Abstract
Age related decline in reproductive performance in women is well documented and apoptosis has been considered as one of the reasons for the decline of primordial follicle reserve. Recently we observed a decline in the efficiency of DNA repair ability in aged rat primordial follicles as demonstrated by decreased mRNA levels of DNA repair genes BRCA1 and H2AX. In the present study, a two-dimensional electrophoresis (2DE) proteomic approach was employed to identify differentially expressed proteins in primordial follicles isolated from ovaries of immature (∼20 days) and aged (∼400–450 days) rats. Using MALDI-TOF/TOF MS, we identified 13 differentially expressed proteins (p < 0.05) which included seven up-regulated and six down-regulated proteins in aged primordial follicles. These proteins are involved in a wide range of biological functions including apoptosis, DNA repair, and the immune system. Interestingly, the differentially expressed proteins such as FIGNL1 (DNA repair) and BOK (apoptotic protein) have not been previously reported in the rat primordial follicles and these proteins can be related to some common features of ovarian aging such as loss of follicle reserve and genome integrity. The quantitative differences of two important proteins BOK and FIGNL1 observed by the proteomic analysis were correlated with the transcript levels, as determined by semi-quantitative RT-PCR. Our results improve the current knowledge about protein factors associated with molecular changes in rat primordial follicles as a function of aging and our understanding of the proteomic processes involved in degenerative changes observed in aging primordial follicles.
Introduction
Age related decline in reproductive performance in women is well documented [American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Practice Committee of the American Society for Reproductive Medicine Citation2014; Spira Citation1988; te Velde and Pearson Citation2002]. One of the main reasons for this decline is the decrease in primordial follicle reserve which is due to progressive apoptosis of the follicles with age [Broekmans et al. Citation2009; Kaipia and Hsueh Citation1997; Vaskivuo and Tapanainen Citation2003]. Studies have estimated that the number of primordial oocytes in the human ovary of a newborn infant is around two million, of which most oocytes die by follicular atresia. During adult human life, about 400 oocytes eventually reach maturation and are ovulated, finally leading to menopause [Baird et al. Citation2005; Faddy et al. Citation1992; Kaipia and Hsueh Citation1997]. As primordial follicles are arrested at dictyotene stage of meiosis for many years, the follicles during this long waiting period undergo degenerative change which is due to the action of environmental factors that can damage DNA [Baird et al. Citation2005; Eichenlaub-Ritter Citation2012; Hunt and Hassold Citation2008; Tarin Citation1996]. These degenerative changes which include DNA double strand breaks (DSBs) due to oxidative stress and other causative agents over time have been hypothesized to be a cause of ovarian aging and oocyte loss [Aitken Citation2014; Kurus et al. Citation2013; Titus et al. Citation2013]. In our recent study [Govindaraj et al. Citation2015], we have observed that a significant decrease in the mRNA levels of DNA DSB repair genes namely BRAC1, ATM, and H2AX in the primordial follicles isolated from aged rats compared to the primordial follicles isolated from immature rats. Based on the results obtained, it has been suggested by us that the decrease in the ability to repair DNA damage by BRCA1 and other related proteins declines with age in rat primordial follicles [Govindaraj et al. Citation2015]. This results in the decrease of the number of oocytes as well as the quality of existing oocytes as a function of age. In the present study we have attempted to compare the profile of proteins of primordial follicles isolated from immature rats with that of primordial follicles isolated from aged rats by two-dimensional electrophoresis (2DE) followed by identification of differently expressed protein spots using MALDI-TOF/TOF mass spectrometry.
Results
Two-dimensional protein expression profiles of young and aged primordial follicles
Two-dimensional electrophoresis (2DE) was carried out as described in the methods section to identify differentially expressed proteins in immature and aged rat primordial follicles. After 2DE separation of proteins and subsequent coomassie staining, gels were scanned to generate images of immature and aged rat primordial follicle protein profile. A representative gel image has been given in . Analysis of gel images by Dymension 2D gel image analysis software revealed approximately 50 protein spots in each gel with molecular weights ranging from 10 to 250 kDa and pI value between 3 and 10. There were no protein spots which were exclusive for either immature or aged rat primordial follicles. We used an independent t-test to calculate the difference in intensity of differentially expressed protein spots. We considered only spots which were found to be differentially expressed with fold changes greater than 1.5 (p < 0.05) for further analysis. There were 13 protein spots (marked with the number) differentially expressed between primordial follicles isolated from immature and aged rats in which seven (spots 1–6 and 10) were up-regulated and six (spots 7, 8, 9, 11, 12, and 13) were down-regulated in aged primordial follicles compared to immature primordial follicles. The close-up images and spot abundance of differentially expressed proteins are presented in .
Identification of differentially expressed proteins by MALDI-TOF/TOF MS
These differentially expressed protein spots were subjected to in-gel digestion and MALDI-TOF/TOF analysis, and mass spectral data were submitted to a database search against the NCBInr database for protein identification using the Mascot search program (http://www.matrixscience.com). The identity of down-regulated and up-regulated proteins in immature and aged rat primordial follicles is presented in . We further used ontological classification to analyze the biological processes and molecular functions which encompass the 13 differentially expressed proteins.
Table 1. Differentially expressed proteins identified by MALDI-TOF/TOF MS.
Functional classification of differentially expressed proteins
The 13 differentially expressed proteins identified by maldi-tof were categorized according to the PANTHER Classification System (http://pantherdb.org/). The list of proteins have been entered into the panther database to identify significantly over- and under-represented pathways, molecular functions, and biological processes, in comparison with a reference list (all genes of Rattus norvegicus in the database). The statistically significant (p < 0.05) pathways, biological processes, and molecular functions relevant to the 13 differentially expressed proteins suggested that of the several biological processes and functions, the most significant was the apoptotic pathway (). In this connection specific mention can be made that the differentially expressed proteins are mainly related to regulation of apoptotic pathway (1.07E-03), regulation of programmed cell death (1.18E-03), and response to reactive oxygen species (3.01E-02).
Table 2. PANTHER GO classification of differentially expressed proteins.
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis of some of the identified proteins
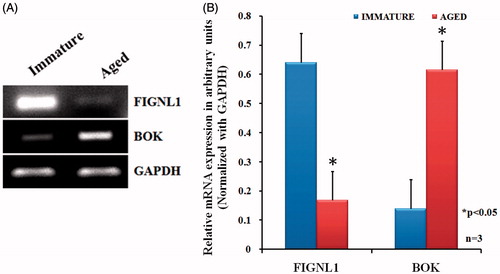
To correlate differential protein expression pattern with their corresponding mRNA expression in immature and aged primordial follicles, we analyzed the expression level of selected proteins by semi-quantitative RT-PCR analysis. We observed a significant down-regulation of FIGNL1 and up-regulation of BOK in primordial follicles isolated from ovaries of aged rats compared to primordial follicles isolated from immature rats (). Our results indicate a positive correlation between mRNA expression and the corresponding protein expression.
Discussion
Considerable evidence is available to conclude that ovarian aging is related to the decline in the expression of enzymes involved in the repair of DNA DSBs with age [Govindaraj et al. Citation2015; Titus et al. Citation2013]. The results obtained in the present study provide additional support that there is a decline in the expression at the level of genes as well as the level of proteins involved in oocytes aging. In an attempt to profile changes in the proteins expressed in the oocytes, the proteomic profiling of the protein extract from primordial follicles isolated from ovaries of immature and aged rats was carried out. The result of our study reveals that significant changes in the expression of several proteins are known to be involved in metabolic pathways, stress response, DNA repair, and apoptosis. The proteins such as 78 kDa glucose regulated protein (Grp78), heat shock cognate 71 kDa (Hsp71C) [Calvert et al. Citation2003; Parsell and Lindquist Citation1993; Wiest et al. Citation1995], calrecticulin [Henson Citation2003; Ramos et al. Citation2011; Wang and Machaty Citation2013; Zhang et al. Citation2010], and protein disulfide isomerase A3 which were down regulated in aged primordial follicles are in agreement with the general observation that synthetic activities like DNA repair, protein folding, and anti-apoptotic properties are on the decline in aged primordial follicles. For example, Grp78 protein is a molecular chaperon protein of the endoplasmic reticulum of the cell. It is an abundant protein and its synthesis is markedly induced under conditions that lead to the accumulation of unfolded polypeptide in the ER which are transported for elimination [Calvert et al. Citation2003; Mao et al. Citation2010]. Similarly the heat shock cognate 71 kDa protein which is also down regulated is known to bind to nascent polypeptides to facilitate correct folding. Most of the heat shock proteins are pro-survival factors and protect against apoptosis [Beere Citation2004] and Grp78 also has been shown to have anti-apoptotic activity [Chang et al. Citation2012; Gutiérrez and Simmen Citation2014]. As protein synthesis is on the decline in primordial follicles of aged rats, as can be expected, the expression of this protein is decreased.
A very interesting observation in the present study is the down-regulation of a FIGNL1 which is a very important protein involved in the repair of DNA DSBs via homologus recombination in mitotic cells and ours is the first study to report the expression of FIGNL1 in primordial follicles. Initially this protein was identified by affinity purification of proteins that interacted with RAD51 in human 293T cells followed by mass spectrometry [Yuan and Chen Citation2013]. Both FIGNL1 and RAD51 are recruited to the sites of DNA damage. It is pertinent to point out that in our earlier studies the expression of RAD51 which interacts with BRAC1 [Cousineau et al. Citation2005], another gene which is involved in DNA DSBs, is also decreased in aged primordial follicles [Govindaraj et al. Citation2015]. However, studies have revealed that similar to RAD51AP1, FIGNL1 and its interacting protein KIAAO146 (a nuclear protein) are recruited for DNA repair that is independent of RAD51 [Yuan and Chen Citation2013].
Figure 1. Two dimensional patterns of proteins from primordial follicles isolated from immature (A) and aged (B) rat ovaries. Proteins were separated in the first dimension on an IPG strip pH 3–10 and in the second dimension on a 10% acrylamide SDS-gel, followed by staining with coomassie stain. An equal quantity (250 µg) of total protein extracts was loaded in each gel. The gels were scanned and the images were analyzed using Dymension 2D gel image analysis software (Syngene, Cambridge, UK). The distribution of 13 differentially expressed spots is numbered and correspond to .
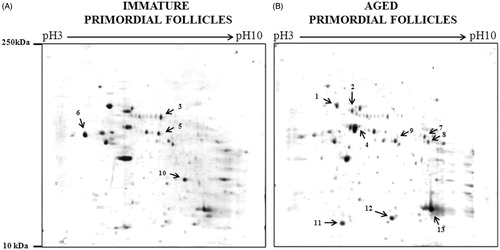
Figure 2. Cropped and enlarged images of 2D gels showing differentially expressed protein spots and graph showing the expression of differentially expressed protein spots. (A) Cropped and enlarged images of 2D gels showing differentially expressed spots. (B) Graph showing the expression of differentially expressed protein spots. Expression is displayed as the average normalized spot volume ratio. The normalized spot volume represents the relative abundance of protein within a spot; the ratio of normalized spot volumes between corresponding spots in a gel equates to changes in abundance of protein within that spot.
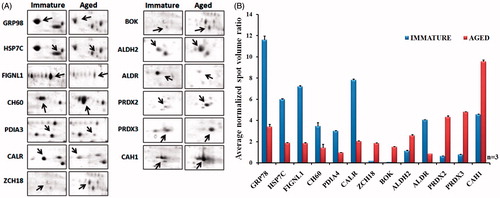
Figure 3. Semi-quantitative RT-PCR for FIGNL1 and BOK. Total RNA isolated from immature and aged rat primordial follicles, reverse transcribed, and the complementary DNA subjected to semi-quantitative RT-PCR in a linear range of amplification with GAPDH as an internal control. Representative image of three independent experiments are shown in Panel A. A graphical representation of results is presented in Panel B. Data from three independent experiments were expressed as arbitrary densitometric units (mean ± SEM). *p < 0.05 compared with immature rat primordial follicles.
Oocytes from immature mice showed resistance to apoptosis and efficient RAD51 associated DNA repair due to lack of BAX, a pro-apoptotic protein, after chemotherapy induced DNA damage [Kujjo et al. Citation2010]. Our studies showed a decrease in the genes and proteins involved in double strand DNA repair, are in agreement with the observation that there is progressive apoptosis in the aged oocytes. Considering this, the increase in the expression of the protein BOK (Bcl2 related ovarian killer protein) identified in the present study as a differentially expressed protein is of significance. BOK has been classified as a member of the Bcl2 protein family showing high sequence similarly to Bak and Bax and the over-expression of BOK triggers apoptosis [Hsu and Hsueh Citation1998]. BOK-deficient mice or the combined loss of BOK and BAK did not show apparent defects, although it remains possible that BOK and BAK have critical roles in developmental cell death that overlap with those of BAX. In contrast to the ovaries from normal aged mice, the ovaries from combined BOK and Bax deficient aged mice had an abnormally increased number of oocytes at different stages of development [Ke et al. Citation2013]. In this connection, it is justified to suggest that the increase in the expression of BOK observed in aged rat primordial follicles in the present study may be responsible for age related decline in primordial follicles.
Peroxiredoxin 2 (PRDX2) and Peroxiredoxin 3 (PRDX3) were up-regulated in primordial follicles isolated from aged compared to immature rats. Peroxiredoxins are known to be involved in antioxidant protection and cell signaling and due to their peroxide reductase activity, these enzymes might be involved in fine-tuning peroxide levels in embryos during in vitro production [Rhee et al. Citation2001]. Studies have established that the oxidative damage increases with age and consequently antioxidant gene expression (peroxiredoxin-3) decreases with age in the mouse ovary [Lim and Luderer Citation2011]. It is possible that the role of these proteins as an antioxidant may improve the biological function of primordial follicles in aged rats as a defensive mechanism. The zinc finger proteins are classified into several different structural families and each one of these proteins has a unique three dimensional structure. These essentially function as interaction modules that bind DNA, RNA, and other molecules, and variations in the structure determined the binding specificity of the particular proteins. In the present study the significance of up regulation of zinc finger protein can be ascertained only if we can establish the identity of a particular type of zinc finger protein. Regarding the significance of up regulation of aldehyde dehydrogenase in the primordial follicle of aged rats, it is possible to assign specific function only if a specific stage of maturation of oocytes as it is known that aldehyde dehydrogenase level varies with maturation. In the current study it is possible that the oocyte in the primordial follicle isolated can be at different stages. Carbonic anhydrases are enzymes which rapidly inter convert CO2 and H2O to bicarbonate and protons. These proteins essentially maintain acid-base balance in cells [Becker et al. Citation2014; Breton Citation2001; Colton and Downs Citation2004].
We observed a down regulation of catalytic enzyme aldose reductase in primordial follicles of aged rat ovaries. This enzyme is NADPH-dependent oxidoreductase mainly known for catalyzing the reduction of glucose to sorbitol in the first step of polyol pathway of glucose metabolism [Petrash Citation2004]. A disturbance in the polyol pathway has been demonstrated in diabetic mice [Colton and Downs Citation2004]. Interestingly, aldose reductase has been shown to be expressed in all the stages of oocytes and granulosa cells [Bhatnagar and Srivastava Citation1992]. Detailed studies using aldose reductase inhibitors in diabetic mice have suggested a role for aldose reductase in FSH-induced meiotic maturation [Colton and Downs Citation2004].
It is well established that oocytes present at birth undergo a progressive process of apoptosis in humans and other mammals as they age and this is associated with reduced fertility and adverse pregnancy outcomes in females [Crawford and Steiner Citation2015]. It is generally accepted that no fresh oocytes are produced other than those present at the time of birth. The follicular reserve is well established in-utero in humans and it declines continuously. Although more than one million oocytes are present at birth in the human ovary only about 500 of these ovulate during reproductive life. The vast majority of these oocytes are removed by apoptosis resulting in almost complete exhaustion of oocytes and eventually this loss leads to menopause by the mean age of 51–52 years. Studies have shown that DNA repair genes in oocytes of mammals including human decline with age, and lack of these genes show higher DNA breaks and increased oocyte death rates [Govindaraj et al. Citation2015; Titus et al. Citation2013]. With the development of assisted reproductive technology (ART) it has been possible to overcome the problems of infertility due to a variety of reasons both in the male and female. In the case of females this essentially involves the retrieval of oocytes from females, they are then matured in vitro, fertilized, then implanted in a suitably prepared uterus of the same woman from whom the oocyte is retrieved or a surrogate woman. This technique is also extensively employed to produce cattle, buffaloes, goats, and sheep which are known for quality of meat, milk yield, and resistance to diseases to mention a few benefits. However, if the defective oocytes harbor chromosomal abnormalities or if DNA DSB repair is deficient the situation becomes complicated. If the change in the characteristics of the oocyte is lethal the embryo will not survive. But if it is not lethal but involves changes resulting in defective embryos the situation needs attention. Thus for example, the first in vitro produced animal ‘Dolly’ the sheep, died young [Wilmut et al. Citation2008]. It was reported that ‘Dolly’ had many metabolic problems including arthritis. It is not clear whether the cloning using the nucleus from an adult animal cell resulted in ‘not so visible’ damage to DNA over time. In the review article which was based on discussions from the sixth Evian Annual Reproduction (EVAR) Workshop Group Meeting in 2011, it is mentioned that “IVF conceived children have lower birth weight and higher peripheral fat, blood pressure, and fasting glucose concentrations than controls and woman undergoing assisted reproduction are often older, increasing their chances of obtaining abnormal gametes that may cause deviations in outcomes between assisted conception and naturally conceived children” [Fauser et al. Citation2014, p. 163]. Considering the above, it is suggested that it is safe to retrieve oocytes from animals (livestock) which are known for good qualities such as quality of meat, milk yield, resistance to diseases, etc. when they are young and only up to certain age.
Conclusions
We have recently shown the decline in the efficiency of DNA repair in aged rat primordial follicles and suggested that DNA DSBs which accumulate with age as a possible cause for accelerating oocyte loss. Using a 2DE proteomic approach, we have identified 13 differentially expressed proteins in primordial follicles isolated from immature and aged rat ovaries. Two important proteins, FIGNL1 and BOK were identified and are involved in DNA repair and apoptosis, respectively. Our results add to our current knowledge about protein factors associated with molecular changes in rat primordial follicles as a function of age.
Materials and Methods
Animals
Immature (∼20 d) and aged (400–450 d) wistar strain albino rats were obtained from the Central Animal Facility (CAF) at the Indian Institute of Science (IISc), Bangalore. Animals were socially housed in polypropylene cages (43 cm length X 27 cm width X 14 cm height) with not more than 3 rats per cage in a 12-h light–dark cycle and provided ad libitum with water and standard laboratory diet (per kg cornstarch (397.48 g), casein (>85%) (200 g), sucrose (100 g), soyabean oil (70 g), fiber-(50 g) mineral mixture (35 g), and vitamin mix (10 g), L-cystine (3 g), choline bitartrate (2.5 g), tert-butylhydroquinon (0.014 g)). In this study, the immature rats used were of age 20 d. It is known that in our colony the maximum fertility of the wistar rats is recorded between 100–250 d, and the animals used for breeding were between around 100–120 d. Although female rats do not exhibit menopause, the absence of a regular estrus cycle (equivalent to menopause which occurs around 51–52 y in human) is observed between 450–540 d but breeding efficiency is reduced well before i.e., ∼250 d (8–10 months) and these rats before this period would have been used for breeding and subjected to hormonal influences during estrous cycle and pregnancy. The rats which are more than one y exhibit late pregnancy, smaller litter, problems during delivery, and more bleeding during delivery and hence are not used for breeding purposes. Eighteen-20 d immature rats were selected because at this age the rats are not exposed to any hormonal influence and consequently the primordial follicles are not exposed to any hormone action. The aged rats (450–540 d) were selected because the effects of hormones are minimal and they are no longer capable of reproduction. It is to be noted that these aged rats used in this study are no longer used for breeding purposes and the maximum of 4 cycles of breeding (21 d of pregnancy and 25–30 d of lactation). The experimental procedures were approved by the Institutional Animal Ethics Committee (Committee Project number: CAF/Ethics/270/2012) constituted under the guidelines of CPCSEA, Ministry of Environment and Forests, Government of India, New Delhi.
Isolation of primordial follicles from immature and aged rats
Primordial follicles were isolated as described by Govindaraj et al. [Citation2015]. Briefly, the ovaries from immature and aged wistar female rats were collected and washed twice in PBS and minced into small pieces, then transferred to a digestion medium containing 4 ml of DMEM supplemented with fetal bovine serum at a final concentration of 4%, and 5 ml of PBS and 2% collagenase (type IV). The suspension was incubated at 37°C for 30 min in a shaking water bath and the follicular digest was filtered through a 70 µm nylon cell strainer into a 50 ml Falcon tube. The follicular filtrate was centrifuged at 3,000 rpm for 5 min at 4°C and the supernatant was decanted. The pellet was re-suspended in fresh 5 ml of PBS and suspension was passed through a 40um nylon cell strainer followed by centrifugation at 3,000 rpm for 5 min at 4°C. The pellet was finally re-suspended in DMEM and checked for presence of the primordial follicles under an inverted phase-contrast microscope. The yield of primordial follicles was about 35,000 per immature rat and a total of 70 immature rats were used for isolation of either protein or RNA for each experiment. In the case of aged rats, a total of 120 rats were used for isolation of primordial follicles for each biological experiment and the yield of primordial follicles was 10,000 per rat.
Total protein extraction
Frozen primordial follicles isolated from immature and aged rat ovaries were homogenized in 500 ul of RIPA buffer (150 mM NaCl, 50 mM Tris, 1% IGEPAL-CA, 0.5% sodium deoxycholate, 0.1% Sodium dodecyl sulfate and 1X complete protease inhibitor cocktail (Roche) using a mechanically driven Teflon-and-glass homogenizer (Roche Diagnosis, Indianapolis, IN, USA). Homogenate was centrifuged at 5,000 x g for 10 min at 4°C to remove particulate debris. The resultant supernatant was considered as total protein. The protein concentration was determined by Lowry’s method [Lowry et al. Citation1951].
2DE and image analysis
Equal quantity of protein (250 µg) from immature and aged primordial follicles was acetone precipitated and the pellet was re-suspended in 250 µl of sample rehydration buffer (7 M Urea, 4% CHAPS, IPG buffer (Catalogue No. 17600088, GE Healthcare, Bio-Sciences AB, Uppsala, Sweden). Immobiline pH gradient (IPG) gel strips -13 cm (Catalogue No. 17600088, GE Healthcare) were rehydrated overnight in 250µl of rehydration buffer containing protein sample. Isoelectric focusing (IEF) was carried out in Ettan IPGPhor 3 (GE Healthcare) at 500 V (Step and Hold) for 1 h, at 1,000 V (Gradient) for one h, at 8,000 V (Gradient) and at 8000 V (Step and Hold) for 2 h and 20 min, respectively. After the first dimension, the gel strips were equilibrated for 15 min in SDS equilibration buffer (6M urea, 50 mM Tris (pH 8.8), 30% v/v glycerol, 2% w/v SDS, 2% w/v DTT). The second dimension electrophoresis was performed using 10% sodium dodecyl sulfate polyacrylamide gel. Gels were stained with coomassie blue R-250 and stained gels were scanned by an HP ScanJet G3110 scanner. Using Dymension 2 (Syngene, UK) 2D gel image analysis software, the cropped gel images were loaded and grouped using the ‘multiplexed experimental wizard’ Region of interest was selected as the whole image and the noise filtering and background correction were performed automatically. Spots of the whole gels were detected with default spot detection settings and individual spot volumes in each gel by density/area integration calculated and normalized to total spot volume on that gel automatically. For all spot intensity calculations, normalized spot volume values were used and the results were expressed as the ratio of the normalized volume of a protein spot in immature primordial follicles divided by the normalized volume of matched protein spot in aged primordial follicles and vice versa. A comparative 3D view of protein profiles is given in Supplementary Figure 1.
In-gel digestion
In-gel digestion was carried out as described by Shevchenko et al. [Citation1996]. Briefly, the differentially expressed protein spots were excised from the gels and were destained with 100 µl of 50% acetonitrile (ACN) in 50 mM ammonium bicarbonate for about 15 min at room temperature. This step was repeated until the gel pieces were colorless which were then dried under vacuum centrifugation and subjected to reduction (10 mM DTT in 100 mM ammonium bicarbonate), alkylation (50 mM Iodoacetamide in 100 mM ammonium bicarbonate) and in-gel trypsin digestion (incubated with 20 µl of 12.5 ng/µl sequencing grade modified trypsin (Promega, USA) at 37°C for 16 h). After digestion, the peptides were extracted once with 5% trifluoro acetic acid (TFA) in 50% ACN, and then twice with 2.5% TFA in 50% ACN. Extracts were pooled and peptides were stored at 20°C until further analysis.
Analysis of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF/TOF-MS)
Mass spectrometric analysis was carried out using UltrafleXtreme MALDI TOF/TOF (Bruker Daltonics, Germany) at Proteomics Facility, Molecular Biophysics Unit, Indian Institute of Science, Bangalore. A solution of CHCA (α-cyano-4-hydroxy-trans-cinnamic acid, Bruker Daltonics) at 10 mg/ml in aqueous 30% ACN/30% MeOH/0.2% TFA was used as a MALDI matrix. A sample (1μl) was deposited on a steel MALDI target plate, allowed to air dry at room temperature, and 1μl of the matrix solution was added. Positive ion mass spectra were measured in a MALDI-TOF mass spectrometer (Bruker Daltonics GmbH, Germany) equipped with a 337nm nitrogen laser. The spectra were acquired in the mass range of 700–4500Da in the reflectron mode and calibrated externally with a seven-point calibration using the peptide calibration standard. For peptide mass fingerprint database searching, peak lists in XML data format were created using the flexAnalysis 2.4 program (Bruker Daltonics) with the Sophisticated Numerical Annotation Procedure (SNAP) algorithm. Statistical calibration was integrated in the program; the highest number of allowed peaks was set at 50. After peak markings, all known signals due to noise were manually removed. The peak lists were searched using the MASCOT search engine (Matrix Science, Boston, MA, USA) against either the Swiss-Prot or the NCBInr database with the following search conditions: peptide tolerance of 100 ppm, missed cleavage site was set to one, fixed carbamidomethylation of cysteine, and variable oxidation of methionine and no limitations were applied on protein molecular weight or pI value. RMS errors for all proteins identified were ≤45 ppm. A list of identified peptides for each protein is given in Supplementary Figure 2.
Gene ontology and network analysis
Protein classification of differentially expressed genes was determined using Protein ANalysis THrough Evolutionary Relationships (PANTHER) Version 9.0 (Website: www.pantherdb.org) [Thomas et al. Citation2003; Thomas et al. Citation2006]. PANTHER classifies genes/proteins by their functions and categorizes them by molecular function and biologic purposes. The protein function and location were determined from the Rattus norvegicus Protein Reference Database.
Semi-quantitative RT-PCR
Total RNA from the primordial follicles was isolated using TRI reagent (Sigma #T9424, Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's recommended protocol. RNA quality and quantity were assessed using UV spectro- photometric analysis, by monitoring A280/A260 ratio. The quality of isolated RNA was also assessed by 1% formaldehyde agarose gel electrophoresis using 1% MOPS as gel running buffer. Total RNA (1 µg) was reverse transcribed according to the manufacturer’s protocol (ReverseAid First Strand cDNA synthesis kit from Thermoscientific, USA – Catalogue No. #1621). PCR reaction was carried out using 2X PCR Master Mix (Thermoscientific, USA – Catalogue No. #K0172) with 1 µL of cDNA and 20 µM of specific forward and reverse primers (Sigma Genosys, Sigma Aldrich, India) in 50 µL reaction. The details of primers and the conditions used in RT-PCR analysis are given in . To confirm PCR amplification, PCR product was mixed with 6X DNA gel loading dye and subjected to electrophoresis on 1% agarose gel containing 1% ethidium bromide (5ul/100 ml) at constant voltage (80 V) for 60 min in 0.5X Tris-Borate-EDTA (TBE) buffer. The amplified PCR product was visualized as a single compact band of expected size under UV light and documented by Alpha DigiDoc Gel Documentation & Image Analysis System (Alpha Innotech Corporation, San Leandro, CA, USA). Densito-metric analysis of the RT-PCR from three independent experiments after GAPDH normalization is shown in the bar graph (mean ± SEM).
Table 3. List of primers used for semi-quantitative RT-PCR.
Statistics
Statistical analysis of arbitrary units derived from densitometric measurements of DNA and protein bands from the study was carried out using SPSS software (Version 13.0; SPSS, Chicago, IL, USA). All the data are presented as the mean ± SD or mean ± SEM (n = 3) of triplicate samples. Differences between groups were calculated by Student’s t test and p < 0.05 was considered statistically significant.
Supplementary material available online
IAAN_1077903_Supplementary_Files.zip
Download Zip (474.7 KB)Acknowledgments
AJR would like to thank the Indian National Science Academy for the award of INSA Senior Scientist Fellowship. The authors wish to thank Professor Dipankar Chatterji, Molecular Biophysics Unit (MBU), Indian Institute of Science, Bangalore for help in carrying out mass spectrometry analysis. The authors also wish to thank Department of Biotechnology, Government of India (Grant No.BT/PR5487/AAQ/1/500/2012) for financial assistance provided during the course of the work and the Department of Biochemistry, Indian Institute of Science, Bangalore, India.
Declaration of interest
The Indian National Science Academy award of INSA Senior Scientist Fellowship to AJR and the Department of Biotechnology, Government of India (Grant No.BT/PR5487/AAQ/1/500/2012). The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
Designed the research study, performed the experiments, analyzed the data, and wrote the paper: VG (Postdoctoral Fellow); Contributed substantially to the research design and critically revised the manuscript: AJR (corresponding author).
References
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Practice Committee of the American Society for Reproductive Medicine. (2014) Female age-related fertility decline. Committee Opinion No. 589. Obstet Gynecol 123:719–721
- Aitken, R.J. (2014) Age, the environment and our reproductive future: Bonking baby boomers and the future of sex. Reproduction 147:S1–S11
- Baird, D.T., Collins, J., Egozcue, J., Evers, L.H., Gianaroli, L., Leridon, H., et al. (2005) Fertility and ageing. Hum Reprod Update 11:261–276
- Becker, H.M., Klier, M. and Deitmer, J.W. (2014) Carbonic anhydrases and their interplay with acid/base-coupled membrane transporters. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease and Industrial Applications. Frost S. and McKenna R, eds. Subcell Biochem 75:105–134
- Beere, H.M. (2004) “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651
- Bhatnagar, A. and Srivastava, S.K. (1992) Aldose reductase: Congenial and injurious profiles of an enigmatic enzyme. Biochem Med Metab Bio 48:91–121
- Breton, S. (2001) The cellular physiology of carbonic anhydrases. See comment in PubMed Commons below JOP 2(4 Suppl):159–164
- Broekmans, F., Soules, M. and Fauser, B. (2009) Ovarian aging: Mechanisms and clinical consequences. Endocr Rev 30:465–493
- Calvert, M.E., Digilio, L.C., Herr, J.C. and Coonrod, S.A. (2003) Oolemmal proteomics–identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane. Reprod Biol Endocrinol 1:27
- Chang, Y.-J., Huang, Y.-P., Li, Z.-L. and Chen, C.-H. (2012) GRP78 knockdown enhances apoptosis via the down-regulation of oxidative stress and Akt pathway after epirubicin treatment in colon cancer DLD-1 cells. PloS One 7:e35123
- Colton, S.A. and Downs, S.M. (2004) Potential role for the sorbitol pathway in the meiotic dysfunction exhibited by oocytes from diabetic mice. J Exp Zool A Comp Exp Biol 301:439–448
- Cousineau, I., Abaji, C. and Belmaaza, A. (2005) BRCA1 regulates RAD51 function in response to DNA damage and suppresses spontaneous sister chromatid replication slippage: Implications for sister chromatid cohesion, genome stability, and carcinogenesis. Cancer Res 65:11384–11391
- Crawford, N.M. and Steiner, A.Z. (2015) Age-related Infertility. Obstet Gynecol Clin North Am 42:15–25
- Eichenlaub-Ritter, U. (2012) Oocyte ageing and its cellular basis. Int J Dev Biol 56:841–852
- Faddy, M., Gosden, R., Gougeon, A., Richardson, S.J. and Nelson, J. (1992) Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum Reprod 7:1342–1346
- Fauser, B., Devroey, P., Diedrich, K., Balaban, B., Bonduelle, M., Delemarre-van de Waal, H., et al. (2014) Health outcomes of children born after IVF/ICSI: A review of current expert opinion and literature. Reprod Biomed Online 28:162–182
- Govindaraj, V., Basavaraju, K.R. and Rao, A.J. (2015) Changes in the expression of DNA double strand break repair genes in primordial follicles from immature and aged rats. Reprod BioMed Online 30:303–310
- Gutiérrez, T. and Simmen, T. (2014) Endoplasmic reticulum chaperones and oxidoreductases: Critical regulators of tumor cell survival and immunorecognition. Front Oncol 4:291
- Henson, P.M. (2003) A role for calreticulin in the clearance of apoptotic cells and in the innate immune system. In: Calreticulin, pp. 151–161. Springer US
- Hsu, S.Y. and Hsueh, A.J. (1998) A splicing variant of the Bcl-2 member Bok with a truncated BH3 domain induces apoptosis but does not dimerize with antiapoptotic Bcl-2 proteins in vitro. J Biol Chem 273:30139–30146
- Hunt, P.A. and Hassold, T.J. (2008) Human female meiosis: What makes a good egg go bad? Trends Genet 24:86–93
- Kaipia, A. and Hsueh, A.J. (1997) Regulation of ovarian follicle atresia. Annu Rev Physiol 59:349–363
- Ke, F., Bouillet, P., Kaufmann, T., Strasser, A., Kerr, J. and Voss, A. (2013) Consequences of the combined loss of BOK and BAK or BOK and BAX. Cell Death Dis 4:e650
- Kujjo, L.L., Laine, T., Pereira, R.J., Kagawa, W., Kurumizaka, H., Yokoyama, S., et al. (2010) Enhancing survival of mouse oocytes following chemotherapy or aging by targeting Bax and Rad51. PloS One 5:e9204
- Kurus, M., Karakaya, C., Karalok, M.H., To, G. and Johnson, J. (2013) The control of oocyte survival by intrinsic and extrinsic factors. Adv Exp Med Biol 761:7–18
- Lim, J. and Luderer, U. (2011) Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod 84:775–782
- Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
- Mao, C., Wang, M., Luo, B., Wey, S., Dong, D., Wesselschmidt, R., et al. (2010) Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PloS One 5:e10852
- Parsell, D. and Lindquist, S. (1993) The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496
- Petrash, J. (2004) All in the family: Aldose reductase and closely related aldo-keto reductases. Cell Mol Life Sci 61:737–749
- Ramos, I.B., Campos, C.B., Sorgine, M.H., De Souza, W. and Machado, E.A. (2011) Calreticulin expression levels and endoplasmic reticulum during late oogenesis and early embryogenesis of Rhodnius prolixus Stahl. Mol Biol Rep 38:1757–1767
- Rhee, S.G., Kang, S.W., Chang, T.S., Jeong, W. and Kim, K. (2001) Peroxiredoxin, a novel family of peroxidases. IUBMB Life 52:35–41
- Shevchenko, A., Wilm, M., Vorm, O. and Mann, M. (1996) Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68:850–858
- Spira, A. (1988) The decline of fecundity with age. Maturitas 10:15–22
- Tarin, J.J. (1996) Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod 2:717–724
- te Velde, E.R. and Pearson, P.L. (2002) The variability of female reproductive ageing. Hum Reprod Update 8:141–154
- Thomas, P.D., Campbell, M.J., Kejariwal, A., Mi, H., Karlak, B., Daverman, R., et al. (2003) PANTHER: A library of protein families and subfamilies indexed by function. Genome Res 13:2129–2141
- Thomas, P.D., Kejariwal, A., Guo, N., Mi, H., Campbell, M.J., Muruganujan, A., et al. (2006) Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res 34:W645–650
- Titus, S., Li, F., Stobezki, R., Akula, K., Unsal, E., Jeong, K., et al. (2013) Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 5:172ra121
- Vaskivuo, T.E. and Tapanainen, J.S. (2003) Apoptosis in the human ovary. Reprod BioMed Online 6:24–35
- Wang, C. and Machaty, Z. (2013) Calcium influx in mammalian eggs. Reproduction 145:R97–R105
- Wiest, D.L., Burgess, W., McKean, D., Kearse, K. and Singer, A. (1995) The molecular chaperone calnexin is expressed on the surface of immature thymocytes in association with clonotype-independent CD3 complexes. EMBO J 14:3425–3433
- Wilmut, I., Sullivan, G. and Taylor, J. (2008) A decade of progress since the birth of Dolly. Reprod Fertil Dev 21:95–100
- Yuan, J. and Chen, J. (2013) FIGNL1-containing protein complex is required for efficient homologous recombination repair. Proceed Natl Acad Sci U S A 110:10640–10645
- Zhang, D.X., Li, X.P., Sun, S.C., Shen, X.H., Cui, X.S. and Kim, N.H. (2010) Involvement of ER–calreticulin–Ca2+ signaling in the regulation of porcine oocyte meiotic maturation and maternal gene expression. Mol Reprod Dev 77:462–471
