Abstract
The data of 3,841 cycles undergoing in vitro fertilization-embryo transfer (IVF-ET) in our reproductive Center between January 2003 and December 2013 were retrospectively analyzed. According to the number of oocytes retrieved, this study was divided into the high ovarian response group (oocyte retrieval ≥20, 842 cycles), the moderate ovarian response group (5< oocyte retrieval <20, 2008 cycles), and the low ovarian response group (oocyte retrieval ≤5, 991 cycles). The treatment outcomes were compared between the patients with an increased progesterone (P) level and the patients where the P level did not increase. With increase in ovarian response, the cut-off values of serum P on the day of human chorionic gonadotrophin (hCG) rose, and respectively were 2.5 ng/ml in the high ovarian response group, 2.25 ng/ml in the moderate ovarian response group, and 1.5 ng/ml in the low ovarian response group. In each group, the clinical pregnancy rate and embryo implantation rate were lower in the patients with an increased P level compared to those where the P level did not increase (all p < 0.05). However, there were no significant difference in the fertilization rate, cleavage rate, and high-quality embryo rate (all p > 0.05). The increased level of P on the day of hCG may affect the treatment outcomes of IVF-ET. The cut-off values of serum P seem to be associated with ovarian response. Increased ovarian response causes the cut-off values of serum P to rise.
Introduction
Although gonadotropin releasing hormone agonists (GnRH-α) have been used in assisted reproductive technology to prevent an early peak of luteinizing hormone (LH), early luteinization still occurs in some patients. This mainly manifests as an increased progesterone (P) level on the day of human chorionic gonadotropin (hCG) administration [Elnashar Citation2010; Venetis et al. Citation2007]. The relation of the increased P level on the day of hCG administration with treatment outcomes of in vitro fertilization-embryo transfer (IVF-ET) attracts a great deal of attention. At present, the effect of an increased P level during the day of hCG administration on the treatment outcomes of IVF-ET remains controversial [Andersen et al. Citation2011; Doldi et al. Citation1999; Liu et al. Citation2013; Martinez et al. Citation2004; Ochsenkühn et al. Citation2012; Papanikolaou et al. Citation2012; Venetis et al. Citation2007; Venetis et al. Citation2013]. Some have not found the negative effects of increased P during the day of hCG administration on the treatment outcomes of IVF-ET [Andersen et al. Citation2011; Martinez et al. Citation2004; Venetis et al. Citation2007]. Venetis et al. [Citation2007] described that the increased level of P on the day of hCG administration might not affect the pregnancy rate of IVF in a meta-analysis including 12 related studies. After that, Venetis et al. [Citation2013] also proposed that the increased P level on the day of hCG administration was likely to decrease the pregnancy rate in a meta-analysis including 63 related studies which is consistent with these results [Liu et al. Citation2013; Ochsenkühn et al. Citation2012; Papanikolaou et al. Citation2012]. Doldi et al. [Citation1999] reported that the increased P level on the day of hCG administration can improve the treatment outcomes of IVF-ET in the patients with polycystic ovary syndrome.
This controversy may be caused by the different definitions of the increased P level. For example, Martínez et al. [Citation2004] failed to find the effect of increased P on treatment outcomes when p > 0.9 ng/ml was regarded as the increased P level. A retrospective analysis of 4,000 cycles indicated that the pregnancy rate significantly decreased when P was more than 1.5 ng/ml [Bosch et al. Citation2010]. These authors in the above studies failed to consider the factor of ovarian response in the definition of the increased P level.
The increased P level on the day of hCG administration may be associated with the accumulated secretion of theca cells caused by the simultaneous development of multiple ovarian follicles during ovarian stimulation [Kyrou et al. Citation2012]. During ovarian stimulation, ovarian response is different in all patients, so the number of developmental ovarian follicles is also different, resulting in different P levels observed on the day of hCG administration. Therefore, when investigating the effects of increased P level on the treatment outcomes of IVF-ET, it is necessary to consider the effects of ovarian response on the increased P level, namely, it is necessary to investigate the relation between P level and the treatment outcomes of IVF-ET in patients with different ovarian responses instead of only considering the P level.
In this study, we retrospectively analyzed the data of 3,841 cycles undergoing IVF-ET in our reproductive Center between January 2003 and December 2013. We first found the cut-off values of serum P in the patients with different ovarian responses, then explored the effect of serum P level during the day of hCG administration on the treatment outcomes of IVF-ET in the patients with different ovarian responses.
Results
According to P levels on the day of hCG administration, the total patients and the patients in each different ovarian response group were respectively divided into the following 8 P intervals: ≤1.00, 1.00–1.25, 1.25–1.5, 1.5–1.75, 1.75–2.00, 2.00–2.25, 2.25–2.5, and >2.5 ng/ml, and then the clinical pregnancy rate in each P interval was calculated. Our results indicated that with the increase of the level of P above a certain threshold, the clinical pregnancy rate decreased (). The cut-off values of serum P were 2.5 ng/ml in the high ovarian response group, 2.25 ng/ml in the moderate ovarian response group, and 1.5 ng/ml in the low ovarian response group, respectively ().
Figure 1. Relation between progesterone (P) level and clinical pregnancy rate in patients according to P levels on the day of hCG administration. The P levels are divided into the following 8 intervals: ≤ 1.00, 1.00–1.25, 1.25–1.5, 1.5–1.75, 1.75–2.00, 2.00–2.25, 2.25–2.5, and > 2.5 ng/ml in the patients. The clinical pregnancy rate in each P interval is calculated to enable the observation of relation between P level and clinical pregnancy rate. (A) total patients; (B) patients with high ovarian response; (C) patients with moderate ovarian response; (D) patients with low ovarian response. On the day of hCG administration: the day of human chorionic gonadotropin administration; P: progesterone.
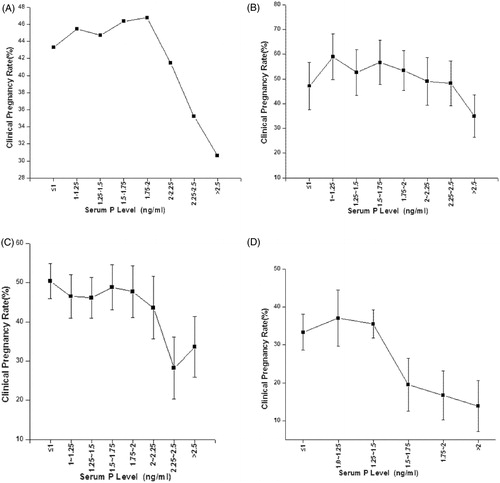
Table 1. Relative OR values obtained by comparison of clinical pregnancy rate between each P interval and the lowest P interval in each group.
Comparisons of general data between the patients with increased P level and the patients in which the P level did not increase
Each group was divided into the patients with an increased P level and the patients in which the P level did not increase according to the cut-off values of serum P. The general data and treatment outcomes of IVF-ET were compared between the patients with increased P level and patients in which the level of P did not increase.
In each group, the number of oocytes retrieved was higher in the patients with increased P than in patients in which the P level did not increase (all p < 0.05). In the moderate and low ovarian response groups, the gonadotropin (Gn) dose was higher in the patients with increased level of P than in the patients in which the P level did not increase (all p < 0.05). In the low ovarian response group, the number of mature oocytes was significantly higher in the patients with an increased P level than in the patients in which the P level did not increase (p < 0.05). In the moderate and low ovarian response groups, there were no statistical differences in the number of mature oocytes between the patients with an increased level of P and the patients in which the P level did not increase (all p > 0.05). There were no statistical differences in female age, duration of infertility, causes of infertility, basal follicle-stimulating hormone (FSH), and Gn days between the patients with an increased level of P and the patients in which the P level did not increase (all p > 0.05; ). In each group, the clinical pregnancy rate and embryo implantation rate were lower in the patients with an increased P level than in the patients in which the P level did not increase (all p < 0.05), but there were no significant differences in the fertilization rate, cleavage rate, high-quality embryo rate, and available embryo rate (all p > 0.05) ().
Table 2. Comparisons of general data between the patients with increased P level and the patients in which the P level did not increase in each group.
Table 3. Comparisons of pregnancy outcomes between the patients with increased progesterone level and the patients in which the progesterone level did not increase in each group.
To identify P level-related factors, age, duration of infertility, causes of infertility, basal FSH, Gn dose, Gn days, and the number of oocytes retrieved underwent logistic regression analysis. Our results indicated that the number of oocytes retrieved and Gn dose were all positively correlated with an increased P level (all p < 0.05) (). ROC curve analysis indicated that the area under ROC curve was 0.702 (95%CI: 0.643–0.761) in the high ovarian response group, 0.66 (95%CI: 0.601–0.718) in the moderate ovarian response group, and 0.71 (95%CI: 0.675–0.744) in the low ovarian response group ().
Figure 2. ROC curve analysis in each group. (A) Area under ROC curve [0.702 (95%CI: 0.643–0.761)] in the high ovarian response group; (B) area under ROC curve [0.66 (95%CI: 0.601–0.718)] in the moderate ovarian response group; (C) area under ROC curve [0.71 (95%CI: 0.675–0.744)] in the low ovarian response group.
![Figure 2. ROC curve analysis in each group. (A) Area under ROC curve [0.702 (95%CI: 0.643–0.761)] in the high ovarian response group; (B) area under ROC curve [0.66 (95%CI: 0.601–0.718)] in the moderate ovarian response group; (C) area under ROC curve [0.71 (95%CI: 0.675–0.744)] in the low ovarian response group.](/cms/asset/f9247113-31d3-44ea-a5d1-24f3e949f35d/iaan_a_1033779_f0002_c.jpg)
Table 4. Logistic regression anaysis of increased P level-related factors in each group.
Discussion
The increased level of P on the day of hCG administration may result from follicular accumulation [Venetis et al. Citation2007]. P secretion is positively correlated with the number of mature follicles in the late follicular phase [Fleming and Jenkins Citation2010]. There is the need to know whether the increased P is associated with ovarian response during controlled ovarian hyperstimulation. Therefore, we first found the cut-off values of serum P in the patients with different ovarian responses, and then explored the effect of increased P on the treatment outcomes of IVF-ET, instead of only observing the relationship between P level and IVF outcomes.
Our results indicated that the cut-off values of serum P to affect the pregnancy rate of IVF-ET varied according to ovarian responses. The cut-off values of serum P were 2.5 ng/ml in the high ovarian response group, 2.25 ng/ml in the moderate ovarian response group, and 1.5 ng/ml in the low ovarian response group. We can see that with an increase in ovarian response, the cut-off values of serum P to affect the treatment outcomes of IVF-ET rise. Griesinger et al. [Citation2013] have reported that with the increase of ovarian response, the P level also rises. Our results also displayed that the increased P level significantly decreased embryo implantation rate and clinical pregnancy rate, demonstrating that the increased P level in late follicular phase may affect treatment outcomes of IVF-ET.
The mechanism that causes the increased P level in the late follicular phase to affect the treatment outcomes is still unclear. Successful pregnancy depends on the interaction between the embryo and endometrium. Melo et al. [Citation2006] established oocyte-donation models, and found that the increased P levels in donors failed to affect the quality of oocytes and there were no significant differences in clinical pregnancy and embryo implantation rates between the doners and other women. These results suggest that the increased P level affects treatment outcomes probably through changing the endometrial implantation window. Xu et al. [Citation2012] found that although the number of mature oocytes increased in the patients with the increased level of P, there was no statistical difference in fertilization rate and cleavage rate between the patients with increased P and the patients in which P did not increase. They hence inferred that the P level may not affect the quality of oocytes. Haouzi et al. [Citation2014] have performed biopsy, DNA sequencing, and qRT-PCR for the endometrium on the days of oocyte retrieval and embryo transfer in 15 patients undergoing IVF-ET, and have found the changes in endometrial gene expression. These changes result in ‘dialogue’ disorders between embryo and the endometrium, affecting the pregnancy rate of IVF-ET. Our results indicated that there were no statistical differences in number of mature oocytes between the patients with an increased P level and the patients in which the P level did not increase in high and moderate ovarian response groups; but in the low response group, the number of mature oocytes was significantly higher in the patients with an increased P level than in the patients in which the P level did not increase. Both embryo implantation rate and clinical pregnancy rate in the patients with an increased P level, no matter the ovarian response, were all significantly decreased as compared to those in the patients in which the P level did not increase; but there were no statistical differences in fertility rate, cleavage rate, high-quality embryo rate, and available embryo rate between the patients with increased P level and the patients in which the P level did not increase. This suggests that an increased P level may fail to affect the quality of embryos and oocytes, but decreases pregnancy rates probably by changing the endometrial receptivity. We will investigate how the different thresholds affect the endometrial receptivity in our further studies.
The phenomenon of the increased P level in some patients undergoing controlled ovarian stimulation is still unclear. There are many hypotheses to this mechanism [Elnashar Citation2010]. For example, the simultaneous development of multiple ovarian follicles results in increased P level; insufficient pituitary-down regulation leads to increased LH level which stimulates P secretion from theca cells; LH receptors have an increased sensitivity to FSH; the patients with low ovarian response have an increased sensitivity to LH; signal pathways between ovarian granulosa cells are injured; the application of large-dose FSH affects steroidogenesis or ovarian function is reduced.
Based on the classic theory of two cells and two gonadotrophic hormones [Moon et al. Citation1978], the combination of LH with LH receptors on theca cells allows intracellular cholesterol to transform into P or 17-hydroxypregnenolone which is further transformed into androgen. The androgen enters granulosa cells. The combination of FSH with FSH receptors on granulosa cells activates aromatizing enzymes which transform androgen into estrogen. Therefore, P is an intermediate product of estrogen synthesis. During controlled ovarian hyperstimulation, P secretion is associated with the number of developmental ovarian follicles, LH stimulation for theca cells, and FSH stimulation for granulosa cells [Fleming and Jenkins Citation2010]. Our logistic regression analysis indicated that the number of oocytes retrieved and Gn dose were all positively correlated with the increased level of P (all p < 0.05), suggesting that the level of P may depend on the number of developmental ovarian follicles and FSH stimulation for granulosa cells.
Our study indicated that the increased level of P in the late follicular phase significantly decreased pregnancy rate and implantation rate of fresh IVF-ET cycles, but failed to affect the quality of oocytes and embryos. In addition the cut-off values of serum P to affect the pregnancy outcomes of IVF-ET varied according to ovarian responses. This suggests that we should take appropriate measures to avoid elevated levels of P based on ovarian responses, or we must provide a suitable strategy of embryo transfer for patients after P elevation.
For the patients with low ovarian response, when there is the diameter of at least one follicle >14 mm, E2 >600 pg/ml, and LH >10 IU/L, administration of antagonists may induce early LH peak and the increased P level [Lainas et al. Citation2005]; when P is more than 1.5 ng/ml, hCG is given in advance to reduce the negative effects of the increased P level on pregnancy outcomes [Harada et al. Citation1996]. For the patients with high ovarian response, such as the patients with polycystic ovary syndrome, recombinant FSH is used with a small starting dose to induce increased P level [Filicori et al. Citation2005].
In addition, with the development of embryo cryopreservation technology, some have proposed embryo freezing to improve pregnancy rate in the patients with increased P level [Venetis et al. Citation2007]. A recent retrospective analysis suggested that the pregnancy rate was higher in freezing cycles than in fresh cycles in the patients with increased P level [Xu et al. Citation2012]. Therefore, based on our results, when the P level is more than 1.5 ng/ml in the patients with low ovarian response, 2.25 ng/ml in the patients with moderate ovarian response, and 2.5 ng/ml in the patients with high ovarian response, these patients should undergo freezing cycles instead of fresh cycles. For the patients with high ovarian response, in particular, freezing cycles improve pregnancy rate but also avoid ovarian hyperstimulation syndrome. The cut-off values of serum P in this study are not suitable for all patients, because some types of patients such as the patients with uterine malformation, uterine polypus, intrauterine adhesion, or endometriosis have been excluded from this study.
In summary, the increased P level on the day of hCG administration is likely to affect the clinical pregnancy rate of IVF-ET. With the increase in ovarian response, the cut-off values of serum P rises. Therefore, the scheme of IVF-ET should be based on ovarian response and P level on the day of hCG administration.
Materials and Methods
This study involving the use of human tissue specimens was approved by the Review Board of the Second Affiliated Hospital of Zhengzhou University. The patient samples used in this study were obtained with informed consent.
Subjects
The patients undergoing IVF-ET in our reproductive Center between January 2003 and December 2013 were enrolled in this study. The exclusion criteria included: (1) uterine malformation, uterine polypus, intrauterine adhesion, and endometriosis; (2) history of operation on ovary; (3) oocyte-donation cycles; (4) sperm-donation cycles; and (5) non-fresh cycles. Finally, a total of 3,841 cycles were enrolled in this study.
Controlled ovarian stimulation scheme
Controlled ovarian stimulation was performed with standard long protocol in all patients. From the midluteal phase, 0.1 mg/d of decapeptyl (0.1 mg/ampoule, Ferring Company, Germany), GnRH-α was subcutaneously injected for 7 d, and then decapeptyl was reduced from 0.1 mg/d to 0.05 mg/d until on the day of hCG administration. When pituitary-down regulation was complete (endometrial thickness ≤5 mm, FSH ≤5 IU/L, E2 ≤50 pg/ml and LH ≤5 IU/L), Gn (recombinant FSH, Gonal-F, 75 IU/ampoule, Serono Company, Switzerland) was given. The starting dose of Gn was between 150 IU/day and 300 IU/day according to patient’s age, body mass index, basal FSH, and the number of antral follicle. The regimen of Gn administration was adjusted based on follicle size and the levels of hormones. When the average diameter of dominant follicles was between 18 mm and 20 mm, 5000–10,000 IU of hCG was intramuscularly injected instead of Gn. Thirty six h later, oocyte retrieval was performed under ultrasonic guidance. Embryo transfer was performed 2–3 d after oocyte retrieval. A total of 60 mg of P was intramuscularly injected every d after oocyte retrieval. Serum β–HCG was determined 14 d after embryo transfer. Clinical pregnancy was diagnosed when B-mode ultrasound showed gestation sac with fetal heart beat 35 d after embryo transfer.
Grouping
According to the number of oocyte retrieval [Bu et al. Citation2014], this study was divided into high ovarian response group (oocyte retrieval ≥20,842 cycles), moderate ovarian response group (5< oocyte retrieval <20,2008 cycles), and low ovarian response (oocyte retrieval ≤5,991 cycles).
Hormone determination
Fasting blood was collected from the ulnar vein on the day of hCG administration, and then P level was determined with chemiluminescent immunoassay (UniCel DxI 800, Beckman, Chaska, Minnesota, USA).
Statistical analysis
According to P levels, the total patients and the patients in each different ovarian response group were respectively divided into the following 8 P intervals: ≤1.00, 1.00–1.25, 1.25–1.5, 1.5–1.75, 1.75–2.00, 2.00–2.25, 2.25–2.5, and >2.5 ng/ml [Xu et al. Citation2012]. Clinical pregnancy rates were calculated for all P intervals. The cut-off values of serum P were determined based on odds ratio (OR) value and 95% confidence interval (CI) obtained by comparison of clinical pregnancy rates between each P interval and the lowest P interval. The analysis of clinical data was performed with t test, nonparametric test, and chi square test. Increased P level-related factors in the patients with different ovarian responses were analyzed with logistic regression. All data were treated with SPSS13.0 software. The size of test α = 0.05. Statistical significance was established at p < 0.05.
| Abbreviations | ||
| GnRH-α | = | gonadotropin releasing hormone agonist |
| LH | = | luteinizing hormone |
| P | = | progesterone |
| hCG | = | human chorionic gonadotropin |
| IVF-ET | = | in vitro fertilization-embryo transfer |
| Gn | = | gonadotropin |
| FSH | = | follicle-stimulating hormone |
| OR | = | odds ratio |
| CI | = | confidence interval |
Author contributions
Conceived and designed the study: P-fL, LT; Performed the study: P-fL, HZ; Analyzed the data: D-mZ, L-yM, Y-gX, DZ, QD, NL; Wrote the manuscript: P-fL.
Declaration of interest
The authors have no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Andersen, Y.C., Bungum, L., Andersen, A.N. and Humaidan, P. (2011) Preovulatory progesterone concentration associates significantly to follicle number and LH concentration but not to pregnancy rate. Reprod Biomed Online 23:187–195
- Bosch, E., Labarta. E., Crespo, J., Simón, C., Remohí, J., Jenkins, J., et al. (2010) Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: Analysis of over 4000 cycles. Hum Reprod 25:2092–20100
- Bu, Z., Zhao, F., Wang, K., Guo, Y., Su, Y., Zhai, J., et al. (2014) Serum progesterone elevation adversely affects cumulative live birth rate in different ovarian responders during in vitro fertilization and embryo transfer: A large retrospective study. PLoS One 9:e100011
- Doldi, N., Marsiglio, E., Destefani, A., Gessi, A., Merati, G. and Ferrari, A. (1999) Elevated serum progesterone on the day of HCG administration in IVF is associated with a higher pregnancy rate in polycystic ovary syndrome. Hum Reprod 14:601–605
- Elnashar, A.M. (2010) Progesterone rise on the on the day of HCG administration (premature luteinization) in IVF: An overdue update. J Assist Reprod Genet 27:149–155
- Filicori, M., Cognigni, G.E., Gamberini, E., Parmegiani, L., Troilo, E. and Roset, B. (2005) Efficacy of low-dose human chorionic gonadotropin alone to complete controlled ovarian stimulation. Fertil Steril 84:394–401
- Fleming, R. and Jenkins, J. (2010) The source and implications of progesterone rise during the follicular phase of assisted reproduction cycles. Reprod Biomed Online 21:446–449
- Griesinger, G., Mannaerts, B., Andersen, C.Y., Witjes, H., Kolibianakis, E.M. and Gordon, K. (2013) Progesterone elevation does not compromise pregnancy rates in high responders: A pooled analysis of in vitro fertilization patients treated with recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in six trials. Fertil Steril 100:1622–1628
- Haouzi, D., Bissonnette, L., Gala, A., Assou, S., Entezami, F., Perrochia, H., et al. (2014) Endometrial receptivity profile in patients with premature progesterone elevation on the day of HCG administration. Biomed Res Int 2014:951937
- Harada, T., Katagiri, C., Takao, N., Toda, T., Mio, Y. and Terakawa, N. (1996) Altering the timing of human chorionic gonadotropin injection according to serum progesterone concentrations improves embryo quality in cycles with subtle P rise. Fertil Steril 65:594–597
- Kyrou, D., Al-Azemi, M., Papanikolaou, E.G., Donoso, P, Tziomalos, K., Devroey, P., et al. (2012) The relationship of premature progesterone rise with serum estradiol levels and number of follicles in GnRH antagonist/recombinant FSH-stimulated cycles. Eur J Obstet Gynecol Reprod Biol 162:165–168
- Lainas, T., Zorzovilis, J., Petsas, G., Stavropoulou, G., Cazlaris, H., Daskalaki, V., et al. (2005) In a flexible antagonist protocol, earlier, criteria-based initiation of GnRH antagonist is associated with increased pregnancy rates in IVF. Hum Reprod 20:2426–2433
- Liu, L., Zhou, F., Lin, X., Li, T., Tong, X., Zhu, H., et al. (2013) Recurrent IVF failure is associated with elevated progesterone on the day of hcg administration. Eur J Obstet Gynecol Reprod Biol 171:78–83
- Martinez, F., Coroleu, B., Clua, E., Tur, R., Buxaderas, R., Parera, N., et al. (2004) Serum progesterone concentrations on the day of hcg administration cannot predict pregnancy in assisted reproduction cycles. Reprod Biomed Online 8:183–190
- Melo, M.A., Meseguer, M., Garrido, N., Bosch, E., Pellicer, A. and Remohí, J. (2006) The significance of premature luteinization in an oocyte-donation programme. Hum Reprod 21:1503–1507
- Moon, Y.S., Tsang, B.K., Simpson, C., Armstrong, D.T. (1978) beta-Estradiol biosynthesis in cultured granulosa and thecal cells of human ovarian follicles: stimulation byfollicle-stimulating hormone. J Clin Endocrindol Metab 47:263–267
- Ochsenkühn, R., Arzberger, A., von Schönfeldt, V., Gallwas, J., Rogenhofer, N., Crispin, A., et al. (2012) Subtle progesterone rise on the day of human chorionic gonadotropin administration is associated with lower live birth rates in women undergoing assisted reproductive technology: A retrospective study with 2555 fresh embryo transfers. Fertil Steril 98:347–354
- Papanikolaou, E.G., Pados, G., Grimbizis, G., Bili, E., Kyriazi, L., Polyzos, N.P., et al. (2012) GnRH—agonist versus GnRH—antagonist IVF cycles: Is the reproductive outcome affected by the incidence of progesterone elevation on the on the day of hcg triggering? A randomized prospective study. Hum Reprod 27:1822–1828
- Venetis, C.A., Kolibianakis, E.M., Bosdou, J.K. and Tarlatzis, B.C. (2013) Progesterone elevation and probability of pregnancy after IVF: A systematic review and meta-analysis of over 60000 cycles. Hum Reprod Update 19:433–457
- Venetis, C.A., Kolibianakis, E.M., Papanikolaou, E., Bontis, J., Devroey, P. and Tarlatzis, B.C. (2007) Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update 13:343–355
- Xu, B., Li, Z., Zhang, H., Jin, L., Li, Y., Ai, J, et al. (2012) Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: An analysis of more than 10,000 cycles. Fertil Steril 97:1321–1327
