testicular function and spermatogenesis [Russell et al. Citation1990]. Furthermore, a reduction in testicular weight also suggests a loss of germ cells [Lanning et al. Citation2002]. The testis shows fast growth during the fetal and neonatal periods but each cell population expands at different phases, under the influence of different factors. In rats, fetal and neonatal periods are very important to establish the size and weight of testis, Sertoli cell population, length of seminiferous tubules, and sperm production in adulthood [Orth Citation1982; Silva Junior et al. Citation2006].
Seminiferous tubules occupy 89% of the testicular parenchyma in rats and it is directly correlated with testis weight, volume of seminiferous tubules and epithelium, length of seminiferous tubules, and sperm production [França et al. Citation2005; Russell and França Citation1995]. The reduction in these parameters shown in our study also indicates a reduction in fertility after exposure to fluoxetine. Oliveira et al. [Citation2013] also observed a reduced volume of seminiferous tubules and epithelium after exposure to fluoxetine at 20 mg/kg via placenta and lactation. Moreover, germ cells and Sertoli cells are located in seminiferous epithelium [Holstein et al. Citation2003], considering that the population of Sertoli cells did not change, the present study also indicates an effect of fluoxetine on germ cells. This corroborates Vieira et al. [Citation2012], which showed a reduction in the number of spermatozoa after exposure to 7.5 mg/kg fluoxetine via placenta and lactation. Furthermore, Aggarwal et al. [Citation2012] also reported that adult male rats treated with 10, 20, or 40 mg/kg fluoxetine for 2 weeks, 4 weeks, and 12 weeks showed a decrease in thickness of germinal epithelium, diameter of seminiferous tubules, and counts of germ cells.
Previous studies published by our research group showed a reduction in the Sertoli cell population after intraperitoneal treatment with fluoxetine 5, 10, and 20 mg/kg [Silva Junior et al. Citation2008] and exposure to 20 mg/kg fluoxetine via the placenta and lactation [Oliveira et al. Citation2013] in young animals. However, in the present study and according to Silva Junior et al. [Citation2013], the Sertoli cell population in adulthood did not change after perinatal exposure to fluoxetine. This indicates that stopping fluoxetine treatment in the young results in testicle recovery, e.g. Sertoli cell patterns.
Previous studies showed that an increase in brain serotonin may change the levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH) due to the inhibition of gonadotrophin releasing hormone (GnRH) [Das et al. 1985]; therefore, fluoxetine as a selective serotonin reuptake inhibitor may impair Leydig cells and change its volume in the testis. The increase in the individual area of Leydig cells after exposure to fluoxetine corroborates the increased testosterone concentration because testosterone levels are directly related to the size of Leydig cells [Ewing and Keeney Citation1993; França et al. Citation2005]. The main intracellular organelles involved in steroidogenesis, the smooth endoplasmic reticulum, and mitochondria, occupy 53% of Leydig cell size [Mendis-Hadagama et al. Citation1988]. Therefore, the increased individual area of Leydig cells observed in the present study may have increased the concentration of testosterone to assure the efficiency of spermatogenesis. According to França et al. [Citation2005], testosterone has a great role for the maintenance of quantitatively normal spermatogenesis. It is interesting to note that treatment with 10 mg/kg fluoxetine from post-natal days 1 to 21 also reduced plasma FSH levels [Silva Junior et al. Citation2008]. Furthermore, human patients treated with SSRI showed changes in serum levels of LH, FSH, and testosterone [Safarinejad Citation2008], moreover exposure to fluoxetine at 17 mg/kg may increase progestagen and glucocorticoid metabolites in gestation and lactation [Müller et al. Citation2013]. Besides the endocrine changes, men treated with 20 mg/day fluoxetine for 6 weeks experienced induced delay in ejaculation [Waldinger et al. Citation1998] and treatment of mice dams with fluoxetine 7.5 mg/kg during pregnancy and lactation may impair sexual motivation in adult male mice descendants [Gouvêa et al. Citation2008].
The populations of round spermatids, spermatocyte, spermatogonia, and Sertoli cells per cross section of seminiferous tubule is related to the spermatogenesis efficiency [França et al. Citation2005]. Despite the changes in testicular parameters important for total fertility, exposure to fluoxetine did not affect the spermatogenesis efficiency, which may be a result of high concentration of testosterone in order to maintain normal spermatogenesis. Moreover, the main spermatogenesis parameter also called daily sperm production per testicle was reduced after exposure to fluoxetine. This parameter indicates the total sperm produced by an individual in one day and this is directly related to fertility potential [França et al. Citation2005; Russell and França Citation1995].
Interestingly, in this study low doses of fluoxetine (5 and 10 mg/kg) induced only minor changes in spermatogenic parameters. This may be due to the long time that the animals were not exposed to fluoxetine between puberty and adulthood. In addition, other studies also showed changes in spermatogenesis of rats even after a long period of time at doses of 5, 7.5, and 10 mg/kg of fluoxetine [Silva Junior et al. Citation2013; Vieira et al. 2013]. Therefore, doses above 5–10 mg/kg of fluoxetine should be considered carefully as they may represent a risk to the fertility of male rats. Fluoxetine prescription in humans ranges from 20–80 mg/day, corresponding to 0.29–1.14 mg/kg [Vieira et al. 2013]. However, we should consider that rats are more resistant to drugs than humans; according to Aggarwal et al. [Citation2012], 10 mg/kg fluoxetine is a mild dose and 20 mg/kg is a moderate dose for rats. Considering the great importance of fluoxetine to treat depression, including treatment for pregnant and nursing women, the present study also suggests an urgent need for more research to better understand the extent of damage caused by fluoxetine and safe dosage for use during pregnancy and lactation.
In conclusion, exposure to 20 mg/kg fluoxetine via the placenta and lactation, induced a reduction in the weight of the testis, epididymis, and seminal gland. Body weight of animals exposed to 20 mg/kg fluoxetine was reduced from post-natal day 9 to 36 compared to controls. Furthermore, volume of seminiferous epithelium and Leydig cells, length of the seminiferous tubules, and daily sperm production per testicle was also reduced in animals exposed to the highest dose of fluoxetine compared to controls. The individual area of Leydig cell and plasma testosterone increased after exposure to fluoxetine. Therefore, exposure to 20 mg/kg fluoxetine via the placenta and lactation may change testosterone and spermatogenesis parameters in adulthood.
Materials and Methods
Experimental design
Twenty pregnant Wistar rats (Rattus norvegicus, var. Albinos) were kept in an environment with controlled temperature (22 ± 2 °C), humidity (60%), and 12 h light/dark cycle in the biotery of the Animal Morphology and Physiology Department in the Federal Rural University of Pernambuco. Animals received standard pellet food (Purina Labina, Paulínea, Brazil) and water ad libidum. The experimental protocol was approved by the Ethics Committee of the Animal Morphology and Physiology Department of Federal Rural University of Pernambuco (CEEA-DMFA/UFRPE N° 0658706) in accordance with the basic principles for research using animals.
Rat dams were treated daily by oral route (gavage) from gestation day 13 to lactation day 21 with fluoxetine chloride (5 mg/kg, 10 mg/kg, and 20 mg/kg) diluted in deionized water or as controls only deionized water (5 dams in each group). The fluoxetine doses were calculated taking into account the weight of the animal measured every day, based on previous experimental studies with fluoxetine [Frank et al. Citation2000; Oliveira et al. Citation2013]. Fluoxetine chloride was obtained from Roval Manipulation Pharmacy (Recife, Brazil).
Offspring analysis
Following birth, litter size was standardized to 6 male pups. The neonatal male rats remained with their mothers and continued to receive the same treatment by lactation up to postnatal day 21. After weaning, animals were divided into batches of six per cage; at 90 days old, animals exposed perinatally to fluoxetine and the controls were analyzed (5 mg/kg, n = 9; 10/kg, n = 8; 20 mg/kg, n = 9; controls n = 9). Animals were weighed daily during the first 21 days of life and weighed weekly after weaning up to postnatal day 90. The weight gain was compared at each time.
Ninety day old rats were anesthetized by intraperitoneal injection of sodium thiopental (50 mg/kg, Cristália, São Paulo, Brazil), heparinized (125 UI per 100 g, Blausiegel, Cotia, Brazil), and blood samples were collected for testosterone analysis.
Thereafter, animals were submitted to intracardiac perfusion with solution of 0.9% NaCl plus heparin (500 IU/L) and died with hypovolemia. The testes, epididymis, seminal gland, and prostate were removed, dissected. and weighed using a scale (Mark 500 BEL Engineering, Monza, Italy) with 0.001 g precision. Gonadosomatic index (GSI) was calculated according to Tenorio et al. [Citation2011].
Histomorphometrical and histopathological analyses of testes
After washing the vascular system, animals were perfused with 4% glutaraldehyde (Vetec, Rio de Janeiro, Brazil) in 0.01 M phosphate buffer (pH 7.4, Merck, Rio de Janeiro, Brazil). Testicular fragments were cut two millimeters thick and re-fixed in the same glutaraldehyde solution for two h. Subsequently, the fragments of testis were dehydrated in an ascending series of alcohols and embedded in plastic resin (glycol methacrylate, Historesin, Leica, Wetzlar, Germany). The histological sections were cut four micrometers thick and stained with 1% toluidine blue, sodium borate [Tenorio et al. Citation2012].
Histomorphometrical and histopathological analyses of the testes were performed as described by de Siqueira Bringel et al. [Citation2013]. Volumes of the components in testicular parenchyma were calculated from 6,615 intersection points per animal at 400 X magnification. The volume of testicular component (µL) was established from the product of the volume density of testicular components and liquid weight of testis. As the testicular density is approximately 1.03 to 1.04, the testis weight was considered similar to the volume. Testicular liquid weight was obtained by subtraction of the albuginea and mediastinum weight (6.5%) from the gross testicular weight.
Mean diameter of seminiferous tubules was obtained from two diametrically opposed measurements of thirty tubules cross sections with circular shape. Measurements were randomly performed using a micrometer (100 X magnification, U-OCMSQ 10/10, Olympus, Tokyo, Japan). Same cross sections were used in the measurements of seminiferous epithelium height; measurements were performed from the basement membrane to the tubular lumen.
The total length of seminiferous tubules (TLST) per testis, expressed in meters, was obtained by dividing the seminiferous tubule absolute volume (STAV) by r2 (r = diameter/2) and π (area of seminiferous tubule cross section = πr2):
Germ cell populations were classified according to the acrosomal method (stage VII of the epithelium cycle). Germ cells nuclei and Sertoli cells nucleoli were counted in ten seminiferous tubules cross sections per animal. The mean nuclear or nucleolar diameters were measured using a micrometer (U-OCMSQ 10/10, Olympus, Tokyo, Japan) at 1,000 X magnification. Number of nuclei of spermatocytes I in preleptotene stage, spermatocytes I in the pachytene stage, round spermatids, and nucleoli of Sertoli cells were counted. Germ or Sertoli cells counts (GSCC) were corrected for nuclear or nucleolar diameter and histological section thickness. The crude counts (CC) were corrected for section thickness (S) and the mean nuclear or nucleolar diameter (ND):
Number of Sertoli cells per testis (NSCT), also called Sertoli cells population, was determined from the corrected count of Sertoli cells per tubule cross section (CSC), section thickness (S) and the total length of seminiferous tubules (TLST):
Daily sperm production per testis was obtained according to the formula:
DSP = daily sperm production; NSCT = total number of Sertoli cells per testis; RSC = round spermatids count in stage VII; RFS VII = relative frequency of stage VII; Stage VII duration (days).
Daily sperm production per gram of testis (DSP/g) was obtained from the relation between daily sperm production and testicular liquid weight.
Plasma testosterone
Blood samples were obtained at postnatal day 90 by puncture of the venous sinus of the vena cava and placed in plastic containers. Following collection, blood samples were centrifuged and the plasma was stored at −20°C. Testosterone concentration analyses were performed by Enzyme Linked Immuno Sorbent Assay (ELISA), using absorbance reading 405 nm, according to Brown et al. [Citation2004]. All samples were evaluated in duplicate, using a coefficient of variation intra and inter-assay less than 10%.
Computational histometry
After producing the testicular histological sections, 50 images (.sfc format, 2048x1536 pixels) per animal were captured using an optical microscope (Motic BA300, Hong Kong, China), coupled to a digital camera MOTICAM 2300 (Motic, Hong Kong, China), connected to a microcomputer [Tenorio et al. Citation2014]. Leydig cell area measurement was performed using the biometric software Images Plus 2.0 (Motic) calibrated for each objective. Areas of 50 Leydig cells were measured for each animal, chosen randomly. Leydig cell area per cross section was measured in 1,000 x magnification, as previously described by Tenorio et al. [Citation2011].
Statistical analysis
The Shapiro-Wilks test was used to check the tendency to normality of the data. Depending on the normality of the results, either parametric or nonparametric tests were used. On data considered normal, we used analysis of variance (ANOVA) followed by Tukey-Kramer post-hoc test. If the data did not follow a normal distribution, we used the nonparametric test of Kruskal-Wallis followed by Dunn's post-hoc test. Data were expressed as mean ( ± ) standard deviation. A p value < 0.05 was considered statistically significant.
Declaration of interest
The authors report no declarations of interest. Funding sources supporting the work described in the manuscript: Federal Rural University of Pernambuco, Recife, Pernambuco, Brazil and Federal University of Paraná, Curitiba, Paraná, Brazil.
Author contributions
Performed the animals treatment with fluoxetine: WOMF; Prepared the histological sections: SMdT; Performed the histopathological analysis: AAdAJ, MJAALA; Performed the plasma testosterone analysis: AJMA, RNdM; Performed the histomorphometrical analyzes and wrote this manuscript: BMT; Oversaw all stages of the present study and wrote this manuscript: VAdSJ.
References
- Aggarwal, A., Jethani, S.L., Rohatgi, R.K. and Kalra, J. (2012) Effects of Fluoxetine on Testis of Albino rats - A Histological Assessment. Int J Sci Eng Res 3:1–5
- Aragón, M.A., Ayala, M.E., Marín, M., Avilés, A., Damián-Matsumura, P. and Domínguez, R. (2005) Serotoninergic system blockage in the prepubertal rat inhibits spermatogenesis development. Reproduction 129:717–27
- Brown, J., Walker, S. E. and Steinmain, K. (2004) Endocrine Manual for the Reproductive Assessment of Domestic and Non-domestic Species. Virginia: Conservation and Research Center, Smithsonian’s National Zoological Park Front Royal
- Cooper, W.O., Willy, M.E., Pont, S.J. and Ray, W.A. (2007) Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol 196:544.e1–e5
- Das, T.K., Mazunder, R. and Biswas, N.M. (1985) Effect of intraventricular injection of 5,6-dihydroxytryptamine on spermatogenesis and plasma testosterone levels in rats. J Endocrinol 106:395--400
- Deiró, T.C.B.J., Manhães de Castro, R., Cabral Filho, J.E., Souza, S.L., Freitas, S., Ferreira, L.M.P., et al. (2004) Neonatal administration of citalopram delays somatic maturation in rats. Braz J Med Bio Res 37:1503–9
- de Siqueira Bringel, S., Amorim Júnior, A.A., Amorim, M.J., Brito, L.T., Morais, R.N., de Torres S.M., et al. (2013) Endocrine and testicular changes induced by olanzapine in adult Wistar rats. J Appl Toxicol 33:24–31
- Ewing, L.L. and Keeney, D.S. (1993) Leydig cell: structure and function. In Cell and Molecular Biology of the Testis, ed Desjardins, C. and Ewing, L.L. Oxford University Press: New York, pp. 137–65
- França, L.R., Avelar, G.F. and Almeida F.F.L. (2005) Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 63:300–3\18
- França, L.R., Silva, V.A., Jr., Chiarini-Garcia, H., Garcia, S.K., and Debeljuk, L. (2000) Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol Reprod 63:1629–36
- Frank, J.L.W., Hendricks, S.E. and Olson, C.H. (2000) Multiple ejaculations and chronic fluoxetine effects on male rat copulatory behavior. Pharmacol Biochem Behav 66:337–42
- Gouvêa, T.S., Morimoto, H.K., Faria, M.J.S.S., Moreira, E.G. and Gerardin, D.C.C. (2008) Maternal exposure to the antidepressant fluoxetine impairs sexual motivation in adult male mice. Pharmacol Biochem Behav 90:416–19
- Harris, S.S., Maciag, D., Simpson, K.L., Lin, R.C. and Paul, I.A. (2012) Dose-dependent effects of neonatal SSRI exposure on adult behavior in the rat. Brain Res 1429:52–60
- Heikkinen, T.M.D., Ekblad, U., Palo, P. and Laine, K. (2003) Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Terap 73:330–7
- Holstein, A.F., Schulze, W. and Davidoff, M. (2003) Understanding spermatogenesis is a prerequisite for treatment. Reprod Biol Endocrinol 1:107
- Homberg, J.R., Schubert, D. and Gaspar, P. (2010) New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci 31:60–5
- Kim, J., Riggs, K.W., Misri, S., Kent, N., Oberlander, T.F., Grunau, R.E., et al. (2006) Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol 61:155–63
- Kristensen, J.H., Ilett, K., Hackett, F.L.P., Yapp, P., Paech, M. and Begg, E. J. (1999) Distribution and excretion of fluoxetine and norfluoxetine in human milk. J Clin Pharmacol 48:521–7
- Lanning, L.L., Creasy, D.M., Chapin, R.E., Mann, P.C., Barlow, N.J., Regan, K.S., et al. (2002) Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol Pathol 30:507–20
- Leigh, B. and Milgrom, J. (2008) Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry 8:24
- Mendes da Silva, C., Souza, S.L., Barreto Medeiros, J.M., Freitas-Silva, S.R., Antunes, D.E.C., Cunha, A.D.U., et al. (2002) Neonatal treatment with fluoxetine reduces depressive behavior induced by forced swim in adult rats. Arq Neuropsquiatr 60:928–31
- Mendis-Hadagama, S.M.L.C., Zirkin, B.R. and Ewing, L.L. (1988) Comparison of components of the interstitium with testosterone secretion in hamster; rat and guinea pig testes perfused in vitro. Am J Anat 181:12–22
- Morrison, J.L., Riggs, K.W. and Rurak, D.W. (2005) Fluoxetine during pregnancy: impact on fetal development. Reprod Fertil Dev 17:641–50
- Mulder, E.J.H., Ververs, F.F.T., Heus, R. and Visser, G.H.A. (2011) Selective Serotonin Reuptake Inhibitors Affect Neurobehavioral Development in the Human Fetus. Neuropsychopharmacol 36:1961–71
- Müller, J.C., Boareto, A.C., Lourenço, E.L., Zaia, R.M., Kienast, M.F., Spercoski, K.M., et al. (2013) In utero and lactational exposure to fluoxetine in wistar rats: pregnancy outcomes and sexual development. Basic Clin Pharmacol Toxicol 113:132–40
- Oberlander, T.F., Warburton, W., Misri, S., Aghajanian, J. and Hertzman, C. (2006) Neonatal Outcomes After Prenatal Exposure to Selective Serotonin Reuptake Inhibitor Antidepressants and Maternal Depression Using Population-Based Linked Health Data. Arch Gen Psychiatry 63:898–906
- Oliveira, W.M., Sá, I.R., Torres, S.M., Morais, R.N., Andrade, A.M., Maia, F.C.L., et al. (2013) Perinatal exposure to fluoxetine via placenta and lactation inhibits the testicular development in male rat offspring. Syst Biol Reprod Med 59:244–50
- Olivier, J.D.A., Blom, T., Arentsen, T. and Homberg, J.R. (2011) The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: A review. Prog Neuro-Psychopharmacol Biol Psychiatry 35:1400–8
- Orth, J. (1982) Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative and autoradiographic study. Anat Rec 203:485–92
- Orth, J.M. (1984) The role of follicle-stimulating hormone in controlling sertoli cell proliferation in testes of fetal rats. Endocrinology 115:1248–55
- Pawluski, J.L., Rayen, I., Niessen, N.A., Kristensen, S., Van Donkelaar, E.L., Balthazart, J., et al. (2012) Developmental fluoxetine exposure differentially alters central and peripheral measures of the HPA system in adolescent male and female offspring. Neuroscience 220:131–41
- Pereira, J.D., Caricati-Neto, A., Jurkiewicz, A. and Jurkiewicz, N. (2007) Decreased noradrenergic and serotonergic reactivity of vas deferens of newborn rats from mothers treated with the serotonin reuptake inhibitor fluoxetine during pregnancy and breast-feeding. Life Sci 81:1501–8
- Pohland, R.C., Byrd, T.K., Hamilton, M. and Koons, J.R. (1989) Placental transfer and fetal distribution of fluoxetine in the rat. Toxicol Appl Pharmacol 98:198–205
- Russel, L.D., Ettlin, R.A., Sinha Hikim, A.P. (1990) Mammalian spermatogenesis. In Histological and Histopathological Evaluation of the Testis ed. Russel, L.D., Ettlin, R.A. and Sinha Hikim, A.P. Clearwater, Fl: Cache River Press, pp. 1–40
- Russell, L.D. and Franca, L.R. (1995) Building a testis. Tissue Cell 27:129–47
- Safarinejad, M.R. (2008) Evaluation of endocrine profile and hypothalamic–pituitary-testis axis in selective serotonin reuptake inhibitor-induced male sexual dysfunction. J Clin Psychopharmacol 28:418–23
- Silva Junior, V.A., Amorim, M.J.A.L., Amorim Júnior, A.A., Pinto, C. F., Deiró, T.C.B.J., Oliveira, J.R.M., et al. (2008) Neonatal administration of fluoxetine decreased final Sertoli cell number in Wistar rats. Int J Morphol 26:51–62
- Silva Junior, V.A., Monteiro Filho, W.O., Pinto, C.F., Torres, S.M. and Tenorio, B.M. (2013) Testis evaluation of adult Wistar rats after neonatal treatment with fluoxetine. Acta Sci 35:115–22
- Silva Junior, V.A., Vieira, A.C., Pinto, C.F., de Paula, T.A., Palma, M.B., Lins Amorim, M.J., et al. (2006) Neonatal treatment with naloxone increases the population of sertoli cells and sperm production in adult rats. Reprod Nutr Dev 46:157–66
- Simansky, K.J. (1996) Serotoninergic control of the organization of feeding and satiety. Behav Brain Res 73:37–42
- Tenorio, B.M., Ferreira Filho, M.B., Jimenez, G.C., de Morais, R.N., Peixoto, C.A., Nogueira, R.A., et al. (2014) Extremely low-frequency magnetic fields can impair spermatogenesis recovery after reversible testicular damage induced by heat. Electromagn Biol Med 33:139–46
- Tenorio, B.M., Jimenez, G.C., Morais, R.N., Peixoto, C.A., Nogueira, R.A. and Silva Junior, V.A. (2012) Evaluation of testicular degeneration induced by low-frequency electromagnetic fields. J Appl Toxicol 32:210–18
- Tenorio, B.M., Jimenez, G.C., Morais, R.N., Torres, S.M., Nogueira, R. A. and Silva Junior, V.A. (2011) Testicular development evaluation in rats exposed to 60 Hz and 1 mT electromagnetic field. J Appl Toxicol 31:223–30
- Vieira, M.L., Hamada, R.Y., Gonzaga, N.I., Bacchi, A.D., Barbieri, M., Moreira, E.G., et al. (2013) Could maternal exposure to the antidepressants fluoxetine and St. John’s Wort induce long-term reproductive effects on male rats? Reprod Toxicol 35:102–7
- Waldinger, M.D., Hengeveld, M.W., Zwinderman, A.H. and Olivier, B. (1998) Effect of SSRI Antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J Clin Psychopharmacol 18:274–81
