Abstract
Four genes involved in DNA double-strand break repair and chromosome synapsis, i.e., testis expressed gene 11 (TEX11), testis expressed gene 15 (TEX15), mutL homolog 1 (MLH1), and homolog 3 (MLH3), play critical roles in genome integrity, meiotic recombination, and gametogenesis. We explored the possible association between single nucleotide polymorphisms (SNPs) in these genes and idiopathic male infertility involving azoospermia or oligozoospermia. A total of 614 fertile control and infertile men were recruited to this study in Sichuan, China. The latter group included 244 men with azoospermia and 72 men with oligozoospermia. Six SNPs in the TEX11, TEX15, MLH1, and MLH3 genes were investigated in both patients and controls by sequencing. The frequency distributions of SNPs rs6525433, rs175080, rs6525433–rs4844247, and rs1800734–rs175080 were found to be significantly different between patients and control groups (p < 0.05), while rs4844247, rs323344, rs323346, and rs1800734 showed no significant difference between the two cohorts. Thus, the SNPs TEX11 rs6525433, MLH3 rs175080, rs6525433–rs4844247, and rs1800734–rs175080 might be associated with male infertility.
Introduction
About 10–15% of couples are known to experience some form of infertility, and among them approximately 50% of the cases are caused by genetic abnormalities [Ferlin et al. Citation2006; Ghorbel et al. Citation2012]. The repair of DNA double-strand breaks (DSBs) is required during homologous chromosome pairing and chromosome synapsis. Correct repair of DSBs and chromosome synapsis enables the maintenance of genome integrity. These processes are essential for gamete formation in sexually reproducing organisms [Inagaki et al. Citation2010].
Testis-expressed gene 11 (TEX11) is an integral component of the synaptonemal complex and ensures the progress of synapsis [Adelman and Petrini Citation2008; Stouffs and Lissens Citation2012]. In a study of Tex11-deficient mice, cells that succeeded in synapsis fail to complete crossover [Yang et al. Citation2008b]. TEX11 promotes the initiation and/or maintenance of crossover formation in a distinct way (). Meiotic homologous recombination is initiated by the formation of DSBs, depending on several accessory factors [La Volpe and Barchi Citation2012]. Testis expressed gene 15 (TEX15) regulates the loading of DNA repair proteins onto sites of DSBs [Inagaki et al. Citation2010]. The mutL homolog 1 (MLH1)/mutL homolog 3 (MLH3) heterodimer, MutLγ, is involved in crossover formation and DNA mismatch repair during the repair of DSBs [Cannavo et al. Citation2005; Li Citation2008].
Figure 1. Synaptonemal complex (SC) formation and repair of DSBs. (A) SC formation. TEX11 and other elements form the SC. TEX11 could contribute to the elongation or stabilization of the SC. (B) DSBs repair. Recombination is initiated by DSBs formation. After DSBs formation, TEX15 regulates DNA repair proteins onto DSBs to insure 5′ to 3′ resection. Then TEX11, MLH1, and MLH3 may be involved in crossover formation. MLH1 and MLH3 also take part in DNA mismatch repair. DSBs: DNA double-strand breaks; TEX11: testis expressed gene 11; TEX15: testis expressed gene 15; MLH1: mutL homolog 1; MLH3: mutL homolog 3.
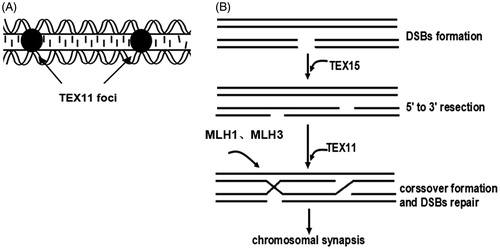
DSB repair and chromosome synapsis play critical roles in the maintenance of genetic integrity. Malfunctions in these processes can lead to various diseases, including infertility [Aparicio et al. Citation2014; Wang and Pan Citation2007]. Studies in gene knockout mice have indicated that Tex11−/Y, Tex15−/−, Mlh1−/−, or Mlh3−/− genotypes result in male infertility [Adelman and Petrini Citation2008; Svetlanov and Cohen Citation2004; Yang et al. Citation2008a; Yang et al. Citation2008b]. TEX11 knockout mice fail to complete synapsis and crossover formation, which might result in male infertility. Loss of function of TEX15 in male mice severely impairs the localization of DNA repair proteins on DSBs and a failure in meiotic recombination. The MLH1 and MLH3 proteins form foci along the synapsed homologs where absence of these proteins leads to defective mismatch repair and sterility in both male and female mice. Based on the important physiological functions of TEX11, TEX15, MLH1, and MLH3, these four genes are good candidates for exploring human male infertility.
Recently, analysis of single-nucleotide polymorphisms (SNPs) in TEX15, MLH1, and MLH3 have helped in understanding the etiology and susceptibility of individual men to male infertility. Studies on the associations of TEX15 rs323344 and rs323346 polymorphisms with male infertility have been reported in populations from Europe, Macedonia, Albania, and Anhui (China) [Aston et al. Citation2010; Plaseski et al. Citation2012; Ruan et al. Citation2012]. MLH1 rs1800734 and MLH3 rs175080 have clinical significance. MLH1 rs1800734 is associated with different disorders, including gender dysfunction, microsatellite instability, and some tumors [Martínez-Urueña et al. Citation2013; Santibanez Koref et al. Citation2010; Svetlanov and Cohen Citation2004], while MLH3 rs175080 is associated with endometrial cancer [Taylor et al. Citation2006]. In the study reported below, we evaluated the associations among six genetic variations in TEX11 rs6525433 (K130A, −344 A/G), TEX11 rs4844247 (E451K, −1306G/A), TEX15 rs323344 (L1337V, −4009T/G), TEX15 rs323346 (I1035V, −3103A/G), MLH1 rs1800734 (c. −93 A > G), and MLH3 rs175080 (P844L, −2531C/T), as a function of male infertility in the Sichuan, Chinese population.
Results
Semen parameters in each study group are shown in . No significant differences (p > 0.05) were observed between cases and controls with respect to age.
Table 1. Semen parameters of the study groups.
Six polymorphisms rs6525433, rs4844247, rs323344, rs323346, rs1800734, and rs175080 in 316 infertile and 298 control fertile men were assessed by sequencing. shows the sequencing results for these polymorphisms in TEX11, TEX15, MLH1, and MLH3. In the control group all were in Hardy–Weinberg equilibrium (p > 0.05). The statistical analysis of the six polymorphisms for two groups is shown in . Two SNPs, rs6525433 and rs175080, exhibited associations with male infertility. The TEX11 rs6525433 homozygous variant genotype (TT) was also associated with male infertility (adjusted OR = 1.517, 95% CI: 1.070–2.150; p = 0.019) when compared with the common homozygous genotype (CC). Moreover, the MLH3 rs175080 variant genotypes (CT/TT) were associated with male infertility compared with the common homozygous TT genotype (adjusted OR = 1.862, 95% CI: 1.319–2.628; p < 0.001).
Figure 2. Sequencing results of the six single nucleotide polymorphisms (SNPs). (A) Sequencing results of the TEX11 rs6525433; (B) Sequencing results of the TEX11 rs4844247; (C) Sequencing results of the TEX15 rs323344; (D) Sequencing results of the TEX15 rs323346; (E) Sequencing results of the MLH1 rs1800734; (F) Sequencing results of the MLH3 rs175080. TEX11: testis expressed gene 11; TEX15: testis expressed gene 15; MLH1: mutL homolog 1; MLH3: mutL homolog 3.
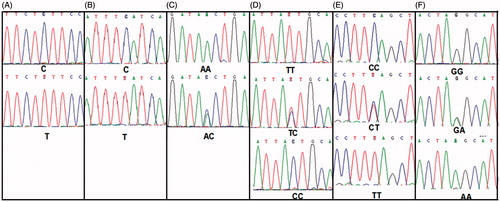
Table 2. Allele frequencies of TEX11, TEX15, MLH1, and MLH3 among the infertile group and controls.
The rs6525433, rs4844247, rs323344, rs323346, rs1800734, and rs175080 variations in the azoospermic group are shown in . There were significant differences among these SNPs. The frequencies of rs175080 CT and CT + TT types were significantly different between the azoospermic group and the fertile controls (rs175080: CT, p < 0.001; CT + TT, p < 0.001). However, the frequencies of the allelic types of rs6525433, rs4844247, rs323344, rs323346, and rs1800734 showed no significant difference.
Table 3. Allele frequencies of TEX11, TEX15, MLH1, and MLH3 among the azoospermic group and controls.
The allelic frequencies of the six SNPs in the oligozoospermic group are listed in . Five of them (rs4844247, rs323344, rs323346, rs1800734, and rs175080) did not demonstrate significant differences between the oligozoospermic group and controls, but rs6525433 showed a difference being much higher in the oligozoospermic group (p = 0.023).
Table 4. Allele frequencies of TEX11, MLH1, and MLH3 among the oligozoospermic group and controls.
Effects of locus-locus interaction analyses among the infertile group and controls
TEX11, TEX15, MLH1, and MLH3 genes are all associated with components of DSB repair and chromosome synapsis. We therefore analyzed the locus-to-locus correlation between the TEX11 rs6525433–rs4844247 and MLH1 rs1800734-MLH3 rs175080 loci among the infertile group and controls. The carriers of rs6525433 CC and rs4844247 TT had an increased risk of male infertility (95% CI: 1.042–2.542; p = 0.031; ). As shown in , rs1800734 TC/TT and rs175080 CT/TT also presented a higher risk of male infertility (95% CI: 1.135–3.582; p = 0.016) compared with those who had both wild-type alleles (rs1800734 CC and rs175080 CC).
Table 5. Interaction analyses for TEX11 rs6525433 and rs4844247 among the infertile group and controls.
Table 6. Interaction analyses for MLH1 rs1800734 and MLH3 rs175080 among the infertile group and controls.
Discussion
We analyzed the association between six SNPs of four genes associated with DSB repair and chromosome synapsis (TEX11, TEX15, MLH1, and MLH3) and male infertility. To our knowledge, this study is the first to provide a comprehensive evaluation of the relationship between polymorphisms in such genes and susceptibility to male infertility. We investigated the polymorphisms of TEX11 rs6525433 and TEX11 rs4844247 for the first time. We also showed that in this population SNPs rs6525433, rs175080, rs6525433-rs4844247, and rs1800734-rs175080 were associated with male infertility.
The TEX11 gene, encoded by the X chromosome, is only expressed in male germ cells [Koslowski et al. Citation2006]. The TEX11 protein promotes chromosomal synapsis and regulates crossover formation in two different ways [Stouffs and Lissens Citation2012; Yu et al. Citation2012]. The frequency of the homozygous genotype TEX11 rs6525433 was significantly associated with general infertility (OR = 1.517, 95% CI: 1.070–2.150, p = 0.019) and with oligozoospermia (OR = 1.858, 95% CI: 1.082–3.192, p = 0.023), indicating that rs6525433 polymorphism plays a role in male infertility in this population. The non-synonymous SNP rs6525433 neutralizes the charge at position 130 of the protein (K130A), which might have a negative effect on protein structure. However, we found no association between the TEX11 rs4844247 and male infertility. To further address the potential combined effects of TEX11 gene SNPs on the risk of male infertility, we analyzed TEX11 rs6525433–rs4844247 variants. The carriers of rs6525433 C and rs4844247 T had an increased risk of infertility (95% CI: 1.042–2.542).
TEX15 is only expressed in the testis and ovary. It regulates the loading of DNA repair proteins onto sites of DSBs and its absence causes a failure in DSB repair [Yang et al. Citation2008a]. Our study on rs323344 and rs323346 showed no association between these variants of TEX15 and male infertility. Similar negative results for rs323344 were shown for populations from Macedonian, Albanian, and Anhui [Plaseski et al. Citation2012; Ruan et al. Citation2012]. However, a positive association between the rs323344 polymorphism and male infertility was observed in a European study [Aston et al. Citation2010]. The rs323346 polymorphism was found to be related to male infertility in a study in Anhui [Ruan et al. Citation2012], but the work of Aston et al. [Citation2010] questioned this association. This inconsistency likely reflects the limited number of samples, and differences in the ethnic and genetic backgrounds studied.
The MLH1 and MLH3 proteins form the MutLγ heterodimer that leads to the formation of DNA crossover and the repair of mismatched DNA through activation of exonuclease-mediated degradation of DNA during DSB repair and chromosome synapsis [Koslowski et al. Citation2006; Mukherjee et al. Citation2010; Svetlanov and Cohen Citation2004]. Present data suggests that rs1800734 affects MLH1 gene expression by adjusting MLH1 promoter trans-factor binding [Martínez-Urueña et al. Citation2013; Mei et al. Citation2010; Santibanez Koref et al. Citation2010]. Methylation of the MLH1 promoter might be significantly associated with gender differentiation, tumor location, tumor differentiation, microsatellite instability, and MLH1 protein expression [Lipkin et al. Citation2000; Santibanez Koref et al. Citation2010; Vogelsang et al. Citation2012]. According to Santibanez Koref’s report, rs1800734 is associated with colorectal cancer risk [Santibanez Koref et al. Citation2010]. Our analyses for polymorphisms in rs1800734 of MLH1 showed that it had no association with male infertility.
The rs175080 SNP is known as a common germ line missense variant (polymorphism) predicted to affect MLH3 protein function [Taylor et al. Citation2006] and has been investigated in a large case-control study of endometrial cancer [Taylor et al. Citation2006]. Here we found that rs175080 was associated with male infertility (OR = 1.862, 95% CI: 1.319–2.628; p < 0.001). A similar result was presented in a study in Hunan, China [Xu et al. Citation2010]. However, future studies are required to determine how rs175080 affects the function of MLH3 and male infertility. In addition, subjects carrying risk genotypes of both rs1800734 TC/TT and rs175080 CT/TT had a two-fold (95% CI: 1.135–3.582; p = 0.016) increase in the risk of male infertility, indicating a significant interaction between the two loci.
In summary, our data suggest that the SNP polymorphisms TEX11 rs6525433, MLH3 rs175080, rs6525433–rs4844247, and rs1800734–rs175080 are associated with idiopathic male infertility presenting as azoospermia or oligozoospermia. However, many different genetic variants and other factors are also likely to influence male infertility. Further studies with larger numbers of subjects and different ethnic populations are needed to confirm these findings.
Materials and Methods
Subjects and collection of blood samples
A test group of 316 infertile men, 244 with idiopathic azoospermia () and 72 with oligozoospermia, were recruited from the Affiliate Hospital of Sichuan Genitalia Hygiene Research Center (Chengdu, Sichuan, China) from October 2012 to January 2014. All samples were de-identified. Patients with chromosomal abnormalities, Y chromosome micro/macro-deletions, hypogonadotropic hypogonadism, infections, obstructive azoospermia, or a history of drug, alcohol, or tobacco use were excluded from the study. The control group included 298 fertile men (>15 × 106 sperm/ml) who had fathered at least one child without needing assisted reproductive technology. The definition of a normal semen sample for sperm concentration (≥15 × 106 sperm/ml), motility (PR, ≥32%), and normal morphology (≥4%) was based on the World Health Organization criteria [Cooper et al. Citation2010]. Semen analyses were performed at least twice for all subjects. All participants were informed about the study according to a protocol that was approved by the institutional ethical review board of Sichuan University (Chengdu, China), and all gave their written consent.
Blood samples (3 ml) were collected in EDTA-coated tubes from participants. Genomic DNA extraction was then performed using the Ezup Column Blood Genomic DNA Purification Kit according to the manufacturer’s recommendations (AnHui U-gene Biotechnology Co., Ltd., China). The concentration of DNA was measured by the s spectrophotometry analysis at 260 nm. All DNA samples were preserved at −20°C.
SNP selection and genotyping
Pairwise linkage disequilibrium (LD) analyses for the SNPs of TEX11, TEX15, MLH1, and MLH3 were performed to optimize the selection according to the public SNP database (http://snpinfo.niehs.nih.gov). This showed that rs6525433, rs4844247, rs323346, rs1800734, and rs175080 were in high LD. The SNPs rs6525433, rs4844247, rs323346, and rs1800734 located at exons of the genes, and rs175080 located at a promoter site, all with minor allele frequencies (MAF) ≥0.10 in the Chinese population, were identified from the NCBI dbSNPs database (http://www.ncbi.nlm.nih.gov/). Based on this pilot genome-wide SNP association study and SNPs with published associations with male infertility, rs323344 was selected to investigate any possible association with idiopathic azoospermia or oligozoospermia [Aston et al. Citation2010].
Six SNPs rs6525433, rs4844247, rs323344, rs323346, rs1800734, and rs175080, were genotyped by sequencing. Polymerase chain reaction amplifications were performed in a total volume of 25µl buffered solution containing about 200 ng of genomic DNA, 0.25 mM of dNTPs (TransGen, Beijing, China), 1.5 mM Mg2+ (Fermentas International Inc., Burlington, Ontario, Canada), 0.2 µM of each primer (Invitrogen, Beijing, China), and 2.5 U Taq polymerase (Fermentas International Inc.). The thermocycling program consisted of 95°C for 2 min; 30 cycles of 95°C for 30 s, annealing temperature () for 30 s, and 72°C for 30 s; a final 7 min extension at 72°C. The products were genotyped by sequencing on an automated sequencer.
Table 7. Primers and conditions used for genotyping analysis of examined polymorphisms.
Statistical analysis
The distributions of rs323344, rs323346, rs1800734, and rs175080 in both cases and controls were examined for deviation from Hardy-Weinberg equilibrium (HWE) using the chi-squared (χ2) tests for each SNP. p Values, Ors, and 95% CIs were calculated using SPSS13.0 software. A p value of <0.05 was considered statistically significant.
| Abbreviations | ||
| DSBs | = | DNA double-strand breaks |
| TEX11 | = | testis expressed gene 11 |
| TEX15 | = | testis expressed gene 15 |
| MLH1 | = | mutL homolog 1 |
| MLH3 | = | mutL homolog 3 |
| SNPs | = | single nucleotide polymorphisms |
Acknowledgments
We are grateful to all who participated in this study.
Declaration of interest
The authors declare that they have no conflict of interest. This research did not receive any specific grants from any funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Conceived and designed the experiments: XHZ, MD, XPD, TJL, HHC. Performed the experiments: XHZ, MD. Analyzed the data: XHZ. Contributed materials: XPD. Wrote the manuscript: XHZ, TJL.
References
- Adelman, C.A. and Petrini, J.H. (2008) ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over. PLoS Genet 4:e1000042
- Aparicio, T., Baer, R. and Gautier, J. (2014) DNA double-strand break repair pathway choice and cancer. DNA Repair 19:169–175
- Aston, K.I., Krausz, C., Laface, I., Ruiz-Castane, E. and Carrell, D.T. (2010) Evaluation of 172 candidate polymorphisms for association with oligozoospermia or azoospermia in a large cohort of men of European descent. Hum Reprod 25:1383–1397
- Cannavo, E., Marra, G., Sabates-Bellver, J., Menigatti, M., Lipkin, S.M., Fischer, F., et al. (2005) Expression of the MutL homologue hMLH3 in human cells and its role in DNA mismatch repair. Cancer Res 65:10759–10766
- Cooper, T.G., Noonan, E., Von Eckardstein, S., Auger, J., Baker, H.G., Behre, H.M., et al. (2010) World Health Organization reference values for human semen characteristics. Hum Reprod Update 16:231–245
- Ferlin, A., Arredi, B. and Foresta, C. (2006) Genetic causes of male infertility. Reprod Toxicol 22:133–141
- Ghorbel, M., Baklouti, S.G., Abdallah, F.B., Zribi, N., Cherif, M., Keskes, R., et al. (2012) Chromosomal defects in infertile men with poor semen quality. J Assist Reprod Gen 29:451–456
- Inagaki, A., Schoenmakers, S. and Baarends, W.M. (2010) DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics-US 5:255–266
- Koslowski, M., Sahin, U., Huber, C. and Türeci, Ö. (2006) The human X chromosome is enriched for germline genes expressed in premeiotic germ cells of both sexes. Hum Mol Genet 15:2392–2399
- La Volpe, A. and Barchi, M. (2012) Meiotic double strand breaks repair in sexually reproducing eukaryotes: we are not all equal. Exp Cell Res 318:1333–1339
- Li, G.-M. (2008) Mechanisms and functions of DNA mismatch repair. Cell Res 18:85–98
- Lipkin, S.M., Wang, V., Jacoby, R., Banerjee-Basu, S., Baxevanis, A.D., Lynch, H.T., et al. (2000) MLH3: a DNA mismatch repair gene associated with mammalian microsatellite instability. Nat Genet 24:27–35
- Martínez-Urueña, N., Macías, L., Pérez-Cabornero, L., Infante, M., Lastra, E., Cruz, J., et al. (2013) Incidence of− 93 MLH1 promoter polymorphism in familial and sporadic colorectal cancer. Colorectal Dis 15:e118–e123
- Mei, M., Liu, D., Dong, S., Ingvarsson, S., Goodfellow, P.J. and Chen, H. (2010) The MLH1 −93 promoter variant influences gene expression. Cancer Epidemiol 34:93–95
- Mukherjee, S., Ridgeway, A. and Lamb, D.J. (2010) DNA mismatch repair and infertility. Curr Opin Urol 20:525–532
- Plaseski, T., Noveski, P., Popeska, Z., Efremov, G.D. and Plaseska-Karanfilska, D. (2012) Association Study of Single-Nucleotide Polymorphisms in FASLG, JMJDIA, LOC203413, TEX15, BRDT, OR2W3, INSR, and TAS2R38 Genes With Male Infertility. J Androl 33:675–683
- Ruan, J., He, X.-J., Du, W.-D., Chen, G., Zhou, Y., Xu, S., et al. (2012) Genetic Variants in TEX15 Gene Conferred Susceptibility to Spermatogenic Failure in the Chinese Han Population. Reprod Sci 19:1190–1196
- Santibanez Koref, M., Wilson, V., Cartwright, N., Cunnington, M.S., Mathers, J.C., Bishop, D.T., et al. (2010) MLH1 Differential allelic expression in mutation carriers and controls. Ann Hum Genet 74:479–488
- Stouffs, K. and Lissens, W. (2012) X chromosomal mutations and spermatogenic failure. BBA-Mol Basis Dis 1822:1864–1872
- Svetlanov, A. and Cohen, P.E. (2004) Mismatch repair proteins, meiosis, and mice: understanding the complexities of mammalian meiosis. Exp Cell Res 296:71–79
- Taylor, N.P., Powell, M.A., Gibb, R.K., Rader, J.S., Huettner, P.C., Thibodeau, S.N., et al. (2006) MLH3 mutation in endometrial cancer. Cancer Res 66:7502–7508
- Vogelsang, M., Wang, Y., Veber, N., Mwapagha, L.M. and Parker, M.I. (2012) The cumulative effects of polymorphisms in the DNA mismatch repair genes and tobacco smoking in oesophageal cancer risk. PloS one 7:e36962
- Wang, P.J. and Pan, J. (2007) The role of spermatogonially expressed germ cell-specific genes in mammalian meiosis. Chromosome Res 15:623–632
- Xu, K., Lu, T., Zhou, H., Bai, L. and Xiang, Y. (2010) The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of male infertility with azoospermia or severe oligozoospermia. Clin Chim Acta 411:49–52
- Yang, F., Eckardt, S., Leu, N.A., McLaughlin, K.J. and Wang, P.J. (2008a) Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J Cell Biol 180:673–679
- Yang, F., Gell, K., van der Heijden, G.W., Eckardt, S., Leu, N.A., Page, D.C., et al. (2008b) Meiotic failure in male mice lacking an X-linked factor. Gene Dev 22:682–691
- Yu, Y.-H., Siao, F.-P., Hsu, L.C.-L. and Yen, P.H. (2012) TEX11 modulates germ cell proliferation by competing with estrogen receptor β for the binding to HPIP. Mol Endocrinol 26:630–642
