Abstract
Semen samples from 40 patients were collected in consecutive fractions. The variability in semen quality of each fraction was then determined. The first ejaculated fraction (FEF) primarily contained prostatic secretions, while the second ejaculate fraction (SEF) held the majority of the spermatozoa suspended in the secretions from the seminal vesicle. Differences in sperm quality were observed when the FEF was compared to the SEF and the total ejaculate fraction (TEF). These included the seminal parameters (volume, sperm concentration, motility) and sperm DNA fragmentation (SDF). When compared to TEF and SEF, the FEF presented a lower volume, higher sperm concentration, higher motility rates, and lower SDF. The data suggest that the first fraction renders an improved subpopulation of spermatozoa, with lower SDF. Spermatozoa from this fraction and hence their use for ART may have a positive effect on fertilization and embryo development.
Introduction
The success of an assisted reproduction treatment is the sum of many variables, gamete quality being a very important. The assessment of the male factor and its influence on the results has been based on seminal parameters. It is understood that these parameters do not provide sufficient information to determine the fertilization ability of the sperm population, whether it is to be used for ICSI, IVF, or intrauterine insemination [Ahmadi and Ng Citation1999]. In comparison, the integrity of sperm DNA is correlated with infertility [Kumar et al. Citation2013; Ribas-Maynou et al. Citation2012] and has a direct impact on embryo quality and its development [Larrson-Cook et al. Citation2003; Virro et al. Citation2004]. Spermatozoal DNA is subject to damage by endogenous and exogenous factors, acquired as post-testicular damage during sperm transport through the epididymis [Ollero et al. Citation2001; Sakkas and Alvarez Citation2010] or iatrogenic damage, caused by the sample handling in the laboratory.
The ejaculate is comprised of several phases including the pre-ejaculatory phase, first ejaculated fraction (FEF), and second ejaculated fraction (SEF). The pre-ejaculatory phase, does not contain sperm, arises from the Cowper and Littré’s glands to minimize urethral acidity. The FEF represents between 15 to 45% of the whole ejaculate. This phase is rich in sperm and contains epididymal and prostatic secretions. The first fraction is rich in acid phosphatase, citric acid, magnesium, and zinc [Arver Citation1982; Mortimer Citation1994], and seems to exert a protective effect on the sperm. In comparison, the SEF, constitutes the remaining 55 to 85% of the whole volume. It exhibits a low sperm count and primarily contains secretions from the seminal vesicles [Arver Citation1982; Mortimer 1984]. The seminal plasma can be a source of reactive oxygen species, which could have a negative impact on sperm DNA integrity [Tremellen Citation2008] as demonstrated by others [Gosálvez et al. Citation2013; Kumar et al. Citation2011].
It has been previously demonstrated that simple and non-invasive strategies such as recurrent ejaculations are effective in increasing the quality of a semen sample which is to be used for ICSI, even increasing the probability of a successful reproductive outcome [Sánchez-Martín et al. Citation2013]. In this study, we have investigated the utility of using separate ejaculate fractions to improve semen sample quality for use in ART. We hypothesized that of the different phases contained in the ejaculate, the FEF would contain the sperm with the best semen parameters and provide a useul method of sperm selection prior to fertilization.
Results and Discussion
Seminal parameters and DNA fragmentation
Sperm parameters, including volume, concentration, and motility, were analyzed in the different ejaculate fractions (FEF, SEF) and the total ejaculate fraction (TEF) of the three study subgroups, normozoospermic, oligozoospermic, and asthenozoospermic patients. As summarized in , the volume was significantly different between both FEF to SEF and FEF to TEF in all of the study groups. The differences in sperm concentration, however, failed to achieve significance (p > 0.05) when FEF and TEF were compared. However, we did observe a significant increase in sperm concentration, in both normozoospermic and asthenozoospermic patients, respectively, when the FEF and SEF were compared. Similar results were observed in sperm motility, with significant differences between the FEF and the SEF in the three study groups. However, no significant differences were observed between FEF and TEF ().
Table 1. Seminal parameters.
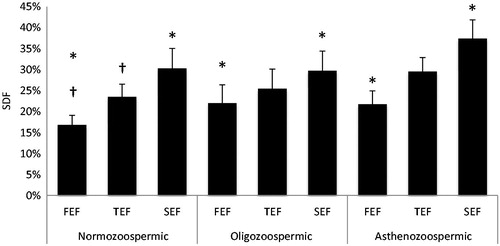
The percentage of sperm with compromised DNA integrity as measured by the sperm chromatin dispersion test was significantly reduced in the first fraction of ejaculate of normozoospermic patients (16.8%) when compared with the second ejaculate fraction (30.3%, p = 0.024) and total ejaculate (23.5%, p = 0.048) (). In oligozoospermic patients, there was also a marked increase in SDF from the FEF (22.2%) to the SEF (27.9%, p = 0.043) and the TEF (25.4%, p = 0.263). In asthenozoospermic patients, we found the same results with FEF fragmentation levels (21.2%) less than those found in SEF (37.4%, p = 0.002) and TEF (29.54, p = 0.414).
Figure 1. Sperm DNA fragmentation. The levels of sperm DNA fragmentation (SDF) were measured by the sperm chromatin decondensation (SCD) assay. The first ejaculate fraction (FEF), total ejaculate fraction (TEF), and second ejaculate fraction (SEF), in normozoospermic, oligozoospermic, and asthenozoospermic patients were compared. SDF presents a similar distribution in all groups, with lower levels in FEF as compared to both SEF and TEF. †p < 0.05; *p < 0.05.
The main objective of this study was to assess differences in sperm quality when performing semen sample collection in two fractions, the first being the initial pulse of ejaculate, and the second, the rest of the ejaculate. Our results showed increased sperm motility, sperm concentration, and reduced DNA fragmentation in the first fraction of the ejaculate as compared to the SEF and, consequently, the complete ejaculate as observed by others [Kumar et al. Citation2011].
The differences observed in the characteristics of the sperm populations in the two ejaculate fractions reflects differences in their composition [Eggert-Kruse et al. Citation2002; Fuse et al. Citation1999; Henkel et al. Citation1999; Wong et al. Citation2001]. For example, prostate secretions like prostate specific antigen (PSA) correlate positively with sperm motility yet have a degradative effect on seminogelins [Lilja et al. Citation1989; Robert and Gagnon Citation1996]. Elements like zinc must also be considered as they are essential for the activation of sorbitol dehydrogenase and lactate dehydrogenase required for sperm motility [[Dhanad et al. Citation1981; Eliasson and Virginia Citation1985].
The ejaculate is treated as a whole even though it is well known that it is composed of many fractions [Kareskoski et al. Citation2011]. In regard to the differences observed in sperm DNA fragmentation, we must consider sources of DNA damage. In addition to the basal damage that occurs during spermiogenesis, e.g., histone replacement, laboratory introduced iatrogenic damage must be considered. Prolonged contact of spermatozoa with seminal vesicle secretions which have chelating agents, alkaline pH, and low zinc availability can affect chromatin stability and hence DNA fragmentation [Arver Citation1982; Mortimer 1984]. To lessen iatrogenic damage, the time between semen delivery and processing should be minimized. As suggested by Kumar et al. [Citation2011] fractioned ejaculate collection is another means of reducing iatrogenic damage. The sperm from the first ejaculate fraction contains prostatic secretions rich in acid phosphatase, magnesium, and zinc, promoting the formation of disulfide bridges, preventing premature chromatin decondensation and inhibiting endonuclease activity [Arver Citation1982]. By collecting the whole ejaculate together, the sperm are exposed to prolonged contact between the components of the first fraction of the ejaculate and the second fraction, with a deleterious result. In comparison, fractioned collection is a simple method that renders a better population of sperm characterized by increased sperm motility, concentration, and most importantly, decreased DNA fragmentation. Whether the separate collection of the FEF and its use influences sygnamy, embryo development, and implantation remains to be critically evaluated.
Materials and Methods
Patient selection criteria
From February 2013 to May 2013 we included a total of 40 IVF patients who consulted Ginemed Reproduction Clinic. We established three study subgroups, with patients who were diagnosed as normozoospermic (n = 10), oligozooespermic (n = 13), and asthenozoospermic (n = 27). For patient classification we followed WHO [1999] criteria which defines oligozoospermia as a sperm concentration < 20 × 106/mL and asthenozoospermia as motility < 50% (a+b). Patients whose semen sample presented both oligo and asthenozoospermic parameters were included in both groups. All patients included were thoroughly informed of the study and written consent was obtained from all the participants before inclusion. The Institutional Ethical Committee approved the study.
Split ejaculate collection
Semen was collected by masturbation into two containers labelled as fraction 1 and fraction 2. Patients were instructed to collect in container 1 the first two organic contractions, which constitute the FEF, and all the subsequent contractions in container 2, corresponding to the SEF. Previously, patients included in the study were instructed on how to perform the fractioned collection of the ejaculate protocol. After collection of the sample according to the protocol, the TEF was reconstituted from proportional aliquots from both fractions. From there, we proceeded to the analysis of the 3 fractions. Patients maintained an ejaculatory abstinence period between 2 and 5 days.
Semen analysis
Sperm count, motility, and morphology were assessed in liquified semen according to WHO [1999] criteria. Normal values for semen variables were sperm concentration >20 million/mL, sperm motility >50% (a+b), and normal morphology >30%. A single observer evaluated all the samples to avoid inter-observer variation in the study. Volume was determined with a 10 mL serological pipette; sperm concentration and motility were assessed in a Makler chamber. SDF was assessed using sperm chromatin dispersion technique by Halosperm (Halotech DNA, Madrid, Spain), following the protocol recommended by the manufacturer. Staining was performed with diff quik and counting was carried out in a bright field microscope under oil immersion at 100 × magnification. A total of 500 spermatozoa were counted in each sample. Sperm with a large or medium halo (halo diameter >1/3 of sperm head size) were considered negative for fragmentation meanwhile sperm with a small or no halo (halo diameter <1/3 of sperm head size) and degraded sperm were considered positive for DNA fragmentation.
Statistical analysis
The data is presented as mean and standard error (mean ± SEM) of the values. The distribution of the samples was checked using the Shapiro-Wilk test of normality. The statistical significance level was calculated with the Student’s t Test for the normally distributed variable (motility) and the nonparametric Mann-Whitney test for those which did not follow a normal distribution, using the software SPSS XVI (SPSS Inc. Released 2007, SPSS for Windows, Version 16.0. Chicago, IL, USA). The graphs were plotted using Microsoft Office Excel 2010 (Microsoft Excel Computer Software, Microsoft, 2003, Redmond, WA, USA).
| Abbreviations | ||
| FEF | = | first ejaculate fraction |
| SEF | = | second ejaculate fraction |
| TEF | = | total ejaculate fraction |
| SDF | = | sperm DNA fragmentation |
Acknowledgments
We would like to thank Mrs. Stephanie Blake for her help in translating the manuscript, and all of the staff of Ginemed Clinics for their help in conducting the study.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Authors contributions
Conceived and designed the experiments, and took part in the writing of the manuscript: MH; Performed the statistical analysis of the data and took part in the writing of the manuscript: MD; Performed the experiments and took part in the writing of the manuscript: MGal; Performed the experiments: MGon; Conceived and designed the experiments, and revised the manuscript before submission: PS-M.
References
- Ahmadi, A. and Ng, S.C. (1999) Development capacity of damaged spermatozoa. Human Reprod 14:2279–2285
- Arver, S. (1982) Zinc and zinc ligands in human seminal plasma III. The principal low molecular weight zinc ligand in prostatic secretion and plasma seminal. Acta Physiol Scand 116:67–73
- Dhanad, O.P., Rao, B.R. and Razdan, M.N. (1981) Sorbitol dehydrogenase and hyaluronidase activity in buffalo semen. Ind J Exp Biol 19:286–289
- Eggert-Kruse, W., Zwick, E.M., Batschulat, K., Rohr, G., Armbruster, P.E., Petzoldt, D., et al. (2002) Are zinc levels in seminal plasma associated with seminal leucocytes and other determinants of semen quality? Fertil Steril 77:260–269
- Eliasson, R. and Virji, N. (1985) LDH-C4 in human seminal plasma and its relationship to testicular function. Int J Androl 8:201–203
- Fuse, H., Kazama, T., Ohta, S. and Fujiuchi, Y. (1999) Relationship between zinc concentrations in seminal plasma and various sperm parameters. Int Urol Nephrol 31:401–408
- Gosálvez, J., Caballero, P., López-Fernández, C., Ortega, L., Guijarro, J.A., Fernández, J.L., et al. (2013) Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICIS of fertile oocyte donors? Asian J Androl 15:821–818
- Henkel, R., Bittner, J., Weber, R., Huther, F. and Miska, W. (1999) Revelance of zinc in human sperm flagella and its relation to motility. Fertl Steril 71:1138–1143
- Kareskoski, M., Sankari, S., Johannisson, A., Kindahl, H., Andersson, M. and Katila, T. (2011) The association of the presence of seminal plasma and its components with sperm longevity in fractionated stallion ejaculates. Reprod Domest Anim 46:1073–1081
- Kumar, D., Kalthur, G., Mascarenhas, C., Kumar, P. and Adiga S.K. (2011) Ejaculate fractions of asthenozoospermic and teratozoospermic patients have differences in the sperm DNA integrity. Andrologia 43:416–421
- Kumar, M., Kumar, K., Jain, S., Hassan, T. and Dada, N. (2013) Insights into the genetic and epigenetic paternal contribution to the human embryo. Clinics (Sao Paulo) 68:5–14
- Larson-Cook, K.L., Brannian, J.D., Hansen, K.A., Kasperson, K.M., Aamold, E.T. and Evenson, D.P. (2003) Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril 80:895–902
- Lilja, H., Abrahamsson, P.A. and Lundwall, A. (1989) Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem 264:1894–1900
- Mortimer, D. (1994) Biochemistry of spermatozoa and seminal plasma. In: Practical Laboratory Andrology, Oxford University Press, New York, USA, pp 89–109
- Ollero, M., Gil-Guzman, E., Lopez, M.C., Sharma, R.K., Agarwal, A., Larson, K., et al (2001) Characterization of subsets of human spermatozoa at different stages of maturation. Implications in the diagnisosis and treatment of male infertility. Human Reprod 16:1912–1921
- Ribas-Maynou, J., Garcia-Peiró, A., Fernandez-Encinas, A., Amengual, M.J., Prada, E., Cortes, P., et al. (2012) Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One 7:e44679
- Robert, M. and Gagnon, C. (1996) Purification and characterization of the active precursor of a human sperm motility inhibitor secreted by the seminal vesicles: identity with semenogelin. Biol Reprod 55:813–821
- Sakkas, D. and Alvarez, J.G. (2010) Sperm DNA fragmentation: mechanism of origin, impact on reproductive outcome, and analysis. Fertil Steril 93:1027–1036
- Sánchez-Martín, P., Sánchez-Martín, F., González-Martínez, M. and Gosálvez, J. (2013) Increased pregnancy after reduced male abstinence. Syst Biol Reprod Med 59:256–260
- Tremellen, K. (2008) Oxidative stress and male infertility – a clinical perspective. Hum Reprod Update 14:243–258
- Virro, M.R., Larson-Cook, K.L., and Evenson, D.P. (2004) Sperm chromatin structure assay (SCSA®) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 81:1289–1295
- Wong, W.Y., Flik, G., Groenen, P.M., Swwinkels, D.W., Thomas, C.M., Copius-Peereboom, J.H., et al. (2001) The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol 15:131–136
- World Health Organization (1999) WHO Laboratory Manual for the Examination of Human Semen and Semen – Cervical Mucus Interaction. 4th edn. Cambridge University Press, Cambridge
