Abstract
Stress is associated with detrimental effects on male reproductive function. It is known that stress increases reactive oxygen species (ROS) generation in the male reproductive tract. High ROS levels may be linked to low sperm quality and male infertility. However, it is still not clear if ROS are generated by stress in the testis. The objective of this study was to characterize the role of oxidative stress induced by cold-water immersion stress in the testis of adult male rats and its relation with alterations in cauda epididymal sperm. Adult male rats were exposed to acute stress or chronic stress by cold-water immersion. Rats were sacrificed at 0, 6, 12, and 24 hours immediately following acute stress exposure, and after 20, 40, and 50 days of chronic stress. ROS production increased only at 6 hours post-stress, while the activity and expression of antioxidant enzymes, lipid peroxidation (LPO), and sperm parameters were not modified in the testis. Corticosterone increased immediately after acute stress, whereas testosterone was not modified. After chronic stress, testicular absolute weight decreased; in addition, ROS production and LPO increased at 20, 40, and 50 days. The activity of superoxide dismutase (SOD) and glutathione peroxidase (GPx) decreased throughout the duration of chronic stress and the activity of catalase (CAT) decreased at 40 and 50 days, and increased at 20 days. The expression of copper/zinc superoxide dismutase (SOD1) and CAT were not modified, but the expression of phospholipid hydroperoxide glutathione peroxidase (GPx-4) decreased at 20 days. Motility, viability, and sperm count decreased, while abnormal sperm increased with chronic stress. These results suggest that during acute stress there is a redox state regulation in the testis since no deleterious effect was observed. In contrast, equilibrium redox is lost during chronic stress, with low enzyme activity but without modifying their expression. In addition, corticosterone increased while testosterone decreased, this decrease is related to the negative effects seen in sperm.
Introduction
Stress is defined as physical and psychological modifications that disrupt homeostasis and balance in organisms [Rivier and Rivest Citation1991]; and the stimuli that challenge homeostasis are designated as stressors. The response to stress depends on the intensity of the stressor, its unpredictability, and uncontrollability [Koolhaas et al. Citation2011]. Stress is subdivided based on duration: acute (single, intermittent, and time-limited exposures) and chronic (intermittent-and-prolonged or continuous exposures) [Pacak et al. Citation1998]. The secretion of glucocorticoids is a classic endocrine response to stress [Sapolsky et al. Citation2000]. Several studies have suggested that stress may cause infertility by affecting the gonads [Al-Joudi and Jamil Citation2012; Chidrawar et al. Citation2011; Kirby et al. Citation2009; McGrady Citation1984]. Both acute and chronic stress cause an increase in glucocorticoid levels, which precedes a decrease in testosterone levels in males [Dhanabalan et al. Citation2011; Dong et al. Citation2004; Srivastava et al. Citation1993]. It has been suggested that glucocorticoids increase oxidative stress in several tissues including the testes. Furthermore, suppression of testicular testosterone inhibits the testicular expression of antioxidant enzymes, leading to an oxidative stress state [Ghosh et al. Citation2002; Zini and Schlegel Citation2003].
Oxidative stress results from the imbalance between the cellular antioxidant defense systems and the production of reactive oxygen species (ROS); they target cells and lead to oxidative damage from the interaction of reactive oxygen with critical cellular macromolecules [Sies Citation1997]. Recently, chronic stress by immobilization has been associated with altering antioxidant enzyme activity, and oxidative damage to membrane lipids and DNA in testes [Nirupama et al. Citation2013; Priya and Reddy Citation2012]. These alterations are explained by the negative effects of ROS, such as superoxide anion radical (), hydrogen peroxide (H2O2) and hydroxyl radical (HO•) [Sikka Citation2001]. Both germ cells and sperm are vulnerable to oxidative stress because they contain high levels of long-chain and very long chain highly unsaturated fatty acids, susceptible of being oxidized and cause cellular damage [Parodi Citation2014; Sikka Citation2001]. Lipid peroxidation (LPO) is highly detrimental to germ cell membrane structure and sperm function inducing numerous cytopathological changes [Collodel et al. Citation2011; Farombi et al. Citation2013; Leong et al. Citation2013] as well as cell death [Maheshwari et al. Citation2009; Mupfiga et al. Citation2013]. In addition, sperm motility is affected by LPO that can compromise the fertilizing capacity of sperm under conditions of oxidative stress [Aitken Citation1999; Aitken and Clarkson Citation1987]. Both morphology and sperm count are other parameters affected by the presence of high ROS concentrations [Agarwal et al. Citation2014].
It is reported that elevated production of ROS in the testes has the propensity to cause significant alteration in tissue physiology or induce oxidative damage to DNA, which is a potential risk to male reproduction [Doreswamy and Muralidhara Citation2005; Sepaniak et al. Citation2004]. Thus, spermatogenesis and sperm function must be protected from oxidative stress. To avoid harmful effects from ROS, the testes mainly use enzymatic copper/zinc superoxide dismutase (Cu/Zn-SOD or SOD1), mitochondrial SOD (Fe/Mn-SOD or SOD2), extracellular SOD (SOD-Ex or SOD3) [Bauche et al. Citation1994; Gu and Hecht Citation1996; Mruk et al. Citation2002], catalase (CAT) [Dastig et al. Citation2011; Luers et al. Citation2006; Nenicu et al. Citation2007], and selenoenzyme phospholipid hydroperoxide glutathione peroxidase (PHGPX or GPx-4) [Baek et al. Citation2007; Kaur et al. Citation2006] and non-enzymatic antioxidants, like reduced glutathione (GSH) [Bilommi et al. Citation2013; Elsharkawy et al. Citation2012]. The cytoplasmic space of sperm is limited and the availability and location of intracellular antioxidant enzymes is restricted, which may be further compromised by the effects of oxidative stress [Weir and Robaire Citation2007].
However, to our knowledge, the effects of acute stress on the oxidative state, as well as the beginning of oxidative stress after chronic stress have not been assessed. Therefore, the aim of this work was to evaluate the effects of acute and chronic stress, at different time points, on the activity of testicular antioxidant enzymes, SOD, CAT, and GPx, as well as the expression of SOD1, CAT, and GPx-4, ROS production, and LPO. Epididymal sperm parameters from the caudal region, as well as serum corticosterone and testosterone were evaluated under the same conditions.
Results
In the present study, we observed that testicular ROS production increased 6 hours after the acute exposure to the stressor, returning to similar levels to those of the control group at 12 and 24 hours after stress (). In contrast, in chronic stress groups, there was a marked increase in ROS production at 20, 40, and 50 days (). Testis LPO remained constant after the males were first exposed to stress (). In comparison, under chronic stress, LPO increased at days 20, 40, and 50 of stress ().
Figure 1. Reactive oxygen species (ROS) production in the testis of rats subjected to acute and chronic stress. (A) In males exposed to one stress session ROS generation increased significantly only at 6 hours post-stress. (B) In males stressed chronically for 20, 40, and 50 consecutive days ROS production increased significantly. The fluorescence relative units of the three control groups were normalized to 1 and are represented as a control group. The values of the graph show the mean ± SD (n = 5). One-way ANOVA. Tukey-Kramer post-test. *p < 0.05 compared with control group.
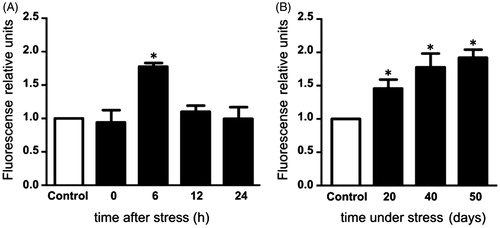
Figure 2. Lipid peroxidation (LPO) in the testis of rats acutely and chronically stressed. (A) LPO was evaluated at 0, 6, 12, and 24 hours after stress exposure in males exposed to one stress session. No significant differences were observed in the concentrations of lipid hydroperoxide. One-way ANOVA. (B) In males stressed chronically for 20, 40, and 50 consecutive days the concentrations of lipid hydroperoxide were significantly increased. The values of the graph show the mean ± SD (n = 5). Two-way ANOVA. Tukey-Kramer post-test. *p < 0.05 compared with their respective control group.
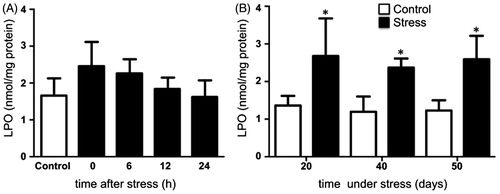
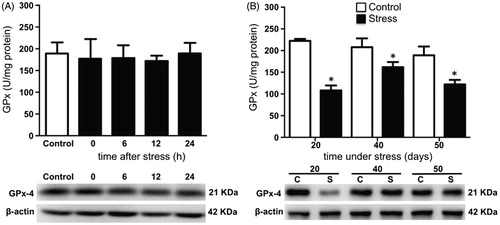
Testicular SOD activity remained unchanged after acute stress when compared with the control group (). Conversely, SOD activity decreased on days 20, 40, and 50 of stress, compared with the control group (). CAT activity was not modified after acute stress (). Its activity increased at day 20 but decreased at days 40 and 50 of chronic stress (). GPx activity was not modified by acute stress (), but decreased with chronic stress at 20, 40, and 50 days (). The expression of testicular SOD1, CAT, and GPx-4 did not change with acute stress (, and , bottom panel). Similarly, the expression of SOD1 and CAT remained unchanged on days 20, 40, and 50 of chronic stress ( and ). The expression of GPx-4 decreased with 20 days of chronic stress, but remained unchanged at 40 and 50 days (, lower panel).
Figure 3. Total superoxide dismutase (SOD) activity and copper/zinc superoxide dismutase (SOD1) expression in the testis of stressed rats. SOD1 expression was determined by Western blot (bottom panel). (A) In males exposed to one stress session no significant differences in the activity and expression of enzyme at 0, 6, 12, and 24 hours post-stress were observed. One-way ANOVA. (B) In males stressed chronically for 20, 40, and 50 consecutive days, total SOD activity decreased at different time points as result of chronic stress. The values of the graph show the mean ± SD (n = 5). Two-way ANOVA. Tukey-Kramer post-test. *p < 0.05 compared with their respective control group.
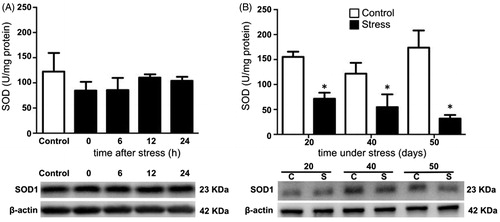
Figure 4. Activity and expression of catalase (CAT) in the testis of stressed rats. (A) No modification of CAT activity was observed in males exposed to one stress session at 0, 6, 12, and 24 hours post-stress. One-way ANOVA. Acute stress did not modify the expression of the enzyme. CAT expression was determined by Western blot (bottom panel). (B) In males stressed chronically for 20, 40, and 50 consecutive days. CAT activity increased after 20 days of stress and decreased at 40 and 50 days. The values of the graph show the mean ± SD (n = 5). Two-way ANOVA. Tukey-Kramer post-test. *p < 0.05 compared with their respective control group.
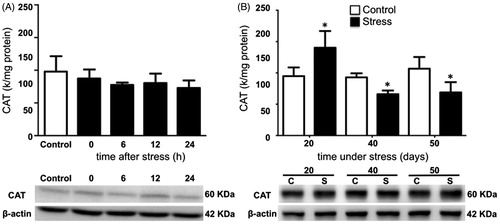
Figure 5. Total glutathione peroxidase (GPx) activity and phospholipid hydroperoxide glutathione peroxidase (GPx-4) expression in the testis of stressed rats. (A) One stress session did not cause modifications in the activity and expression of enzyme at 0, 6, 12, and 24 hours post-stress. One-way ANOVA. (B) Total GPx activity in the testes of males stressed chronically for 20, 40, and 50 consecutive days decreased significantly. The expression of the GPx-4 was determined by Western blot (bottom panel). Western blot analysis showed that solely at 20 days of stress decreased the GPx-4 expression. The values of the graph show the mean ± SD (n = 5). Two-way ANOVA. Tukey-Kramer post-test. *p < 0.05 compared with their respective control group.
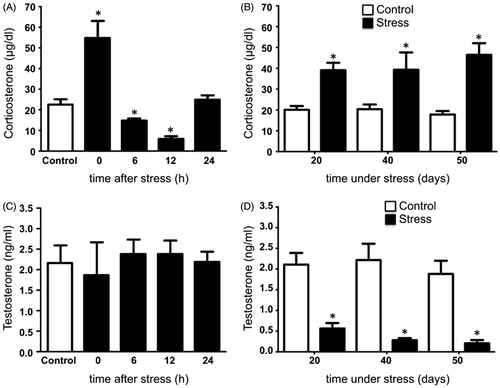
Plasma levels of corticosterone increased significantly in rats exposed to cold-water immersion stress at hour 0, while at 6 and 12 hours post-stress corticosterone decreased compared to control males (). In males subjected to chronic stress, a significant increase of corticosterone occurred as compared to control males (). Acute stress did not modify testosterone in rats exposed to the stressor (), whereas testosterone was reduced at 20, 40, and 50 days of stress compared with the control group ().
Figure 6. Serum corticosterone and testosterone levels in rats under acute and chronic stress. (A) Corticosterone increased significantly immediately after the exposure to the stressor and decreased at 6 and 12 hours post-stress, returning at 24 hours to similar concentrations to the control group. One-way ANOVA. (B) Corticosterone increased significantly in males stressed chronically for 20, 40, and 50 consecutive days. (C) Testosterone in males exposed to acute stress was not modified at 0, 6, 12, and 24 hours post-stress. One-way ANOVA. (D) Males stressed chronically for 20, 40, and 50 consecutive days showed a significant decrease in testosterone. The values of the graph show the mean ± SD (n = 5). Two-way ANOVA. Tukey-Kramer post-test. *p < 0.05 compared with the control group.
In this study, we observed that testes weights of rats in the stress group at day 50 decreased significantly compared with the control group ().
Table 1. Effect of cold water immersion stress on testicular weight.
Acute stress did not modify any of the sperm parameters analyzed (). The effect of chronic stress on motility, viability, total sperm count, and number of abnormal sperm cells is shown in . A significant decrease was observed in total motility, viability, and total sperm count in males exposed to stress for 20, 40, and 50 days as compared to control rats. A significant increase in the number of abnormal sperm was also observed in rats exposed to chronic stress.
Table 2. Effect of acute stress on epididymal sperm parameters.
Table 3. Effect of chronic stress on epididymal sperm parameters.
The increase in serum corticosterone correlated positively with ROS and LPO in males under chronic stress. CAT activity correlated positively with corticosterone only at day 20 of stress. A negative correlation was observed with both SOD and GPx activities at all days of chronic stress. CAT activity at days 40 and 50 of stress, as well as testosterone also correlated negatively with corticosterone (). The decrease in testosterone correlated positively with motility, viability, and sperm count, but negatively with the number of abnormal sperm ().
Table 4. Pearson correlation between serum corticosterone and oxidative stress markers (ROS, LPO, SOD, CAT, GPx), and serum testosterone levels at different time points.
Table 5. Pearson correlation between serum testosterone and sperm parameters (sperm total motility, sperm viability, sperm count, and abnormal sperm) at different time points.
Discussion
To date, the role played by increased ROS in the male reproductive organs has not been fully investigated. The effects of stress on the activity of antioxidant enzymes and LPO have been examined in the testes of rats subjected to chronic stress for 60 days. In the present work, we studied whether acute stress or shorter periods of chronic stress can cause oxidative stress in the testis and disruptions in sperm. In this study, it was shown that just one exposure to stress by cold-water immersion has an effect on ROS generation in the testis and that it is increased at 6 hours post-stress. However, the increase in ROS is not sustained and is reversible, reaching control levels after 12 hours. This ROS increment is not enough to cause oxidative stress since no LPO was observed after its increase. The activity and expression of SOD, CAT, and GPx enzymes were not modified at any time after acute stress. These results indicate that a single exposure to a stressor is not sufficient to cause oxidative stress in the testis, rather it causes a transient seemingly harmless modification of the cellular redox state.
Hormone level results showed that corticosterone rose immediately after the first exposure to stress; whereas, testosterone remained unaltered, indicating that acute stress does not compromise the steroidogenic function of the testis. In contrast, chronic exposure to stress caused an increase of corticosterone at 20, 40, and 50 days and a concomitant decrease of testosterone. The increase in serum corticosterone is an important indicator of stress [Sapolsky et al. Citation2000], and prolonged exposure to stress has been reported to cause oxidative damage in many tissues [Caro et al. Citation2007; Long et al. Citation2008; Sahin and Gumuslu Citation2007; Zafir and Banu Citation2009], including the testes [Nirupama et al. Citation2013; Priya and Reddy Citation2012]. The positive correlation between increased corticosterone and ROS and LPO, as well as SOD, CAT, and GPx activities suggests that increased glucocorticoids could be related with the decrease in antioxidant enzyme activity, thus increasing ROS and damage to lipids in the testis. These findings also indicate that chronic stress response causes damage to membrane lipids in testicular cells through the increase in ROS, thus affecting testicular steroidogenesis.
In addition, we found that ROS production increased in rats subjected to chronic stress, from day 20 to 50, suggesting that corticosterone may cause prolonged dysregulation of the redox state in the testis, leading to a condition of oxidative stress, as previously stated [Nayanatara Citation2005]. However, LPO and protein oxidation are inconclusive, since LPO increased significantly in rats exposed to stress from 20 to 50 days, nevertheless protein oxidation was not modified in any of those days (data not shown), suggesting that the damage to the testis is caused by a deregulation of the redox state and not by oxidative stress.
It has been reported that stress by immobilization for 60 days causes a decrease in the activity of SOD, CAT, and GPx in the testis [Nirupama et al. Citation2013]. Our results demonstrate that SOD activity decreases from day 20 of stress and remains so until day 50 of chronic stress. The decrease in SOD activity could be due to oxidation of its polypeptide chains during ROS increase [Goldstone et al. Citation2006; Hodgson and Fridovich Citation1975; MacMillan-Crow et al. Citation1998]. Additionally, we observed that chronic stress for 20 days caused an increase in CAT activity and a decrease in GPx activity. At this same time, ROS increased and GPx-4 expression decreased. It is likely that H2O2 generated during chronic stress could have been metabolized, mainly by CAT and not by GPx [Forstrom et al. Citation1979], since CAT activity increases with high levels of H2O2, whereas GPx activates with low levels of H2O2 [Cohen and Hochstein Citation1963]. This could explain the differences observed in the activity of CAT and GPx enzymes. ROS increased at 40 and 50 days of chronic stress. This increase could be due to the production of H2O2 by a direct reduction of O2. Peroxisomes in Leydig, Sertoli and peritubular cells, as well as in spermatogonia, spermatocytes, and round and elongated spermatids in the seminiferous tubules are another important source of H2O2, as they contain a large number of oxidases, which generate H2O2 [Nenicu et al. Citation2007]. This could explain the increase in ROS production from days 20 to 50 of stress and not by H2O2 originating from the dismutation of in the presence of SOD. Reduction in the activity of antioxidant enzymes indicates that exposure to chronic stress only alters their activity as a result of the imbalance of the redox state, since the expression of the enzymes was unchanged. The constant expression of enzymes could reflect a compensatory effect of the imbalance of the redox state.
In addition, we found that absolute weights of the testes decreased in rats stressed for 50 consecutive days; this indicates that these organs are vulnerable to prolonged stress, which has been considered an important parameter to assess the risk of toxic effects in the male reproductive system [Yavasoglu et al. Citation2008]. Along with testicular weight, chronic stress causes damage and loss of Leydig cells by apoptosis [Bitgul et al. Citation2013; Hou et al. Citation2014] and therefore, a decrease in testosterone levels. It is known that testosterone deprivation causes low motility and fertilizing capacity, and sperm death [Dyson and Orgebin-Crist Citation1973]. Androgen receptors are found throughout the epididymis of different species, including the rat [Goyal et al. Citation1997], and are mainly located in the principal cells, but can also be found in basal and apical cells [Zhou et al. Citation2002; Zhu et al. Citation2000]. Principal cells synthesize proteins necessary for sperm maturation [Hermo Citation1995; Hermo and Robaire 2002], and are the most sensitive epididymal cells to testosterone deprivation [Moore and Bedford Citation1979]. Since testosterone did not decrease after acute stress, this could explain the lack of effect on sperm parameters. In our study, after the rats were exposed to chronic stress, we observed a positive correlation between decreased testosterone and motility, viability, and sperm count, as well as a negative correlation with the number of abnormal sperm. A minimum period in the caput and corpus epididymis is required for the maturation processes of sperm [Franca et al. Citation2005]. The reduction in testosterone by stress accelerated the transit time of sperm through the epididymis contributing to the alteration of sperm parameters, since transit time has an important role in sperm maturation [Fernandez et al. Citation2008]. The diminution in motility, viability, and sperm count of chronically stressed males could be due to the acceleration of sperm transit time in the epididymis, as it has been suggested in other studies [Klinefelter Citation2002]. In addition, we observed an increase in flagellar abnormalities, presence of cytoplasmic droplets, and alterations in the connecting piece that can lead to the separation of head and flagella. The detection of morphological abnormalities in sperm and their evaluation, allows inferences regarding changes in the functionality of testes that could affect the process of spermatogenesis, and/or epididymis functionality, particularly the sperm maturation process. In this study, we observed only flagella; the heads had been phagocytized by Sertoli cells or by epididymis macrophages [Baccetti et al. Citation1989; Chemes et al. Citation1999]. Droplets in sperm can induce swelling and flagellar angulation, both of which inhibit progressive motility and are associated with infertility [Cooper et al. Citation2004]. These results indicate that chronic stress alters the maturation process of sperm cells during their transit through the epididymis. It is also possible that the observed disturbances in motility, morphology, and sperm count can be correlated with high ROS levels in sperm, as previously reported in infertile patients [Agarwal et al. Citation2014].
Markers of oxidative stress were modified having undergone 20 days of chronic stress, so it is possible that the imbalance in the redox state in the testis could be started with fewer days of chronic stress. Spermatogenesis extends over approximately 52 days in the rat, and during 20 days of chronic stress the sperm parameters are altered, which would mean that the sperm formed during spermatogenesis are affected by chronic stress both in the late stage of spermatogenesis and in the epididymal environment.
In conclusion, our work shows that a single exposure to stress does not affect the antioxidant status in the testes, while chronic stress by cold water immersion significantly alters the redox state and impairs various testicular functions. This suggests weak testicular adaptation to constant or sustained challenges. These effects correlate with corticosterone increase. Finally, altered sperm parameters due to chronic stress might be a result of a decrease in the level of testosterone.
Materials and Methods
Animals and experimental design
A total of 55 adult male Wistar rats (90 day old, 300-320 g) were housed in polypropylene cages (50 cm x 30 cm x 20 cm). Rats were maintained under controlled temperature (24 ± 1°C) and lighting conditions (12/12 reversed light/dark cycle, lights off at 0900 h). Food and water were available ad libitum throughout the study. Rats were assigned randomly to one of the following groups: control (n = 20), acute stress (n = 20), and chronic stress (n = 15). Control rats remained undisturbed in their cages throughout the experiment. The rats from the experimental groups were submitted to stress by cold-water immersion. Adequate measures were taken to minimize pain or discomfort in the experimental animals. Management of the rats throughout the experiments as well as the method of euthanasia, were in accordance to international standards on animal welfare (NIH guidelines) and to Mexican Official guidelines (NOM-062-ZOO-1999).
Stressor
This model combines physical stress (due to low temperature and shaking) and psychological stress (due to the impossibility to escape). Rats were placed individually in a covered tank of cold water (temperature = 15°C, depth = 15.5 cm) for 15 min to produce cold-water immersion stress. The rats either swam or remained in an upright position, keeping their head above water level. Acute stress was induced by exposing the rats once to the stressor at the onset of the dark phase (0900 h) of the light/dark cycle to avoid alterations in corticosterone levels. Chronic stress was induced by exposing the rats daily to the stressor for 20, 40, or 50 consecutive days at the onset of the dark phase of the cycle. Males stressed acutely were sacrificed at 0, 6, 12, and 24 h after exposure to the stressor. Animals exposed repeatedly to the stressor, were sacrificed at 20, 40, and 50 days, immediately after the last stress session. Five animals from each experimental group were anesthetized with a single injection of sodium pentobarbital (25 mg/kg body weight, i.p.) and subsequently sacrificed by exsanguination. The same procedure was carried out with the animals of the control groups.
Biological samples
Blood samples were collected in glass centrifuge tubes by cardiac puncture. Blood was allowed to clot at room temperature and centrifuged (2000 x g, 4°C, 15 min) to separate serum. Serum samples were stored at −20°C until the time of corticosterone and testosterone extraction and determination by high-performance liquid chromatography (HPLC). The left testis was removed and washed with saline solution (0.9%, w/v), weighed, decapsulated, and clipped to evaluate ROS production, enzymatic activity, antioxidant enzyme expression, and LPO. Tissue destined to evaluate enzyme activity and expression was frozen in liquid nitrogen (−190°C) and kept at −80°C until use. The cauda epididymis was ligated. The ligature was placed between the distal corpus and the proximal cauda, and between the distal cauda and the vas deferens to avoid contamination of sperm from different regions of the caudal region. The caudal region was removed and washed with saline solution. Sperm were obtained from the cauda epididymis for qualitative and quantitative analysis.
Reactive oxygen species measurement
ROS determination in testis was based on a modified fluorometric assay using 2′,7′-dichlrofluorecin diacetate (DCFH-DA, SIGMA) as a ROS and cellular marker of oxidative stress [Kumar and Muralidhara Citation2007; LeBel et al. Citation1992]. Briefly, 0.1 g testis was homogenized in 600 µl 0.1 M phosphate buffer saline, pH 7.4. A total of 200 µl of homogenate was pre-incubated with DCFH-DA (10 µM) at 37°C to allow the probe to be incorporated into any membrane-bound vesicles, and the diacetate group cleaved by esterases. After 30 min of incubation, the conversion of DCFH to the fluorescent product 2′,7′-dichlorofluorescein (DCF) was recorded using a fluorescent spectrophotometer DTX 880 multimodal detector (Beckman Coulter, Atlanta, GA, USA) with excitation at 480 nm and emission at 520 nm. Samples were recovered from each well and protein content in each sample was determined. The results were expressed as relative fluorescence units.
Determination of lipid peroxidation
Lipid peroxidation was determined by using a LPO assay kit (Calbiochem, USA) which measures lipid hydro peroxides (LOOHs) directly utilizing the redox reactions with ferrous ions [Mihaljevic et al. Citation1996]. Briefly, to 0.1 g testicular tissue freshly collected was added 400 µl of HPLC-grade water and homogenized. A total of 300 μl of homogenate was added to 600 µl of chloroform and 300 µl of methanol. The solution was mixed thoroughly and then centrifuged (1500 x g, 0°C, 5 min) to extract LOOHs from the chloroform layer. Then, 500 μl of extract was mixed with 450 μl of chloroform:methanol solvent and 50 μl of freshly prepared chromogen (4.5 mM ferrous sulfate in 0.2 M HCl and 3% ammonium thiocyanate) in a glass tube. Absorbance was measured at 500 nm after 5 min incubation. LOOHs levels in the samples were calculated from a standard curve of LOOHs. Each serum was analyzed in duplicate, and LPO levels were expressed as nmol/mg protein.
Assessment of antioxidant enzymes
Testis were homogenized in 0.1 M HEPES buffer, pH 7.4 and centrifuged (5000 x g, 4°C, 10 min) [Arenas-Rios et al. Citation2007]. The aqueous phase was recovered and used for determination of enzymatic activity, as described.
Total SOD [EC 1.15.1.1] activity was measured employing generated superoxide radicals which react with chloride 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyl tetrazolium (INT) to form red formazan [Arthur and Boyne Citation1985]. SOD activity was measured by the degree of inhibition of this reaction. Total SOD activity was expressed as U/mg protein at 37°C (U = amount of enzyme which inhibits 50% of formazan). CAT [EC 1.11.1.6] activity was measured using hydrogen peroxide as substrate. The principle consists in reducing the color due to peroxidation reaction caused by H2O2 to KMnO4, and maintenance of color in the presence of CAT [Cohen et al. Citation1970]. Enzymatic activity was expressed as k/mg protein (k = first-order reaction rate constant) to 37°C. Total GPx [EC 1.11.1.9] activity was assayed using cumene hydroperoxide as substrate. This method measures the GPx activity indirectly by a coupled reaction to glutathione reductase [Paglia and Valentine Citation1967]. Enzyme activity was expressed as U/mg protein at 37°C (U = amount of enzyme that oxidizes 1.0 nmol of NADPH to NADP+/min).
Western blot analysis
Western blot analysis was performed as previously described [Gomez-Quiroz et al. Citation2008]. Western blot was also performed using antibodies against SOD1 (Santa Cruz, TX, USA (sc-11407) 1:3,000), GPx-4 (Santa Cruz (sc-50497) 1:200), or CAT (Sigma Aldrich 1:3,000). Monoclonal β-actin was used as loading control (Sigma Aldrich, St Louis, MO, USA, (A3854), 1:3,000). Immune complexes were detected using the enhanced chemiluminescence system (Pierce, IL, USA). Bands were visualized using a digital imaging system KODAK Gel Logic 1500 (New York, USA).
High-performance liquid chromatography analysis of hormones
Corticosterone and testosterone were extracted from serum and quantified by HPLC using a modification of the method reported by Woodward and Emery Citation1987 [Woodward and Emery Citation1987]. Serum (1 ml) was mixed with 100 µL of 19-nortestosterone solution (5 μg/ml in methanol/water, 60:40, v/v) as an internal standard. Steroids were extracted into 5 ml of diethyl ether:dichloromethane (60:40 v/v) by vortex mixing and immediately centrifuged (1050 x g, 4°C, 5 min). The organic phase was vortex mixed with 1 ml of HPLC-grade water. After a second centrifugation, organic phase (3 ml) was evaporated at room temperature. The residue was re-dissolved in 100 µl of methanol:water (60:40 v/v). The guard column (Symmetry C18, particle size 3.5 μm, 2.1 mm x 10 mm; Waters Corp., Milford, Massachusetts, USA) and the column were equilibrated using HPLC-grade water:acetonitrile (65:35 v/v) at a flow rate of 0.4 ml/min. Separations were made at a temperature of 40°C, in a Waters Symmetry C18 column (particle size 5 μm; column size 2.0 mm x 150 mm; Waters Corp., Milford, Massachusetts, USA). A Waters 600–MS system controller was used to flush the mobile phase and the steroids were assessed using a 486 Water UV absorbance detector (fitted at 250 nm). The results were analyzed using the Millennium 32 software (Waters Corp., Milford, Massachusetts, USA). A series of standards covering the range of 0–50 µg/dl to corticosterone and 0–34 ng/ml to testosterone were used in daily work. The regression line between the peak height and the levels of corticosterone and testosterone were calculated and used for determining the unknowns. Regression line for corticosterone was: y = 0.6464 + 0.01655x (r2 = 0.99986). For testosterone, the regression line was: y = 0.774 + 0.2181x (r2 = 0.99893). The detection limit of the assay for corticosterone was 0.05 µg/dl and 0.05 ng/ml for testosterone.
Protein content determination
Protein concentration was determined using a bicinchoninic acid kit (Pierce Chemical), according to the manufacturer’s instructions.
Sperm parameters
Sperm were obtained from the cauda epididymis by the chopped method using surgical scissors [Kaabi et al. Citation2003], allowing the exit of sperm. Sperm were diluted in 1 ml of saline solution, pre-warmed at 37°C. Sperm motility was assessed by counting motile and non-motile sperm from a total of 200. Sperm motility was expressed as percentage of motile sperm of the total sperm counted. Sperm viability was determined by eosin-nigrosin assay. Sperm viability was assessed by using the one-step eosin-nigrosin staining technique [Dott and Foster Citation1972]. A sample of 10 µl of sperm suspension was mixed with 10 µl of 0.67% eosin/10% nigrosin stain and placed on a pre-warmed slide. Sperm viability was expressed as the percentage of unstained sperm of the total sperm counted (200). Sperm count was performed using a Neubauer’s chamber as described [Nirupama et al. Citation2013]. The data were expressed as 106/ml for sperm count. Sperm with morphological abnormalities in head and flagellum were counted. Morphological abnormalities were expressed as percentage of a total of 500 sperm counted.
Data analysis
Data were expressed as mean ± standard deviation (SD). Hormone concentrations, LPO levels, antioxidant enzyme activity, sperm parameters (acute stress), and ROS generation (acute and chronic stress) were analyzed by one-way analysis of variance (ANOVA). Testicular weight, hormone concentrations, LPO levels, antioxidant enzyme activity, and sperm parameters under chronic stress were analyzed by two-way ANOVA, with condition and days as factors. Tukey-Kramer test was used for multiple comparisons. Pearson correlation was used to evaluate the correlation between corticosterone and oxidative stress markers, and testosterone with sperm parameters. The level of significance was fixed at p < 0.05.
| Abbreviations | ||
| ROS | = | reactive oxygen species |
| LPO | = | lipid peroxidation |
| SOD | = | superoxide dismutase |
| GPx | = | glutathione peroxidase |
| CAT | = | catalase |
| = | superoxide anion radical | |
| H2O2 | = | hydrogen peroxide |
| HO• | = | hydroxyl radical |
| Cu/Zn-SOD or SOD1 | = | copper/zinc superoxide dismutase |
| Fe/Mn-SOD or SOD2 | = | mitochondrial SOD |
| SOD-Ex or SOD3 | = | extracellular SOD |
| PHGPX or GPx-4 | = | phospholipid hydroperoxide glutathione peroxidase |
| GSH | = | reduced glutathione |
| DCFH-DA | = | 2′,7′-dicholofluorecin diacetate |
| DCF | = | 2′,7′-dichlorofluorescein |
| INT | = | chloride 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyl tetrazolium |
| HPLC | = | high-performance liquid chromatography |
| SD | = | standard deviation |
| ANOVA | = | analysis of variance |
Author contributions
Study design: conceived, designed, and coordinated the study, evaluated steroid hormones, and participated in the critical review of the manuscript: MSIRM; Carried out the assessment of enzymatic antioxidants, western blot, LPO, ROS evaluation, statistical data analysis, and drafted the manuscript: ECGD; Participated in the critical review of the manuscript: LEGQ, EAR, AMA, JAIA. All authors read and approved the final version of the manuscript.
Acknowledgments
We thank CONACyT for financial support to ECG-D for her Ph.D. studies. The authors also express their gratitude to Edith Monroy for her advice on the language of the text.
Declaration of interest
This study was partially funded by Consejo Nacional de Ciencia y Tecnología through agreement 309-0, code C/PFPN-2002-35-32; by ECGD (CVU/becario) 229367/212815, by PROMEP (103.5/09/1247) grants from MdSRM, and by the Universidad Autónoma Metropolitana-Iztapalapa. Financial support was received from CONACyT to ECG-D for Ph.D. studies. The authors report no conflicts of interest.
References
- Agarwal, A., Mulgund, A., Sharma, R., and Sabanegh, E. (2014) Mechanisms of oligozoospermia: An oxidative stress perspective. Syst Biol Reprod Med 60:206–16
- Aitken, R.J. (1999) The Amoroso Lecture. The human spermatozoon—a cell in crisis? J Reprod Fertil 115:1–7
- Aitken, R.J., and Clarkson, J.S. (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 81:459–69
- Al-Joudi, F.S., and Jamil, J.A. (2012) Imprisonment-associated sperm clumping and male infertility. J Int Med Res 40:393–7
- Arenas-Rios, E., Leon-Galvan, M.A., Mercado, P.E., Lopez-Wilchis, R., Cervantes, D.L., and Rosado, A. (2007) Superoxide dismutase, catalase, and glutathione peroxidase in the testis of the Mexican big-eared bat (Corynorhinus mexicanus) during its annual reproductive cycle. Comp Biochem Physiol A Mol Integr Physiol 148:150–8
- Arthur, J.R., and Boyne, R. (1985) Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci 36:1569–75
- Baccetti, B., Burrini, A.G., Collodel, G., Magnano, A.R., Piomboni, P., Renieri, T., et al. (1989) Morphogenesis of the decapitated and decaudated sperm defect in two brothers. Gamete Res 23:181–8
- Baek, I.J., Seo, D.S., Yon, J.M., Lee, S.R., Jin, Y., Nahm, S.S., et al. (2007) Tissue expression and cellular localization of phospholipid hydroperoxide glutathione peroxidase (PHGPx) mRNA in male mice. J Mol Histol 38:237–44
- Bauche, F., Fouchard, M.H., and Jegou, B. (1994) Antioxidant system in rat testicular cells. FEBS Lett 349:392–6
- Bilommi, R., Nawas, B.A., Kusmayadi, D.D., Diposarosa, R., Chairul, A., and Hernowo, B.S. (2013) The effects of glutathione on malondialdehyde expression and seminiferous tubule damage in experimental testicular torsion-detorsion in Wistar rats. J Pediatr Urol 9:1059–63
- Bitgul, G., Tekmen, I., Keles, D., and Oktay, G. (2013) Protective Effects of Resveratrol against Chronic Immobilization Stress on Testis. ISRN Urol 2013:278720
- Caro, P., Gomez, J., Sanz, A., Portero-Otin, M., Pamplona, R., and Barja, G. (2007) Effect of graded corticosterone treatment on aging-related markers of oxidative stress in rat liver mitochondria. Biogerontology 8:1–11
- Chemes, H.E., Puigdomenech, E.T., Carizza, C., Olmedo, S.B., Zanchetti, F., and Hermes, R. (1999) Acephalic spermatozoa and abnormal development of the head-neck attachment: A human syndrome of genetic origin. Hum Reprod 14:1811–18
- Chidrawar, V., Chitme, H., Patel, K., Patel, N., Racharla, V., Dhoraji, N., et al. (2011) Effects of Cynodon dactylon on Stress-Induced Infertility in Male Rats. J Young Pharm 3:26–35
- Cohen, G., Dembiec, D., and Marcus, J. (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–8
- Cohen, G., and Hochstein, P. (1963) Glutathione Peroxidase: The Primary Agent for the Elimination of Hydrogen Peroxide in Erythrocytes. Biochemistry 2:1420–8
- Collodel, G., Federico, M.G., Geminiani, M., Martini, S., Bonechi, C., Rossi, C., et al. (2011) Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod Toxicol 31:239–46
- Cooper, T.G., Yeung, C.H., Wagenfeld, A., Nieschlag, E., Poutanen, M., Huhtaniemi, I., et al. (2004) Mouse models of infertility due to swollen spermatozoa. Mol Cell Endocrinol 216:55–63
- Dastig, S., Nenicu, A., Otte, D.M., Zimmer, A., Seitz, J., Baumgart-Vogt, E., et al. (2011) Germ cells of male mice express genes for peroxisomal metabolic pathways implicated in the regulation of spermatogenesis and the protection against oxidative stress. Histochem Cell Biol 136:413–25
- Dhanabalan, S., Jubendradass, R., Latha, P., and Mathur, P.P. (2011) Effect of restraint stress on 2,3,7,8 tetrachloro dibenzo-p-dioxin induced testicular and epididymal toxicity in rats. Hum Exp Toxicol 30:567–78
- Dong, Q., Salva, A., Sottas, C.M., Niu, E., Holmes, M., and Hardy, M.P. (2004) Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl 25:973–81
- Doreswamy, K., and Muralidhara. (2005) Genotoxic consequences associated with oxidative damage in testis of mice subjected to iron intoxication. Toxicology 206:169–78
- Dott, H.M., and Foster, G.C. (1972) A technique for studying the morphology of mammalian spermatozoa which are eosinophilic in a differential ‘life-dead’ stain. J Reprod Fertil 29:443–5
- Dyson, A.L., and Orgebin-Crist, M.C. (1973) Effect of hypophysectomy, castration and androgen replacement upon the fertilizing ability of rat epididymal spermatozoa. Endocrinology 93:391–402
- Elsharkawy, E.E., Yahia, D., and El-Nisr, N.A. (2012) Chlorpyrifos induced testicular damage in rats: Ameliorative effect of glutathione antioxidant. Environ Toxicol 29:1011–19
- Farombi, E.O., Abarikwu, S.O., Adesiyan, A.C., and Oyejola, T.O. (2013) Quercetin exacerbates the effects of subacute treatment of atrazine on reproductive tissue antioxidant defence system, lipid peroxidation and sperm quality in rats. Andrologia 45:256–65
- Fernandez, C.D., Porto, E.M., Arena, A.C., and Kempinas Wde, G. (2008) Effects of altered epididymal sperm transit time on sperm quality. Int J Androl 31:427–37
- Forstrom, J.W., Stults, F.H., and Tappel, A.L. (1979) Rat liver cytosolic glutathione peroxidase: Reactivity with linoleic acid hydroperoxide and cumene hydroperoxide. Arch Biochem Biophys 193:51–5
- Franca, L.R., Avelar, G.F., and Almeida, F.F. (2005) Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 63:300–18
- Ghosh, D., Das, U.B., Ghosh, S., Mallick, M., and Debnath, J. (2002) Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: A correlative study with testicular oxidative stress. Drug Chem Toxicol 25:281–92
- Goldstone, A.B., Liochev, S.I., and Fridovich, I. (2006) Inactivation of copper, zinc superoxide dismutase by H2O2: Mechanism of protection. Free Radic Biol Med 41:1860–3
- Gomez-Quiroz, L.E., Factor, V.M., Kaposi-Novak, P., Coulouarn, C., Conner, E.A., and Thorgeirsson, S.S. (2008) Hepatocyte-specific c-Met deletion disrupts redox homeostasis and sensitizes to Fas-mediated apoptosis. J Biol Chem 283:14581–9
- Goyal, H.O., Bartol, F.F., Wiley, A.A., Khalil, M.K., Chiu, J., and Vig, M.M. (1997) Immunolocalization of androgen receptor and estrogen receptor in the developing testis and excurrent ducts of goats. Anat Rec 249:54–62
- Gu, W., and Hecht, N.R. (1996) Translation of a testis-specific Cu/Zn superoxide dismutase (SOD-1) mRNA is regulated by a 65-kilodalton protein which binds to its 5′ untranslated region. Mol Cell Biol 16:4535–43
- Hermo, L. (1995) Structural features and functions of principal cells of the intermediate zone of the epididymis of adult rats. Anat Rec 242:515–30
- Hermo, L.R., and Robaire, B. (2002) Epididymis cell types and their function. In: The Epididymis: From Molecules to Clinical Practice. Robaire, B., and Hinton, B.T., eds. New York: Kluwer Academic/Plenum, pp. 81–102
- Hodgson, E.K., and Fridovich, I. (1975) The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: Inactivation of the enzyme. Biochemistry 14:5294–9
- Hou, G., Xiong, W., Wang, Chen, X., and Yuan, T.F. (2014) Chronic stress influences sexual motivation and causes damage to testicular cells in male rats. J Sex Med 11:653–63
- Kaabi, M., Paz, P., Alvarez, M., Anel, E., Boixo, J.C., Rouissi, H., et al. (2003) Effect of epididymis handling conditions on the quality of ram spermatozoa recovered post-mortem. Theriogenology 60:1249–59
- Kaur, P., Kaur, G., and Bansal, M.P. (2006) Tertiary-butyl hydroperoxide induced oxidative stress and male reproductive activity in mice: Role of transcription factor NF-kappaB and testicular antioxidant enzymes. Reprod Toxicol 22:479–84
- Kirby, E.D., Geraghty, A.C., Ubuka, T., Bentley, G.E., and Kaufer, D. (2009) Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A 106:11324–9
- Klinefelter, G.R. (2002) Actions of toxicants on the structure and function of the epididymis. In: The Epididymis: From Molecules to Clinical Practice. Robaire, B., and Hinton, B.T., eds. Kluwer Academic Plenum Publishers, pp. 353–69
- Koolhaas, J.M., Bartolomucci, A., Buwalda, B., de Boer, S.F., Flugge, G., Korte, S.M., et al. (2011) Stress revisited: A critical evaluation of the stress concept. Neurosci Biobehav Rev 35:1291–301
- Kumar, T.R., and Muralidhara. (2007) Induction of oxidative stress by organic hydroperoxides in testis and epididymal sperm of rats in vivo. J Androl 28:77–85
- LeBel, C.P., Ischiropoulos, H., and Bondy, S.C. (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–31
- Leong, C.T., D’Souza, U.J., Iqbal, M., and Mustapha, Z.A. (2013) Lipid peroxidation and decline in antioxidant status as one of the toxicity measures of diazinon in the testis. Redox Rep 18:155–64
- Long, F., Wang, Y., Qi, H.H., Zhou, X., and Jin, X.Q. (2008) Rapid non-genomic effects of glucocorticoids on oxidative stress in a guinea pig model of asthma. Respirology 13:227–32
- Luers, G.H., Thiele, S., Schad, A., Volkl, A., Yokota, S., and Seitz, J. (2006) Peroxisomes are present in murine spermatogonia and disappear during the course of spermatogenesis. Histochem Cell Biol 125:693–703
- MacMillan-Crow, L.A., Crow, J.P., and Thompson, J.A. (1998) Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 37:1613–22
- Maheshwari, A., Misro, M.M., Aggarwal, A., Sharma, R.K., and Nandan, D. (2009) Pathways involved in testicular germ cell apoptosis induced by H2O2 in vitro. FEBS J 276:870–81
- McGrady, A.V. (1984) Effects of psychological stress on male reproduction: A review. Arch Androl 13:1–7
- Mihaljevic, B., Katusin-Razem, B., and Razem, D. (1996) The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free Radic Biol Med 21:53–63
- Moore, H.D., and Bedford, J.M. (1979) Short-term effects of androgen withdrawal on the structure of different epithelial cells in the rat epididymis. Anat Rec 193:293–311
- Mruk, D.D., Silvestrini, B., Mo, M.Y., and Cheng, C.Y. (2002) Antioxidant superoxide dismutase – a review: Its function, regulation in the testis, and role in male fertility. Contraception 65:305–11
- Mupfiga, C., Fisher, D., Kruger, T., and Henkel, R. (2013) The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst Biol Reprod Med 59:304–11
- Nayanatara, A.K., Nagaraja, H.S., Anupama, B.K. (2005) The effect of repeated swimming stress on organ weights and lipid peroxidation in rats. Thai J Pharm Sci 18:3–9
- Nenicu, A., Luers, G.H., Kovacs, W., David, M., Zimmer, A., Bergmann, M., et al. (2007) Peroxisomes in human and mouse testis: Differential expression of peroxisomal proteins in germ cells and distinct somatic cell types of the testis. Biol Reprod 77:1060–72
- Nirupama, M., Devaki, M., Nirupama, R., and Yajurvedi, H.N. (2013) Chronic intermittent stress-induced alterations in the spermatogenesis and antioxidant status of the testis are irreversible in albino rat. J Physiol Biochem 69:59–68
- Pacak, K., Palkovits, M., Yadid, G., Kvetnansky, R., Kopin, I.J., and Goldstein, D.S. (1998) Heterogeneous neurochemical responses to different stressors: A test of Selye’s doctrine of nonspecificity. Am J Physiol 275:R1247–55
- Paglia, D.E., and Valentine, W.N. (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–69
- Parodi, J. (2014) Motility, viability, and calcium in the sperm cells. Syst Biol Reprod Med 60:65–71
- Priya, P.H., and Reddy, P.S. (2012) Effect of restraint stress on lead-induced male reproductive toxicity in rats. J Exp Zool A Ecol Genet Physiol 317:455–65
- Rivier, C., and Rivest, S. (1991) Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: Peripheral and central mechanisms. Biol Reprod 45:523–32
- Sahin, E., and Gumuslu, S. (2007) Immobilization stress in rat tissues: Alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comp Biochem Physiol C Toxicol Pharmacol 144:342–7
- Sapolsky, R.M., Romero, L.M., and Munck, A.U. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
- Sepaniak, S., Forges, T., Fontaine, B., Gerard, H., Foliguet, B., Guillet-May, F., et al. (2004) [Negative impact of cigarette smoking on male fertility: From spermatozoa to the offspring]. J Gynecol Obstet Biol Reprod (Paris) 33:384–90
- Sies, H. (1997) Oxidative stress: Oxidants and antioxidants. Exp Physiol 82:291–5
- Sikka, S.C. (2001) Relative impact of oxidative stress on male reproductive function. Curr Med Chem 8:851–62
- Srivastava, R.K., Taylor, M.F., and Mann, D.R. (1993) Effect of immobilization stress on plasma luteinizing hormone, testosterone, and corticosterone concentrations and on 3 beta-hydroxysteroid dehydrogenase activity in the testes of adult rats. Proc Soc Exp Biol Med 204:231–5
- Weir, C.P., and Robaire, B. (2007) Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J Androl 28:229–40
- Woodward, C.J. and Emery, P.W. (1987) Determination of plasma corticosterone using high-performance liquid chromatography. J Chromatogr 419:280–4
- Yavasoglu, A., Karaaslan, M.A., Uyanikgil, Y., Sayim, F., Ates, U., and Yavasoglu, N.U. (2008) Toxic effects of anatoxin-a on testes and sperm counts of male mice. Exp Toxicol Pathol 60:391–6
- Zafir, A. and Banu, N. (2009) Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J Biochem Biophys 46:53–8
- Zhou, Q., Nie, R., Prins, G.S., Saunders, P.T., Katzenellenbogen, B.S., and Hess, R.A. (2002) Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23:870–81
- Zhu, L.J., Hardy, M.P., Inigo, I.V., Huhtaniemi, I., Bardin, C.W., and Moo-Young, A.J. (2000) Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: A quantitative immunohistochemical study. Biol Reprod 63:368–76
- Zini, A. and Schlegel, P.N. (2003) Effect of hormonal manipulation on mRNA expression of antioxidant enzymes in the rat testis. J Urol 169:767–71
