Abstract
A routine computer-assisted sperm analysis is an important diagnostic test in the andrology laboratory. To evaluate the accuracy and precision of the different types of counting chambers for human semen analysis in combination with a computer-assisted semen analyzer (CASA), a quality-control study that compared human sperm analysis results obtained using different counting chambers (Makler chamber, disposable 8-cell GoldCyto chamber, disposable 4-cell Leja chamber, a plain glass slide, and a tissue culture dish cover with a 24 × 24 mm2 coverslip) in conjunction with the CASA systems was performed. Significantly higher counts of sperm concentration were obtained from the reusable Makler chamber than from the other counting chambers. Sperm motility from drop loaded counting chambers was significantly higher than that of capillary-loaded chambers. A plain glass slide and a tissue culture dish cover used with a coverslip showed rather better performance in semen assessment. Disposable chambers are suitable for routine semen analysis with CASA in a diagnostic andrology setting. With the proper workflow and quality control, a plain glass slide and the tissue culture dish cover are acceptable alternatives for routine counting chambers with CASA as necessary. The type of counting chamber should be specified in test reports.
Introduction
Semen analysis is used to monitor spermatogenesis and establish male fertility status, providing useful information for clinicians and investigators [WHO Citation2010].Visual estimation of semen quality is inexpensive and simple, but the accuracy and reliability depend on subjective assessment by the technician. Conventional manual assessment of human sperm parameters is influenced by numerous factors, including the skill and experience of the operator [Klimowicz et al. Citation2008; Makler Citation2000; Matson et al. Citation1999; Vyt et al. Citation2004]. These potential sources of imprecision could affect the results of semen analysis, leading to erroneous clinical diagnostic information during a male fertility workup thereby misinforming therapeutic recommendations.
Recently, with the development and improvement of modern computer technology, more objective and reliable computer-assisted sperm analysis (CASA) systems have emerged, allowing accurate, rapid, and simultaneous assessment of various semen characteristics such as motion, velocity, and morphology [Hoogewijs et al. Citation2012; Rijsselaere et al. Citation2012; Tomlinson et al. Citation2001; Verstegen et al. Citation2002]. Reusable and disposable capillary-loaded counting chambers using CASA for male semen analysis have become commercially available, which can overcome the subjectivity and variability of manual semen analysis to some extent [Hoogewijs et al. Citation2012], and even disposable plastic counting chambers can be used for semen analysis [Kirkman-Brown and Bjorndahl Citation2009].
Discrepancies in sperm concentration estimates among different counting chambers or slides have been widely recognized. For instance, the sperm concentration by Makler chamber filled by dropping was higher than that of other disposable counting chambers [Brazil et al. Citation2004; Christensen et al. Citation2005; Coetzee and Menkveld Citation2001; Mallidis et al. Citation2012; Tomlinson et al. Citation2001; Vyt et al. Citation2004], while counting chambers filled by capillary forces yielded a lower sperm concentration and lower motility parameters [Hoogewijs et al. Citation2012; Tomlinson et al. Citation2001].
In the present study, we assessed three different commercially available counting chambers for semen analysis, including reusable Makler chamber (drop filled slide with a coverslip, Sefi Medical Instruments Ltd., Haifa, Israel), disposable 8-cell GoldCyto chamber (Microptic S.L., Barcelona, Spain), and disposable 4-cell Leja chamber (LEJA, Nieuw-Vennep, The Netherlands) with capillarity loading. Moreover, a plain slide-coverslip, hereafter referred to as “Slide” and even the tissue culture dish cover (Becton Dickinson (B.D.), NJ, USA), hereafter referred to as “Cover”, that were loaded upon which a coverslip was placed were tested and assessed in this program. Furthermore, sperm measurements including sperm concentration, motility, and kinematics were determined and assessed by all counting chambers, including Slide and Cover, which were coupled with a sperm class analyzer (SCA) (Microptic S.L.).
Results
Validation of the counting chambers using the standard latex bead concentrations
In the current study, we compared the accuracy and reproducibility of all the counting chambers, Slide, and Cover against the pre-calibrated standard latex bead solutions (23.5 million/mL and 51.7 million/mL, Institute of Geoffrey Sperm Measurement and Technology, Nanjing, China). GoldCyto and Leja chambers showed small variations and accurately estimated the latex bead concentrations. Moreover, the performance of Slide and Cover chambers was better in spite of their large standard deviation, while the Makler chamber in the same test had a tendency to overestimate the bead concentration (), with the concentration twice as much as that by other counting chambers ( and ). The data from GoldCyto and Leja counting chambers provided a very similar and accurate estimation of the latex bead concentrations.
Figure 1. Validation of the counting chambers. Validation of the counting chambers against the standard latex bead concentrations (A) 23.5 × 106/mL sample and (B) 51.7 × 106/mL sample. Significantly higher counts of two kinds of standard latex bead concentrations were obtained from Makler than from other counting chambers. Similar descripitive and comparative stastical analysis of all counting chambers with CASA in fresh and separated sperm concentration test (C and D); (C) fresh sperm sample and (D) separated sperm sample. Assessments of sperm motility by various counting chambers with CASA (E and F); (E) fresh sperm sample and (F) separated sperm sample. Significantly higher motility percent of fresh sperm from dropping loaded coverslips (including Makler, Slide, and Cover) than that of capillary-loaded (including GoldCyto and Leja). The standard deviations in C, D, E, and F (including in ), are relatively higher because of the statistical data from different semen samples. CON: concentration, MOT: motility.
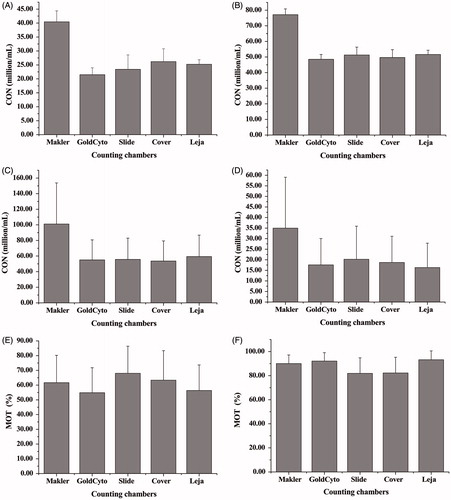
Table1. The standard latex bead concentrations (mean ± SD × 106/mL) obtained by different counting chambers.
Sperm concentrations
The average concentration of 56 human semen samples was determined by CASA using different chambers. Both fresh samples and separated samples were used. In the separated samples, the debris and other cells that may interfere with evaluation by the CASA were removed. The mean concentration of fresh and separated sperm by Makler, GoldCyto, Slide, Cover, and Leja is shown, respectively, in . The sperm concentration similarity between all counting chambers in fresh sperm and separated sperm () was consistent as comparing to the performance in the standard latex bead concentration tests ( and ).
Table 2. Descriptive and comparative sperm concentrations (CON) (×106/mL), motility (MOT %) assessment, and kinematic measurements from 56 patients by different counting chambers (mean ± SD).
Sperm motility and kinematics
Comparison of the motility and kinetics of sperm by the different counting chambers showed that the different counting chambers yielded different results as in the tests of sperm concentration. The mean percentage of motile cells of fresh and separated sperm from Makler, GoldCyto, Slide, Cover, and Leja is presented, respectively, in . Other kinematic parameters including progressive motility (PR), non-progressive motility (NP), immotile spermatozoa (IM), curvilinear velocity (VCL), straightline velocity (VSL), average path velocity (VAP), linearity (LIN), straightness (STR), and wobble (WOB) are also included in . As expected, the data of motility of fresh sperm obtained from drop loading counting chambers (Makler, Slide, and Cover) were higher than those from capillary-loaded chambers (GoldCyto and Leja) (). The result was further confirmed by the ancillary fresh sperm tests.
Correlation between sperm concentration and motility, PR, and NP
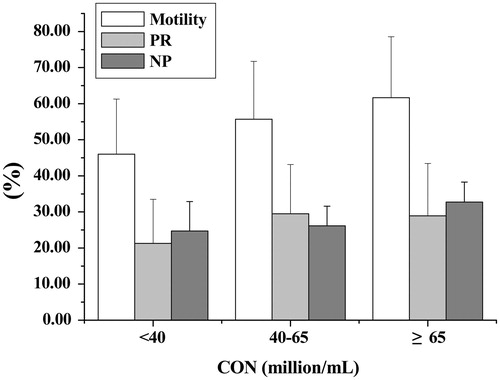
We assessed the total motility, PR, and NP of low (<40 × 106/mL), median (40–60 × 106/mL), and high concentration (>60 × 106/mL) fresh sperm. Analysis of motility and kinematics of fresh sperm under the different concentrations, are summarized in Supplementary Table 2. A high positive correlation was observed between the sperm total motility (including NP) and concentration in all counting chambers in the three groups while the percentage of progressive sperm did not exhibit linear correlation with concentration. Only the data from GoldCyto is presented (). The higher the sperm concentration, the greater the frequency of collisions between sperm. Consequently, non-progressive movement of immotile or dead sperm around those motile sperm occurred more frequently.
Ancillary measurements
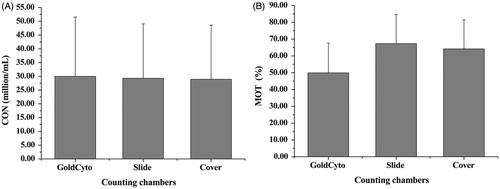
The data obtained using the Slide and Cover fluctuated to some extent in the analysis of sperm motility and kinematics, especially in the separated sperm test and can present very low values. The quality of the coverslip may play an important role in fluctuation. We performed the ancillary tests by GoldCyto, Slide, and Cover with better coverslips (CITOGLAS, Citotest Labware Manufacturing CO., LTD, Jiangsu, China) in 30 fresh sperm samples. We observed more consistent outcomes of sperm motility and kinematics between Slide and Cover ().
Figure 3. Ancillary tests of 32 fresh sperm samples using three counting chambers, including GoldCyto, Slide, and Cover. (A) Analysis of concentrations (p > 0.05) and (B) measurements of motility (p value between GoldCyto and Slide less than 0.05, p value between GoldCyto and Cover less than 0.05, p value between Slide and Cover more than 0.05). CON: concentration; MOT: motility.
Discussion
Generally, the semen analysis is the routine first andrology laboratory test given in male infertility clinics [Tsuji et al. Citation2002]. The results of semen analysis play an important role in evaluating male fertility, to determine sperm function, and to provide a reference point for the use of an assisted reproductive technology (ART) procedure.
Our data from this work clearly demonstrates that the reusable Makler chamber significantly overestimated the latex bead and sperm concentrations compared with other counting chambers, including Slide and Cover. Several reports from different andrology laboratories in IVF centers and veterinary practices have indicated that Makler overestimates sperm concentration [Christensen et al. Citation2005; Coetzee and Menkveld Citation2001; Ginsburg et al. Citation1990; Lenz et al. Citation2011; Seaman et al. Citation1996; Verstegen et al. Citation2002]. We found that the CASA sometimes could not separate sperm from the bright grids in a Makler counting chamber with use of routine phase-contrast optics, which may be one of the causes of overestimation for sample concentration. A previous report suggested that the Makler chamber should only be used when there is no need for high precision and accuracy [Christensen et al. Citation2005]. Manual modification and verification must be done during the CASA procedure using a Makler chamber. Amnon Makler, the designer of the Makler counting chamber, concluded that the “operator should use the Makler counting chamber correctly in order to avoid errors” [Makler et al. Citation2000].
In the pre-calibrated standard latex bead solution tests, the disposable chambers GoldCyto and Leja, and Slide and Cover, showed satisfactory performance compared with the Makler counting chamber. Though there was a statistical difference among the four groups (p < 0.05), all readings were close to the pre-calibrated concentration. Consistently, similar results were obtained in the measurement of sperm concentration.
For sperm motility, we found that the motility from a drop loaded coverslip fresh sample including Makler, Slide, and Cover was significantly higher than that of capillary-loaded GoldCyto and Leja. Consistent with our expectation, the trend of corresponding readings from separated sample did not agree with fresh sample (). The errors could have been due to the collisions among the sperm with a high percent motility such as the separated sample, and erroneous judgment of dead or immotile sperm as motile sperm by CASA. suggested that errors of motility evaluation increase with higher sperm concentrations. For motility assessment by CASA, sperm concentration should be between 2 × 106 /mL and 50 × 106 /mL, sperm sample with high concentration of sperm should be diluted with seminal plasma from the same man [WHO Citation2010].
The thin, capillary-loaded counting chambers (such as GoldCyto and Leja) with a fixed cover can induce Poiseuille flow and Segre–Silberberg effect. The partial sperm concentration was lower than the actual total concentration because of Segre–Silberberg effect. This effect can cause significantly lower counting of latex beads in the central and proximal areas of the chambers than distal areas of the same chambers [Dearing et al. 2014; Douglas-Hamilton et al. Citation2005a; Citation2005b; Kuster Citation2005], similar to the above results. In addition, the adhesive such as glue and paint used for these chambers could be toxic for sperm and damage the sperm membrane; capillary action can also damage the sperm, resulting in decreased sperm motility [Gloria et al. Citation2013; Hoogewijs et al. Citation2012; Tomlinson et al. Citation2001]. These results are in complete accordance with the performances of GoldCyto and Leja in this investigation. If necessary, equipment with clean, nontoxic, and high quality glass slide coverslips, or plain glass slide, or clean tissue-culture dish cover could provide satisfactory sperm concentration measurements and motility assessments.
Currently, CASA systems are frequently used in combination with 20 μm deep disposable capillary-loaded counting chambers. The most common cause of errors in sperm analysis using these counting chambers are capillary forces [Christensen et al. Citation2005]. Moreover, positive displacement pipettes should be used for semen samples with abnormal viscosity. There is no strict “gold” standard of human semen analysis with CASA systems in the 5th WHO [Citation2010] guidelines, and different CASAs and various counting chambers are used all over the world. There are no perfect CASA systems and counting chambers available; standardization, validation, and optimization of protocols must be carried out before using CASA in the andrology laboratory [ESHRE Andrology Special Interest Group Citation1998]. Furthermore, accuracy, precision, and repeatability need to be critically assessed and a regular quality control program needs to be implemented. At present, the application of the standard latex bead sample in performance of External Quality Assurance (EQA) and Internal Quality Control (IQC), can contribute to more objective assessment of semen analysis [Zuvela et al. Citation2011].
Oligozoospermia may be diagnosed using a Leja chamber, but the concentration of the same sample obtained from a Makler chamber appear above the lower reference limit of the WHO [Citation2010] guideline. The type of counting chamber to be used in CASA for semen analysis should be included, and more information on instruments and devices may be helpful for clinicians and laboratory technologists.
Materials and Methods
The study was approved by the Ethics Committee People’s Hospital of Luohu District, Shenzhen, China. We studied 56 men, aged 38 ± 5.9 years, whose partners were undergoing IVF at our institution from February to May, 2014, and informed written consent was obtained from all patients.
The main parameters (concentration, motility, and kinematics) were measured using freshly ejaculated and separated semen specimens. Separated sperm samples were obtained by density centrifugation. In brief, fresh semen was overlaid on a two-layer PureCeption with 40% and 80% gradients (SAGE, In-Vitro Fertilization, Trumbull, CT, USA); after centrifugation at 500 g × g for 10 min at room temperature, sperm pellet was washed with Quinn’s Advantage Fertilization solution (SAGE, In-Vitro Fertilization) two times by centrifugation at 300 × g for 5 min at room temperature, the sperm pellet was re-suspended by Quinn’s Advantage Fertilization solution before the assessment.
The SCA (Microptic S.L., Barcelona, Spain) and a phase contrast microscope (Nikon ECLIPSE E200MV R, Nikon, Tokyo, Japan) were used for semen analysis. The maximum difference between two counts from the same sample by CASA that must be expected to occur in 95% of limits of confidence before the data collection for final analysis. The standard setting for the parameters of CASA was programmed according to the manufacturer’s recommendations. Briefly, no less than 200 sperm tracks from at least three fields were analyzed by capturing 25 frames/field at a rate of 25 frames/s; the velocity limit for slow and medium sperm was 15 and 35 μm/s, respectively, and the minimal linearity for progressive fast sperm was 80%, and the maximal percentage of linearity was 50%. Except for sperm concentration (106/mL), the following sperm motility parameters were analyzed by CASA: PR (%), NP (%), IM (%), and total motility (PR + NP %). Moreover, the following kinematic parameters of spermatozoa were assessed: VCL (μm/s), VSL (μm/s), VAP (μm/s), the percentage LIN (%) (VSL/VCL), STR (%) (VSL/VAP), and WOB (%) (VAP/VCL).
To avoid subjective errors by individuals and reduce interobserver variations, semen analysis was performed by a single experienced andrologist using CASA with the routine protocols. We compared the accuracy and reproducibility of all counting chambers by the standard latex bead concentrations tests before semen analysis, In order to reduce the error, semen with abnormal viscosity were excluded, all samples were analyzed within 30 min after sample production, and all the liquefied samples were incubated at 37°C for 5 min prior to analysis. Before the counting chambers were loaded, each sample was mixed gently again by shaking for at least 10 s and air-displacement pipettes were used. A 5-μL drop of semen was placed on the loading area of a Makler chamber and the coverslip was applied immediately [Christensen et al. Citation2005; Matson et al. Citation1999; Tsuji et al. Citation2002]. A 3-μL drop of semen was placed on the entry area of GoldCyto and Leja counting chambers according to the manufacturers’ instructions. In order to achieve a preparation depth of 20 µm, one drop of 11.5-μL of semen was placed on a plain glass slide and tissue culture dish cover and covered immediately with a coverslip of 24 × 24 mm2 [Comhaire and Vermeulen Citation1995; Mallidis et al. Citation2012; WHO Citation2010]. If air bubbles were present in the counting area, the loading procedure was repeated. All samples of selecting fields for CASA analysis must be homogenous, including neither viscosity nor agglutination/aggregation; all counting chambers were kept warm at 37°C during the procedure and were analyzed by CASA approximately 30 s after all cells stopped drifting. The sequence of loading and analysis of samples among all counting chambers were random; to be consistent, all subsequent procedures and operations remained unchanged.
All statistical analyses were performed using the SPSS 19.0 software. The significance of differences in semen assessment among counting chambers was analyzed using ANOVA, LSD post-hoc test was used for multiple comparison between groups, confidence limit for the 95% confidence internal. Differences were considered to be statistically significant when p < 0.05.
| Abbreviations | ||
| CASA | = | computer-assisted semen analyzer |
| PR | = | progressive motility |
| NP | = | non-progressive motility |
| IM | = | immotile spermatozoa |
| VCL | = | curvilinear velocity |
| VSL | = | straightline velocity |
| VAP | = | average path velocity |
| LIN | = | linearity |
| STR | = | straightness |
| WOB | = | wobble |
| WHO | = | World Health Organization |
| ANOVA | = | Analysis of variance |
| ESHRE | = | the European Society of Human Reproduction and Embryology |
Supplementary materials available online
Supplementary Tables
Supplementary File
Download Zip (45.5 KB)Acknowledgments
The authors thank Professor Y.C. Xu for providing standard latex bead solutions, and thank Professor H.L. Feng for his critical revision of the manuscript.
Declaration of interest
None of the authors have any potential conflicts of interest of this work and the manuscript for publication.
Author contributions
Performed the research and wrote the paper: NP; Analyzed and interpreted the data: XZ; Designed the research study and wrote the paper: LL. All authors read and approved the final manuscript.
References
- Brazil, C., Swan, S.H., Drobnis, E.Z., Liu, F., Wang, C., Redmon, J.B., et al. (2004) Standardized methods for semen evaluation in a multicenter research study. J Androl 25:635–644
- Christensen, P., Stryhn, H. and Hansen, C. (2005) Discrepancies in the determination of sperm concentration using Bürker-Türk, Thoma and Makler counting chambers. Theriogenology 63:992–1003
- Coetzee, K. and Menkveld, R. (2001) Validation of a new disposable counting chamber. Arch Androl 47:153–156
- Comhaire, F. and Vermeulen, L. (1995) Human semen analysis. Hum Reprod Update 4:343–362
- Dearing, C.G., Kilburn, S. and Lindsay, K.S. (2014) Validation of the sperm class analyser CASA system for sperm counting in a busy diagnostic semen analysis laboratory. Hum Fertil 17:37–44
- Douglas-Hamilton, D.H., Smith, N.G., Kuster, C.E., Vermeiden, J.P. and Althouse, G.C. (2005a) Capillary-loaded particle fluid dynamics: effect on estimation of sperm concentration. J Androl 26:115–122
- Douglas-Hamilton, D.H., Smith, N.G., Kuster, C.E., Vermeiden, J.P. and Althouse, G.C. (2005b) Particle distribution in low-volume capillary-loaded chambers. J Androl 26:107–114
- ESHRE Andrology Special Interest Group. (1998) Guidelines on the application of CASA technology in the analysis of spermatozoa. Hum Reprod 13:142–145
- Ginsburg, K.A. and Armant, D.R. (1990) The influence of chamber characteristics on the reliability of sperm concentration and movement measurements obtained by manual and videomicrographic analysis. Fertil Steril 53:882–887
- Gloria, A., Carluccio, A., Contri, A., Wegher, L., Valorz, C. and Robbe, D. (2013) The effect of the chamber on kinetic results in cryopreserved bull spermatozoa. Andrology 1: 879–885
- Hoogewijs, M.K., de Vliegher, S.P., Govaere, J.L., de Schauwer, C., de Kruif, A. and Van Soom, A. (2012) Influence of counting chamber type on CASA outcomes of equine semen analysis. Equine Vet J 44:542–549
- Kirkman-Brown, J. and Bjorndahl, L. (2009) Evaluation of a disposable plastic Neubauer counting chamber for semen analysis. Fertil Steril 91:627–631
- Klimowicz, M.D., Nizanski, W., Batkowski, F. and Savic, M.A. (2008) The comparison of assessment of pigeon semen motility and sperm concentration by conventional methods and the CASA system (HTM IVOS). Theriogenology 70:77–82
- Kuster, C. (2005) Sperm concentration determination between hemacytometric and CASA systems: Why they can be different. Theriogenology 64:614–617
- Lenz, R.W., Kjelland, M.E., VonderHaar, K., Swannack, T.M. and Moreno, J.F. (2011) A comparison of bovine seminal quality assessments using different viewing chambers with a computer-assisted semen analyzer. J Anim Sci 89:383–388
- Makler, A. (2000) Potential sources of error with the Makler counting chamber. Fertil Steril 73:1066–1067
- Mallidis, C., Cooper, T.G., Hellenkemper, B., Lablans, M., Uckert, F. and Nieschlag, E. (2012) Ten years' experience with an external quality control program for semen analysis. Fertil Steril 98:611–616
- Matson, P., Irving, J., Zuvela, E. and Hughes, R. (1999) Delay in the application of the cover glass is a potential source of error with the Makler counting chamber. Fertil Steril 72:559–561
- Rijsselaere, T., Van Soom, A., Maes, D. and Nizanski, W. (2012) Computer-assisted sperm analysis in dogs and cats: an update after 20 years. Reprod Domest Anim 47 (Suppl. 6):204–207
- Seaman, E.K., Goluboff, E., BarChama, N. and Fisch, H. (1996) Accuracy of semen counting chambers as determined by the use of latex beads. Fertil Steril 66:662–665
- Tomlinson, M., Turner, J., Powell, G. and Sakkas, D. (2001) One-step disposable chambers for sperm concentration and motility assessment: how do they compare with the World Health Organization’s recommended methods? Hum Reprod 16:121–124
- Tsuji, T., Okada, H., Fujisawa, M., Hamaguchi, Y. and Kamidono, S. (2002) Automated sperm concentration analysis with a new flow cytometry–based device, S-FCM. Am J Clin Pathol 117:401–408
- Verstegen, J., Iguer-Ouada, M. and Onclin, K.C. (2002) Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 57:149–179
- Vyt, P., Maes, D., Rijsselaere, T., Dejonckheere, E., Castryck, F. and Van Soom, A. (2004) Motility assessment of porcine spermatozoa: a comparison of methods. Reprod Domest Anim 39:447–453
- WHO (2010) WHO laboratory manual for the examination and processing of human semen (5th Edition), World Health Organization, Cambridge University Press: Cambridge, UK
- Zuvela, E., Junk, S., Moska, N. and Matson, P. (2011) The use of latex beads in external quality assurance and internal quality control for routine semen analysis. Reprod Biol 11:264–275