Abstract
When sperm cannot be retrieved from the testes of patients with azoospermia due to spermatogenic dysfunction (ASD), there is no rational way for the patient to become a biological father. We investigated the possibility of inducing spermatogenesis in such patients by hormonal therapy with recombinant human follicle-stimulating hormone (rhFSH) alone. Twenty-six ASD patients who could not obtain spermatozoa by microdissection testicular sperm extraction (micro-TESE) were confirmed to have arrested spermatogenesis at the late stage of maturation arrest. They were subsequently treated with 75–150 IU two times/week rhFSH alone for 12 months. The primary endpoint was the appearance of sperm in ejaculate, and we followed the patients to determine the outcome of inseminating their partners. After rhFSH treatment, mature spermatozoa were found in the ejaculate in five of 26 (19.2%) patients, all of whom showed histology of non-uniform type maturation arrest. Intracytoplasmic sperm injection of the mature spermatozoa resulted in two ongoing clinical pregnancies (insemination success rate, 40.0%). Recombinant human follicle-stimulating hormone treatment can be used as an advanced assisted reproductive technology to improve spermatogenesis in some azoospermic patients with maturation arrest of spermatogenesis and is a potential treatment option after unsuccessful micro-TESE.
Introduction
The most certain method for retrieving testicular sperm in azoospermia due to spermatogenic dysfunction (ASD) is microdissection testicular sperm extraction (micro-TESE) [Okada et al. Citation2002]. This procedure is performed under an operative microscope and is widely considered the best method for sperm retrieval in ASD. In addition, it can be used to identify larger and more opaque seminiferous tubules, presumably with active spermatogenesis, which makes it possible to remove a minimal amount of testicular tissue to obtain spermatozoa, thereby minimizing damage to the testes. Micro-TESE, in combination with intracytoplasmic injection, is applicable to all cases of ASD, including those with Klinefelter syndrome. However, even with micro-TESE, the rate of successful spermatozoa retrieval in ASD is only around 40% [Okada et al. Citation2002]. Follicle-stimulating hormone (FSH) plays an important role in the initiation and maintenance of spermatogenesis [Nieschlag et al. Citation1999]. Recombinant human FSH (rhFSH) has been used in the treatment of male infertility and several studies have reported successful results in separate patient groups with limited numbers of patients, including those with hypogonadotropic hypogonadism, oligozoospermia, idiopathic infertility, and maturation arrest [Selman et al. Citation2006]. Another study reported that men with maturation arrest on testicular biopsy had spermatozoa in the ejaculate after rhFSH treatment [Efesoy et al. Citation2009]. Theoretically, rhFSH may be effective when serum FSH levels are below the normal range. However, studies have not investigated the effect of rhFSH as a treatment for azoospermic patients with maturation arrest diagnosed on the basis of failed micro-TESE. Therefore, the aim of this study was to investigate the efficacy of rhFSH as a treatment for normal or hyper gonadotropin hormone levels azoospermia in patients with a pathologic diagnosis of maturation arrest.
Results
All patients were followed for at least 12 months after beginning rhFSH treatment. They underwent gonadotropin therapy of 75 IU rhFSH twice/week for the first three months. From month four, the dose of rhFSH was increased to 150 IU twice/week. After treatment, small amounts of sperm (less than one million/ml) were found consistently in the ejaculate in five of 26 (19.2%) patients (). Semen parameters, fecundity of partner, and advanced events were evaluated every month. Semen volume did not change after this treatment. Four of the patients had normal levels of androgen and gonadotropin before treatment and one patient, presenting a serum FSH of 14.4 mIU/ml, exhibited a normal level of androgen but was hyper gonadotropic. Mean Johnsen score count data was 5.37 (). The treatment period until the first appearance of spermatozoa in the ejaculate varied from two to six months. Their chromosomal analysis of peripheral blood lymphocytes indicated normal male karyotype in all patients (46,XY). The genetic screening of azoospermia factor (AZF) was not examined. Intracytoplasmic injection of the spermatozoa obtained from the ejaculates resulted in two ongoing clinical pregnancies from the five subjects. One spouse gave birth.
Figure 1. Spermatids in seminiferous tubules (A) and sperm in the ejaculate after rhFSH treatment (B). Patient of case 3 who could not obtain spermatozoa by microdissection testicular sperm extraction was confirmed to have arrested spermatogenesis at late stage of maturation arrest (A). Mature spermatozoa were found in the ejaculate after rhFSH administration (B). rhFSH: recombinant human follicle-stimulating hormone.
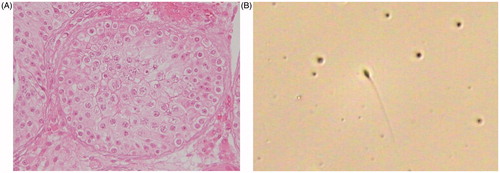
Table 1. Background of patients with sperm in ejaculate after rhFSH and hCG therapy.
Discussion
Micro-TESE is a powerful tool for overcoming infertility in about one-half of the patients presenting as azoospermic. Conventional testicular biopsy can be limiting making it difficult to resolve the affected tubules. There is a significant chance of misdiagnosing maturation arrest in place of hypospermatogenesis, where some seminiferous tubules contain mature spermatozoa. However, using micro-TESE we could provide a definitive diagnosis of maturation arrest. We were able to confirm that all seminferous tubules primarily harbored Sertoli cells (Sertoli cell-only syndrome) and the stage at which spermatogenes is stopped in the tubules.
Hormone therapy with hCG and rhFSH for maturation arrest has been reported [Shiraishi et al. Citation2012]. This group treated patients diagnosed with maturation arrest by micro-TESE and successfully retrieved sperm on a second micro-TESE attempt. Hormone therapy appears to be worthwhile as, after rhFSH treatment, we were also able to obtain sperm in the ejaculate. rhFSH treatment can result in an increase in the number of premeiotic germ cells (spermatogonia, spermatocytes), Sertoli cells, and round spermatids in animal models [Singh et al. Citation1996]. In addition, high dose treatment of rhFSH led to an evident improvement of spermatogenesis in normogonadotropic infertile patients [Paradisi et al. Citation2006].
Maturation arrest is divided into two distinct types, normal uniform maturation arrest and non-uniform maturation arrest [Hung et al. Citation2007]. In this study, only patients with non-uniform maturation arrest benefited from rhFSH treatment. Therefore, it is important to explore molecular histological profiles to determine a patient’s maturation arrest type.
Despite the improved success rate of sperm retrieval by micro-TESE, methods to stimulate spermatogenesis in men with ASD remain unexplored. Until recently, treatment for azoospermic patients entailed ‘looking for sperm’. However, we anticipate that the treatment for these patients may shift in the future to ‘creating sperm’. It was previously reported that hCG-based hormonal therapy prior to micro-TESE was effective in men with hypospermatogenesis [Hung et al. Citation2007]. The present study suggests that treatment with rhFSH-based hormonal therapy might also improve the success of testicular sperm retrieval in men with non-obstructive azoospermia with normal–hyper levels of FSH. It is assumed that the effectiveness of rhFSH therapy for normo-hyper gonadotorophic patients of ASD reflects a functional deficiency of the FSH subtype, however this has not reported in men [Kottler et al. Citation2010] and will be explored by next generation sequencing.
Neonatal mouse testes, which contain only gonocytes or primitive spermatogonia, can produce spermatids and sperm in vitro with serum-free culture media [Sato et al. Citation2011]. In addition, several studies have suggested that spermatozoa derived from induced pluripotent stem cells are a potential source of male gametes in patients with ASD [Sarrate and Anton Citation2009; Yao et al. Citation2011]. In the future, we may be able to induce spermatogenesis in azoospermic patients in vitro. Gonadotropin treatment can improve spermatogenesis in some normo–hypergonadotropic azoospermic patients with maturation arrest of spermatogenesis.
As described in our study an acceptable alternative to push spermatogenesis forward from maturation arrest may be afforded by rhFSH. These results should contribute to further developments in advanced assisted reproductive technology.
Patients and Methods
Between 2009 and 2012, a total of 253 ASD patients underwent micro-TESE at our institution and affiliated hospitals. The patients with obstructive azoospermia were excluded from the study. Semen analyses were repeated at least twice before a diagnosis of azoospermia was made. To exclude the presence of sperm, the ejaculate was centrifuged and thoroughly examined. All of the patients were shown to have a complete absence of spermatozoa in the ejaculate before micro-TESE.
When no sperm could be retrieved by micro-TESE of bilateral testes and histologic diagnosis confirmed late (spermatid) stage maturation arrest, administration of rhFSH (follitropin alpha, Gonalef, Merck Serono Co. Ltd., Tokyo, Japan) was proposed as a further therapeutic option. A total of 26 ASD patients whose spermatogenesis was arrested at around the spermatid stage consulted us about rhFSH treatment after unsuccessful micro-TESE. The procedure was explained to the patients, and all patients gave informed consent before treatment. The procedure was approved by the ethics committee of our hospital.
Gonadotropin and androgen hormone levels were assessed before treatment using standard immunoassay kits. All patients underwent gonadotropin therapy by subcutaneous administration of 75 IU rhFSH twice/week for the first 3 months. From month 4, the dose of rhFSH was increased to 150 IU twice/week. All patients were followed for at least 12 months after beginning of rhFSH treatment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author contributions
Wrote the manuscript: YK, HO; Collected data from four patients (cases 1–4): KS, TI, TS; Collected data from two patients (cases 5 and 6): RS, KN, HY, GA, SS.
References
- Efesoy, O., Cayan, S. and Akbay, E. (2009) The efficacy of recombinant human follicle-stimulating hormone in the treatment of various types of male-factor infertility at a single university hospital. J Androl 30:679–684
- Hung, A.J., King, P. and Shlegerd, P.N. (2007) Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol 178:608–612
- Kottler, M.L., Chou, Y.Y., Chabre, O., Richard, N., Polge, C., Brailly-Tabard, S., et al. (2010) A new FSHbeta mutation in a 29-year-old woman with primary amenorrhea and isolated FSH deficiency: functional characterization and ovarian response to human recombinant FSH. Eur J Endocrinol 162:633–641
- Nieschlag, E., Simoni, M., Gromoll, J. and Weinbauer, G.F. (1999) Role of FSH in the regulation of spermatogenesis: clinical aspects. Clin Endocrinol 51:139–146
- Okada, H., Dobashi, M., Yamazaki, T., Hara, I., Fujisawa, M., Arakawa, S., et al. (2002) Conventional versus microdissection testicular sperm extraction for nonobstructive azoospermia. J Urol 168:1063–1067
- Paradisi, R., Busacchi, P., Seracchioli, R., Porcu, E. and Venturoli, S. (2006) Effects of high doses of recombinant human follicle-stimulating hormone in the treatment of male factor infertility: results of a pilot study. Fertil Steril 86:728–731
- Sarrate, Z. and Anton, E. (2009) Fluorescence in situ hybridization (FISH) protocol in human sperm. J Vis Exp 31:1405
- Sato, T., Katagiri, K., Gohbara, A., Kimiko, I., Narumi, O. Atsuo, O., et al. (2011) In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471:504–507
- Selman, H., De Santo, M., Stertzik, K., Cipollone, G., Aragona, C. and El-Danasouri, I. (2006) Rescue of spermatogenesis arrest in azoospermic men after long-term gonadotropin treatment. Fertil Steril 86:466–468
- Shiraishi, K., Ohmi, C., Shimabukuro, T. and Matsuyama, H. (2012) Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod 27:331–339
- Singh, J. and Handelsman, D.J. (1996) The effects of recombinant FSH on testosterone-induced spermatogenesis in gonadotrophin-deficient (hpg) mice. J Androl 17:382–393
- Yao, L., Yu, X., Hui, N. and Liu, S. (2011) Application of iPS in assisted reproductive technology: sperm from somatic cells? Stem Cell Rev 7:714–721
