Abstract
Optimizing culture conditions lead to the improvement of oocyte developmental competence and additives with anti-oxidative activity in culture media improved embryonic development. Royal jelly (RJ) is a product from the cephalic glands of nurse bees that has considerable health effects. The aim of this study was to investigate the effect of different concentrations of RJ on the maturation, cleavage, and blastocyst rates and gene expression in the oocyte and cumulus cells during in vitro maturation (IVM) of sheep oocyte. IVM of oocyte was performed in the presence of control (RJ0), 2.5 (RJ2.5), 5 (RJ5), 10 (RJ10), 20 (RJ20), and 40 (RJ40) mg/mL of RJ. Following the maturation period, parthenogenetic activation was carried out in two treatment groups (RJ0 and RJ10) and embryonic development was examined three and eight days thereafter. Moreover, the relative expression of BCL2 and BAX in oocyte as well as BCL2, BAX, HAS2, PTGS2, and STAR in cumulus cells were assessed. The results indicated that the addition of 10 mg/mL of RJ (90 ± 4.51%) to the maturation medium linearly increased the oocyte maturation rate compared to the control group (57 ± 2.42%), then it remained constant to the RJ40 (93 ± 3.10%) group. The higher RJ concentrations were associated with increased (p < 0.01) cleavage (53.3 ± 1.55% to 82.3 ± 2.82%) and blastocyst rate (15.5 ± 1.16% to 33.8 ± 3.09%) from the RJ0 to the RJ10 group. The relative mRNA expression of BCL2 and BAX in the oocyte was higher at RJ10. In cumulus cells, the expression of BCL2 was not affected, but that of BAX decreased, and expression of HAS2, PTGS2, and STAR were increased following the addition of RJ to the maturation media. In conclusion, the addition of 10 mg/mL of RJ to maturation medium improved blastocyst formation and decreased the apoptotic incidence in sheep cumulus cells and the oocyte during the in vitro development.
Introduction
Maturation of oocytes is the most critical step to successful in vitro production of embryos. Culture systems, in which oocytes are pooled to mature, not only affect the rate of maturation, but also may influence the subsequent embryonic development [Combelles et al. Citation2009; Goud et al. Citation1998]. With the ultimate aim of providing the in vivo conditions as close to the follicular milieu as possible, numerous culture systems with or without co-culture of helper cells have been tested for in vitro maturation of oocytes [Rizos et al. Citation2008]. Several studies have tried to supplement the maturation medium with a wide range of nutrients such as growth factors, hormones, carbohydrates, proteins, and other regulated substances that support the oocyte during maturation [Abd-Allah Citation2012b; Assidi et al. Citation2008; Elvin et al. Citation2000; Goud et al. Citation1998; Guo et al. Citation2009; Herrick et al. Citation2006; Marei et al. Citation2012; Olson and Seidel Citation2000; Shimada et al. Citation2006; Wang et al. Citation2006]. Nonetheless, the competences of in vitro matured (IVM) oocytes are still behind those of in vivo-matured oocytes.
Oxidative stress is one of the factors for compromising oocyte competence and accelerating the onset of apoptosis, especially in in vitro conditions [Agarwal et al. Citation2005]. The stress is mainly caused by the incongruity between production of reactive oxygen species (ROS) and antioxidant activity, especially that of glutathione peroxidase [Agarwal et al. Citation2005; Tatemoto et al. Citation2000]. A group of compounds with antioxidant property has been reported to protect the oocyte and embryo confronting ROS during maturation and early stage of development [Agarwal et al. Citation2005; Jumnik et al. Citation2007; Nagai and Inoue Citation2004; Nagai et al. Citation2006; Olson and Seidel Citation2000]; however, the role of antioxidants in the competence of the oocyte or embryo is controversial [Combelles et al. Citation2009].
Royal jelly (RJ) is the most important beehive product, secreted from the hypo-pharyngeal and mandibular glands of the worker honey bees (Apis mellifera) [Takenaka and Echigo Citation1982]. Having pharmaceutical and nutritive features, RJ has often been regarded as a functional food for humans. The RJ is a complex mixture of proteins (18%), amino acids, carbohydrates (15%), lipids (3–6%), fatty acids (mainly 10-hydroxy-2- decenoic acid; 10-HAD with antibacterial properties), vitamins (A, C, D, E and all the B complex vitamins), beneficial minerals (1.5%), and other less characterized compounds [Bincolotto et al. Citation2005; Boselli et al. Citation2003; Lucas Citation1942; Lerker et al. Citation1986; Mateescu and Barbulescu Citation1999; Nagai and Inoue Citation2004; Okamoto et al. Citation2003; Pavel et al. Citation2011; Suzuki et al. Citation2008; Tamura et al. Citation2009]. Also, fatty acids and a sterol isolated from RJ has estrogenic activity [Suzuki et al. Citation2008] and exert estrogenic effects in vivo and in vitro, similar to those evoked by 17β-estradiol (E2) [Kridli et al. Citation2003; Mishima et al. Citation2005]. The prevailing free amino acids in RJ are proline, lysine, glutamic acid, phenylalanine, aspartate, serine, cysteine, lysine, and arginine [Boselli et al. Citation2003]. About 15% of RJ is made up of carbohydrates such as fructose, glucose, and sucrose, with some traces of maltose, trehalose, and ribose [Lerker et al. Citation1986; Sabatini et al. Citation2009]. Nakajima et al. [Citation2009] indicated that RJ activity against free radicals is weaker than that of vitamin E. Also, RJ may bring about allergic responses (asthma and fatal anaphylaxis) in humans, but the incidence of allergic side effect in individuals who use RJ is not well understood [Thien et al. Citation1996]. However, despite the biological impacts of RJ on the reproductive system, information on the mechanism of action of chemical and bioactive compounds of RJ is not well known [Pavel et al. Citation2011].
Over the years, the anti-oxidative and anti-apoptotic properties of RJ [Nagai et al. Citation2001; Nagai and Inoue Citation2004; Nagai et al. Citation2006] have been experimentally indicated, owing to its improving effect on traumatic spinal cord injury and cisplatin-induced testes damage in rabbits [Aslan et al. Citation2012], longevity in mice [Silici et al. Citation2010], the oocyte maturation rate in sheep [Abd-Allah Citation2012b] and goat [Mazangi et al. Citation2015], sperm freezing ability in buffalo [Abd-Allah Citation2012a], and summer infertility in male rabbit [Elnagar Citation2010]. Although such improvements were previously achieved by supplementation of other compounds such as ascorbic acid [Tatemoto et al. Citation2000], vitamin E [Marsh and Coombes Citation2006; Olson and Seidel Citation2000], and α-lipoic acid [Marsh and Coombes Citation2006], the plausible beneficial effects of RJ during the development of ovine embryos have yet to be addressed.
The main factors comprised in apoptosis are the B cell/lymphoma-2 (BCL2) family proteins, including inhibitors (BCL2 and Bcl-XL) and promoters (Bid, BAX, Bak, and Bad) of apoptosis [Yang and Rajamahendran, Citation2002]. BCL2 works as an anti-apoptotic gene to promote cell survival, whereas BCL2-associated X protein (BAX) acts as a pro-apoptotic gene to induce cell death [Zhang et al. Citation2013]. The ratio of BCL2 to BAX could be used to predict the fate of cumulus oocyte complexes (COCs) after various treatments [Oltvai et al. Citation1993; Gursoy et al. Citation2008] and it could also be associated with the ability of oocytes to complete nuclear maturation [Filali et al. Citation2009]. Hyaluronan synthase 2 (HAS2), prostaglandin endoperoxide synthase 2 (PTGS2), and steroidogenic acute regulatory protein (STAR) genes expressed especially in cumulus cells during in vitro maturation that have been associated with developmental competence of oocytes and were suggested as predictors of embryo quality in women and cow [Assidi et al. Citation2008; Calder et al. Citation2001; Fair and Lonergan Citation2012; Nuttinck et al. Citation2002].
We hypothesized that supplementary RJ would (i) improve the oocyte nuclear maturation and embryonic development, and (ii) affect the mRNA abundance of the transcripts involved in oocyte and cumulus cell apoptosis.
Results
Effect of RJ in maturation media on oocyte meiotic competence
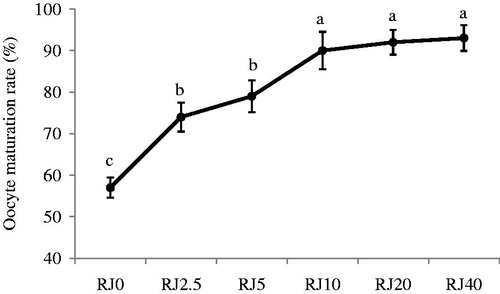
Data on the effect of RJ supplementation to the maturation media on oocyte nuclear maturation are presented in . In our preliminary test, we examined the effect of six concentrations of RJ on the oocyte maturation rate (0.0, 2.5, 5, 10, 20, and 40 mg/mL). Following 27 hours of culture of sheep cumulus oocyte complexes, the percentage of oocytes reaching to the MII stage increased (p < 0.01) from RJ0 to the RJ10 group (57 ± 2.42% vs. 90 ± 4.51%). There were no significant differences among the 10 (90 ± 4.51%), 20 (92 ± 2.95%), and 40 (93 ± 3.10%) mg/mL of RJ and based on these results we chose the 10 mg/mL of RJ as a maximum level for further experiments.
Figure 1. Effect of different concentrations of royal jelly (RJ) in maturation media on sheep oocyte meiotic competence. RJ0, RJ2.5, RJ5, RJ10, RJ20, and RJ40 represent the 0.0, 2.5, 5, 10, 20, and 40 mg/mL of RJ in the maturation medium, respectively. Data are presented as the mean ± SE of six samples. a–cMeans without a common superscript differed (p < 0.01).
Impact of RJ in maturation media on expression of apoptosis-related genes in oocytes
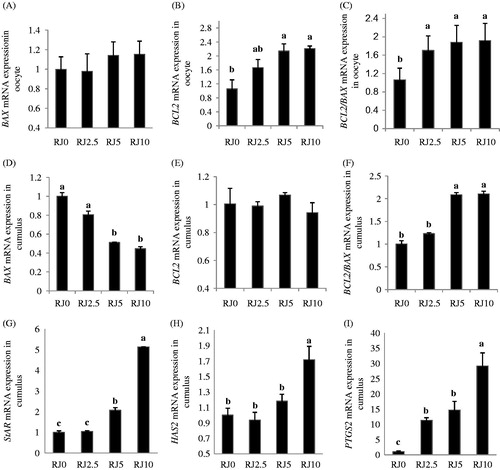
Supplementary RJ during IVM did not influence the transcript abundance of BAX (), but the relative expression of BCL2 mRNA increased (p < 0.01) by RJ in the oocyte (). Similar to BCL2 transcripts, the ratio of the BCL2/BAX transcript on the oocyte was increased in groups receiving RJ ().
Figure 2. Effect of different concentrations of royal jelly (RJ) on gene expression levels in in vitro matured sheep oocyte and cumulus cells. Real-time RT-PCR analysis of BCL2 (B cell/lymphoma-2), BAX (BCL2-associated X protein) in oocyte (A–C) and BCL2, BAX, HAS2 (hyaluronan synthase 2), PTGS2 (prostaglandin endoperoxide synthase 2), and STAR (steroidogenic acute regulatory protein) in cumulus cells (D–I) at the end of IVM. RJ0, RJ2.5, RJ5, RJ10 represent the 0.0, 2.5, 5 and 10 mg/mL of royal jelly (RJ) in the maturation medium, respectively. Relative levels of mRNA expression are presented as a mean ± SE of six independent samples. a–cMeans without a common superscript differed (p < 0.01).
Impact of RJ in maturation media on expression of candidate genes in cumulus cells
, E, and F illustrate the effect of supplementary RJ on BCL2, BAX, and BCL2/BAX transcripts in cumulus cells, respectively. The higher the RJ concentration (RJ10), the lower the relative expression of BAX mRNA in cumulus cells (p < 0.01) at the end of maturation (). The relative expression pattern of BCL2 mRNA in cumulus cells was not changed (); however, the ratio of BCL2/BAX transcript was increased (p < 0.01) by the different concentrations of RJ and reached to the highest level in the RJ10 group ().
By increasing the RJ concentrations from control to RJ10, the relative expressions of STAR (), HAS2 (), and PTGS2 mRNA () in cumulus cells were increased (p < 0.01) at the end of IVM.
Effect of RJ in maturation media on oocyte developmental potential
Data for cleavage and embryonic development are summarized in . Cleavage rate was different between control (53.3 ± 1.55%) and RJ10 (82.3 ± 2.82%) groups (p < 0.01). The blastocyst rate of RJ10 (33.8 ± 3.09%) was also higher as compared to that of the control (15.5 ± 1.16%) group (p < 0.01).
Table 1. Effect of royal jelly on sheep parthenogenetic embryo development.
Discussion
The results of the present study showed that RJ supplementation was associated with an enhancement in oocyte meiotic competence in a dose dependent manner. Treatment of sheep oocytes with RJ during IVM, linearly increased the nuclear maturation rate from the control to 10 mg/mL of RJ, then it remained constant to the higher concentration of RJ (40 mg/mL). Results in the previous studies have also shown that addition of various concentrations of RJ to sheep maturation media [Abd-Allah Citation2012b] and buffalo sperm extender [Abd-Allah Citation2012a] enhanced the rate of oocyte nuclear maturation and freezing capacity of cryopreserved spermatozoa, respectively. Supplementation of RJ to maturation medium at an optimum concentration improved the nuclear maturation rate, glutathione synthesis, and apoptosis-related genes in goat oocytes [Mazangi et al. Citation2015]. Our results showed that RJ treatment improved the cleavage and blastocyst rate following the parthenogenetic activation. Recently, Elnagar [Citation2010] indicated that oral administration of RJ in rabbits reduced the incidence of summer infertility and enhanced the physiological status of the male animals. Also, intramuscular administration of RJ ameliorated the pregnancy rate and reproductive performance in ewes [Husein and Haddad Citation2006; Kridli et al. Citation2003]. RJ is a mixture of numerous constituents, including proteins, free amino acids, carbohydrates, lipids, enzymes, antibiotic components, vitamins, mineral salts, sterols, phosphorous compounds, acetylcholine, and hormone-rich substance [Boselli et al. Citation2003; Echigo Citation1982; Lucas Citation1942; Mateescu and Barbulescu Citation1999; Okamoto et al. Citation2003; Pavel et al. Citation2011; Suzuki et al. Citation2008; Takenaka and Lerker et al. Citation1986]. Fatty acids and a sterol isolated from RJ has estrogenic activity [Suzuki et al. Citation2008] and exert estrogenic effects in vivo and in vitro, similar to those evoked by 17β-estradiol (E2) [Kridli et al. Citation2003; Mishima et al. Citation2005]. Given this, it has been shown that the beneficial activities of RJ in protecting female and male gametes might be attributed to its antioxidant property and especially its peptides [Guo et al. Citation2009] and free amino acid content [Boselli et al. Citation2003], and other bioactive compounds [Nagai and Inoue Citation2004; Silici et al. Citation2010; Tamura et al. Citation2009].
The present work clearly showed that the addition of RJ to maturation media increased the expression of BCL2, but not BAX transcripts in the mature oocytes. The relative expression of BCL2 mRNA differed between RJ treated groups and control group, reaching to the highest level at RJ10. Similar to BCL2, the ratio of BCL2 to BAX transcript was higher in groups treated with RJ compared to their control counterparts. This improvement might have been due to the anti-oxidant activity and scavenging ability against free radicals of the RJ [Aslan et al. Citation2012; Nagai et al. Citation2001; Nagai and Inoue Citation2004]. In further support of this finding, Inoue et al. [Citation2003] indicated that the 16-week-long feeding of RJ to mice reduced 8-hydroxy-2′-deoxyguanosine (a biomarker of oxidative stress on DNA) and increased the average longevity of the mice by the mechanism of reducing oxidative damage. The present data on the apoptosis-related genes showed a lower cumulus expression in the BAX transcript of RJ10 and the ratio of BCL2 to BAX transcripts in the cumulus cells was elevated (p < 0.01) at the higher RJ group. Jamnik et al. [Citation2007] reported that RJ treatment impeded the formation of intracellular ROS and, in turn, the oxidative damages of cell components, an effect possibly related to its anti-aging effect. Also, others reported that the water extract of RJ has a high amount of proteins [Nagai and Inoue Citation2004] and peptides [Guo et al. Citation2009] which contain strong antioxidantive activity and hydroxyl radical scavenging property. Abd-Allah [Citation2012b] reported that some components in RJ such as gonadotropins, steroid hormones, and essential amino acids that stimulate DNA and RNA synthesis and enhance cell division in addition, increases the level of intracellular cAMP, the activity of the hyaluronic acid synthesis enzyme system and induced cumulus expansion in intact complexes. It can be said that the effect of several proteins [Lucas Citation1942; Mateescu and Barbulescu Citation1999; Nagai and Inoue Citation2004], peptides [Guo et al. Citation2009], and hormones [Abd-Allah Citation2012b; Lucas Citation1942; Mishima et al. Citation2005; Suzuki et al. Citation2008] in the RJ may be improved apoptosis-related gene expression in cumulus cells and embryonic development of sheep oocytes.
Some other embryo quality-related candidate genes (STAR, HAS2, and PTGS2) have been analyzed in cumulus cells following the COCs exposure to RJ. Higher levels of STAR, HAS2, and PTGS2 mRNAs were observed at RJ10 compared with the control group. Although this is the first report unraveling such an association of these candidate genes with RJ supplementation in maturation medium, higher expression of HAS2 and PTGS2 genes in cumulus cells was recently found to be associated with the quality of the embryos produced [Assidi et al. Citation2008]. Expression of HAS2 transcripts in the cumulus cells has been previously considered as a valuable and indirect marker of oocyte competence in bovine and its mRNA abundance in COCs was found to be increased following the culture with gonadotropins [Assidi et al. Citation2008; Marei et al. Citation2012]. There is a report suggesting that in vitro maturation and expansion of cumulus cells were related to mRNA abundance of the PTGS2 gene in the cumulus cells [Calder et al. Citation2001]. Feuerstein et al. [Citation2007] indicated that nuclear maturation of human oocytes was associated with increased expression of STAR and PTGS2 transcripts in the cumulus cells. Other researchers have proposed that prostaglandins interact with the steroid biosynthetic pathway during the preovulatory differentiation of the COCs [Fair and Lonergon Citation2012; Shimada et al. Citation2006]. Interestingly, other investigators have found that the addition of progesterone receptor blockers to the maturation media turned off the resumption of oocyte meiosis and cumulus expansion in pig [Shimada et al. Citation2004] and cattle [Wang et al. Citation2006]. Previous reports also confirmed that increased expression of STAR, HAS2, and PTGS2 genes in the cumulus cell has been related to the developmental competence of oocytes in human and bovine [Assidi et al. Citation2008; Feuerstein et al. Citation2007], so that these genes have been suggested to be considered as predictors of human and bovine embryonic quality. This suggests that progesterone, progesterone receptor, and PTGS2 are essential factors in the cumulus cell expansion and oocyte maturation during the ovulation process [Fair and Lonergon Citation2012].
To create an optimal in vivo and in vitro environment for oocyte maturation, it is necessary to prevent oxidative damage or support the proper pro- or antioxidant balance in the body and culture media, respectively [Combelles et al. Citation2009]. In our present study, we hypothesized that supplementation of the maturation media with RJ would improve nuclear maturation and embryonic development of sheep oocytes.
RJ has been broadly used as a medicinal food with antioxidant properties. At present, the demand for health foods is rising, and RJ is one of them. Although there are numerous pharmacological reports on RJ, there is little information about its antioxidant properties [Nagai and Inoue Citation2004]. Recently, Nakajima et al. [Citation2009] demonstrated that compared to RJ vitamin E has a greater ability to neutralize oxygen free radicals. To substantiate the beneficial effect of RJ, future studies should be conducted for comparison between the RJ and the antioxidant constituents such as acid ascorbic (vitamin C) and alpha-tocopherol (vitamin E), as suitable additives during IVM. Also, since the chemical compositions and bioactive compounds of RJ are not fully elucidated, further studies are required to fully characterize what constitutes the antioxidant capacity of this product.
Collating the findings, supplementation of maturation medium with 10 mg/mL of RJ during IVM has begun to optimize the COC environment and improve the in vitro nuclear maturation and developmental potential of sheep oocytes. Treatment with RJ, also, up-regulated the expression of HAS2, STAR, and PTGS2 transcripts in the cumulus cells after the IVM. This improvement may be associated with amelioration of apoptosis-related gene status in both mature oocytes and their surrounding cumulus cells.
Materials and Methods
All chemicals were purchased from the Sigma Chemical Co. (St. Louis, MO, USA) and Gibco (Grand Island, NY, USA), unless otherwise stated. This study was approved by the institutional ethics committee and the different stages of this research were approved by the Animal Care Committee of Sari Agricultural Sciences and Natural Resources University.
Oocyte recovery
Sheep ovaries were collected from a local abattoir and transported to the laboratory within 2–3 h after collection in physiological saline (30–35°C) containing 100 μg/mL streptomycin and 100 IU/mL penicillin. Follicles with 2 to 6 mm diameter were dissected under sterile conditions using a surgical blade in a plastic Petri dish containing warm HEPES-buffered SOF supplemented with 1.5 mM glucose, 0.33 mM pyruvate, 1.0 mM glutamine, 3.0 mM L-lactate, 1X nonessential minimal essential medium (MEM) amino acids, 1X essential MEM amino acids, and 1.0 mg/mL BSA [Herrick et al. Citation2006]. The COCs with multilayered compact cumulus investment and evenly granulated cytoplasm were selected and randomly allocated to the experiments.
Preparation of RJ
Capsulated pure RJ was provided by Natural Life (Brookvale, NSW, Australia). According to manufacturer's reports, the RJ was produced from the purest beehives which were free from chemicals or antibiotic sprays. To prepare a stock solution of RJ, the contents of each capsule (1,000 mg) were dissolved in 10 mL deionized water (W/V) to obtain a concentration of 100 mg of RJ/mL [Abd-Allah Citation2012b]. The RJ was added to the deionized water and mixed thoroughly in a shaker at 4°C overnight. The prepared mixture was sterilized using a 0.22 µm filter and stored at −20°C.
In vitro maturation
The COCs were selected randomly and cultured in groups of 10 in 50 µL of medium 199 supplemented with 10% FBS, 2.0 mM glutamine, 50 mg/mL gentamicin, and 0.01 U/mL of ovine LH (Sigma-L5269) and ovine FSH (Sigma-F8174) under mineral oil. The incubation conditions during in vitro maturation (IVM) were 5% CO2 at 38.5°C with maximum humidity (98%) for 27 h.
Nuclear chromatin evaluation
At the end of maturation in vitro, COCs were mechanically denuded by repeated pipetting and then fixed by placing in 2.5% paraformaldehyde for 30 min at 37°C. The fixed oocytes were washed twice in warm PBS and incubated in PBS containing 1 µg/mL Hoechst 33342 for 5 min away from light at 37°C and then washed in PBS for 15 min at 37°C. Nuclear maturation status of stained oocytes was evaluated under an epifluorescence microscope (Nikon TE-300; Nikon, Tokyo, Japan) at 400X. Nuclear status was evaluated in individual oocyte for metaphase-II (MII) stage [Mohammadi-Sangcheshmeh et al. Citation2012].
Parthenogenetic activation and in vitro culture
Following the IVM, a number of COCs were denuded. The denuded oocytes, then washed in TCM-199 which were supplemented with 10% FBS and activated chemically through exposure to 2.5 μM ionomycin (diluted in TCM-199) for 1 min followed by a 3-h exposure to 2 mM 6-dimethylaminopurine (6-DMAP) (diluted in CR1aa medium). The activated oocytes were washed twice in CR1aa. Each group of 10 oocytes was cultured in 50 µL of CR1aa under mineral oil at 38.5°C, maximum humidity (98%), and 5% CO2 for 8 d [Mohammadi-Sangcheshmeh et al. Citation2014]. To prevent toxic accumulation of ammonium, a half of the culture medium was substituted at 48 h intervals during the culture period. Developmental data were recorded for cleavage and embryonic development at days 3 and 8 post-activation, respectively.
RNA isolation and cDNA synthesis
Six pools of biological replicates, each containing ten denuded mature oocytes were used for total RNA isolation. The cumulus cells of mature oocytes were separated and pooled in six biological replicates for total RNA extraction. Total RNA was isolated using the RNAeasy Micro kit (Cat. No. 74004; Qiagen, GmbH, Germany) according to the manufacturer's instructions and stored at −80°C pending cDNA synthesis. For each sample, cDNA synthesis was carried out using the QuantiTec Reverse Transcription Kit (Cat. No. 205311; Qiagen). The cDNA synthesis reaction conditions were 42°C for 30 min and 95°C for 3 min.
Quantitative real-time PCR (qPCR)
Quantitative real-time polymerase chain reaction (qPCR) was implemented to determine the relative transcripts of BAX, BCL2, and BCL2/BAX ratio in the sheep oocytes as well as the relative transcripts of BAX, BCL2, HAS2, STAR, PTGS2, and the ratio of BCL2/BAX in cumulus cells. Details of primer sequences are provided in . Expression of Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta polypeptide (YWHAZ) transcript was used as an internal housekeeping gene [O'Connor et al. Citation2013]. Quantification of all transcripts was performed using QuantiFast SYBR Green PCR Kit (Cat. No. 204052; Qiagen) in a 20 μL reaction volume containing 1 μL single-strand cDNA, 10 μL of master mix, 0.5 μL of each forward and reverse primers and 8 μL of distilled H2O in 20 μL by a Rotor-Gene 6000 Real-Time PCR software (Corbett Research, Sydney, Australia). The program used for the amplification of genes consisted of a denaturing cycle of 5 min at 95°C, followed by 40 cycles (95°C for 15 s, annealing and extension at 60°C for 40 s). At the end of each PCR, a melting curve analysis was performed at the rate of 0.1°C/s for all genes to check the specificity of the products. Standard curves of efficiency of each primer pair were determined with five series of 10-fold dilution of positive control cDNA as a template. The efficiency of the assays (E) was ≥95%, and standard curve R2 was ≥0.999. The relative levels of mRNA were analyzed by the 2 −ΔΔCt method [Livak and Schmittgen Citation2001].
Table 2. Quantitative real time PCR primer sequences.
Experimental design
To assess the effect of RJ in maturation media on oocyte meiotic competence, ocytes were matured in maturation media supplemented with control (RJ0), 2.5 (RJ2.5), 5 (RJ5), 10 (RJ10), 20 (RJ20), and 40 (RJ40) mg/mL of RJ. Six replicates were analyzed for this experiment. To assess the effect of RJ in maturation media on the expression of apoptosis-related genes in the oocyte, in vitro matured oocytes subjected to different concentrations of RJ (0.0, 2.5, 5, and 10 mg/mL) were evaluated for the expression of BAX and BCL2 genes and also for the ratio of BCL2/BAX transcript. Six replicates were performed per treatment. To assess the effect of RJ in maturation media on the expression of candidate genes in cumulus cells, following IVM, cumulus cells were separated from mature oocytes and were analyzed for the transcript abundance of BAX, BCL2, HAS2, PTGS2, and STAR genes. The ratio of the BCL2/BAX transcript was also evaluated. Six replicates were carried out per treatment. To assess the effect of RJ in maturation media on oocyte developmental potential RJ effects on subsequent embryonic development, matured COCs obtained from the optimum range of RJ (10 mg/mL in experiment 1) and the control group was activated, and cultured in vitro. On days 3 and 8 of in vitro culture, subsequent developmental capacity in terms of cleavage and development to the blastocyst stage of the treated group was recorded and compared with the control group. This experiment was executed in four replicates.
Statistical analysis
Data were analyzed as a completely randomized design using SAS software (Version 9.1, SAS Institute, Cary, NC, USA) by the General Linear Model (GLM) procedure. Analysis among treatments for nuclear maturation of oocyte and embryonic development were performed using analysis of variance (ANOVA) followed by Tukey's test. The relative expression data were analyzed by ANOVA. Comparisons were considered significantly different if p < 0.01. Data were expressed as a mean ± standard error.
| Abbreviations | ||
| RJ | = | royal jelly |
| ROS | = | reactive oxygen species |
| IVM | = | in vitro maturation |
| COC | = | cumulus oocyte complex |
| BCL2 | = | B cell/lymphoma-2 |
| BAX | = | BCL2-associated X protein |
| HAS2 | = | hyaluronan synthase 2 |
| PTGS2 | = | prostaglandin endoperoxide synthase 2 |
| STAR | = | steroidogenic acute regulatory protein |
| YWHAZ | = | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta polypeptide |
Acknowledgments
The authors thank the members of their own laboratories for their helpful discussion.
Declaration of interest
This work was supported by a grant from the Department of Animal Science, College of Animal Science and Fisheries, Sari Agricultural Science and Natural Resources University, Sari, Iran. The authors declare no potential conflicts of interest with respect to the authorship and publication of the article. Also, the authors declare that none of the authors (Hamid Deldar, Mohammad Valiollahpoor, and Zarbakht Ansari Pirsaraei) received any financial support from the Iranian Government.
Author contribution
Participated in conception and design, data collection, statistical analysis, data interpretation, and writing and critical revision of the manuscript: HD; Participated in conception, design, and data interpretation of the manuscript: ZAP; Conducted the study as part of his graduate degree: MVA.
References
- Abd-Allah, S.M. (2012a) Effect of royal jelly on the fertilizing ability of buffalo spermatozoa in vitro. J Buffalo Sci 1:1–4
- Abd-Allah, S.M. (2012b) Effect of royal jelly on viability and in vitro maturation of Egyptian sheep oocytes in serum supplemented medium. Brit J Pharmacol Toxicol 3:29–32
- Agarwal, A., Gupta, S., Sharma, R. (2005) Oxidative stress and its implications in female infertility – a clinician’s perspective. Reprod Biomed Online 11:641–650
- Aslan, A., Cemek, M., Buyukokuroglu, M. E., Altunbas, K., Bas, O., Yurumez, Y. (2012) RJ can diminish secondary neuronal damage after experimental spinal cord injury in rabbits. Food Chem Toxicol 50:2554–2559
- Assidi, M., Dufort, I., Ali, A., Hamel, M., Algriany, O., Dielemann, S., et al. (2008) Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod 79:209–222
- Bincolotto, C., Eberlin, S., Figueiredo, C.A.V., Luengo, M.B., Qeiroz, M.L.S. (2005) Effects produced by royal jelly on haematopoiesis: relation with host resistance against Ehrlich ascites tumour challenge. Int Immunopharmacol 5:679–688
- Boselli, E., Caboni, M.F., Sabatini, A.G., Marcazzan, G.L., Lercker, G. (2003) Determination and changes of free amino acids in royal jelly during storage. Apidologie 34:1–7
- Calder, M. D., Caveney, A.N., Westhusin, M. E., Watson, A.J. (2001) Cyclooxygenase-2 and prostaglandin E2 (PGE2) receptor messenger RNAs are affected by bovine oocyte maturation time and cumulus-oocyte complex quality, and PGE2 induces moderate expansion of the bovine cumulus in vitro. Biol Reprod 65:135–140
- Combelles, C.M.H., Gupta, S., Agarwal, A. (2009) Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online 18:864–880
- Elnagar, S.A. (2010) Royal jelly counteracts bucks “summer infertility". Anim Reprod Sci 121:174–180
- Elvin, J.A., Yan, C, Matzuk, M.M. (2000) Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci U S A 97:10288–10293
- Fair, T., Lonergan, P. (2012) The role of progesterone in oocyte acquisition of developmental competence. Reprod Dom Anim 47:142–147
- Feuerstein, P., Cadoret, V., Dalbies-Tran, R., Guerif, F., Bidault, R., Royere, D. (2007) Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod 22:3069–3077
- Filali, M., Frydman, N., Belot, M.P., Hesters, L., Gaudin, F., Tachdjian, G., et al. (2009) Oocyte in-vitro maturation: BCL2 mRNA content in cumulus cells reflects oocyte competency. Reprod Biomed Online 19 (Suppl 4): 4309
- Goud, P.T., Goud, A.P., Qian, C., Laverge, H., Van der Elst, J., De Sutter, P., et al. (1998) In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod 13:1638–1644
- Guo, H., Kouzuma, Y., Yonekura, M. (2009) Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem 113:238–245
- Gursoy, E., Ergin, K., Basaloglu, H., Koca, Y., Seyrek, K. (2008) Expression and localisation of BCL2 and BAX proteins in developing rat ovary. Res Vet Sci 84:56–61
- Herrick, J. R., Lane, M., Gardner, D.K., Behboodi, E., Memili, E., Blash, S., et al. (2006) Metabolism, protein content, and in vitro embryonic development of goat cumulus-oocyte complexes matured with physiological concentrations of glucose and L-actate. Mol Reprod Dev 73:256–266
- Husein, M., Haddad, S.G. (2006) A new approach to enhance reproductive performance in sheep using royal jelly in comparison with equine chorionic gonadotropin. Anim Reprod Sci 93:24–33
- Inoue, S., Koya-Miyata, S., Ushio, S., Iwaki, K., Ikeda, M., Kurimoto, M. (2003) Royal jelly prolongs the life span of C3H/HeJ mice: correlation with reduced DNA damage. Exp Gerontol 38:965–969
- Jamnik, P., Goranovic D., Raspor, P. (2007) Antioxidative action of royal jelly in the yeast cell. Exp Gerontol 42:594–600
- Kridli, R.T., Husein, M.Q., Humphrey, W.D. (2003) Effect of royal jelly and GnRH on the estrus synchronization and pregnancy rate in ewes using intravaginal sponges. Small Rumin Res 49:25–30
- Lerker, G., Savioll, S., Vecchi, M.A., Sabatini, A.G., Nanetti, A., Piana, L. (1986) Carbohydrate determination of royal jelly by high resolution gas chromatography (HRGC). Food Chem 19:255–64
- Livak, K.J., Schmittgen, T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
- Lucas, C.C. (1942) Chemical examination of royal jelly. Can Med Assoc J 47:406
- Marei, W.F., Ghafari, F., Fouladi-Nashta, A.A. (2012) Role of hyaluronic acid in maturation and further early embryo development of bovine oocytes. Theriogenology 78:670–677
- Marsh, S.A., Coombes, J.S. (2006) Vitamin E and a-lipoic acid supplementation increase bleeding tendency via an intrinsic coagulation pathway. Clin Appl Thromb Hemost 12:169–173
- Mateescu, C., Barbulescu, D. (1999) Enhanced nutritive, functional and therapeutic action of combined bee products in complex food supplements. Roumanian Biotechnol Lett 4:163–172
- Mazangi H.R, Deldar H., Kashan, N.E., Mohammadi-Sangcheshmeh, A. (2015) Royal jelly treatment during oocyte maturation improves in vitro meiotic competence of goat oocytes by influencing intracellular glutathione synthesis and apoptosis gene expression. Reprod Fertil Dev 27(1):241–241
- Mishima, S., Suzuki, K.M., Isohama, Y., Kuratsu, N., Araki, Y., Inoue, M., et al. (2005) Royal jelly has estrogenic effects in vitro and in vivo. J Ethnopharmacol 101:215–220
- Mohammadi-Sangcheshmeh, A., Soleimani, M., Deldar, H., Salehi, M., Soudi, S., Hashemi, S. M., et al. (2012) Prediction of oocyte developmental competence in ovine using glucose-6-phosphate dehydrogenase (G6PDH) activity determined at retrieval time. J Assist Reprod Genet 29:153–158
- Mohammadi-Sangcheshmeh, A., Veshkini, A., Hajarizadeh, A., Jamshidi-Adegani, F., Zhandi, M., Abazari-kia, A.H., et al. (2014) Association of glucose-6-phosphate dehydrogenase activity with oocyte cytoplasmic lipid content, developmental competence, and expression of candidate genes in a sheep model. J Assist Reprod Genet 31:1089–1098
- Nagai, T., Inoue, R. (2004) Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem 84:181–186
- Nagai, T., Inoue, R., Suzuki, N., Nagashima, T. (2006) Antioxidant properties of enzymatic hydrolysates from royal jelly. J Med Food 9:363–367
- Nagai, T., Sakai, M., Inoue, R., Inoue, H., Suzuki, N. (2001) Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem 75:237–240
- Nakajima, Y., Tsuruma, K., Shimazawa, M., Mishima, S., Hara, H. (2009) Comparison of bee products based on assays of antioxidant capacities. BMC Complement Alternat Med 9:4
- Nuttinck, F., Reinaud, P., Tricoire, H., Vigneron, C., Peynot, N., Mialot, J.P., et al. (2002) Cyclooxygenase-2 is expressed by cumulus cells during oocyte maturation in cattle. Mol Reprod Dev 61:93–101
- O'Connor, T., Wilmut, I., Taylor, J. (2013) Quantitative evaluation of reference genes for real-time PCR during in vitro maturation of ovine oocytes. Reprod Dom Anim 48:477–483
- Okamoto, I., Taniguchi, Y., Kunikata, T., Kohno, K., Iwaki, K., Ikeda, M., et al. (2003) Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci 73:2029–2045
- Olson, S.E., Seidel, G.E. (2000) Culture of in vitro-produced bovine embryos with vitamin E improves development in vitro and after transfer to recipients. Biol Reprod 62:248–252
- Oltvai, Z.N., Milliman, C.L., Korsmeyer, S.J. (1993) BCL2 heterodimerizes in vitro with a conserved homology, BAX, that accelerates programmed cell death. Cell 74:609–619
- Pavel, C.I., Marghitas, L.A., Bobis, O., Dezmirean, D.S., Sapcaliu, A., Radoi, I., et al. (2011) Biological activities of royal jelly - Review. Anim Sci Biotechnol 44:108–118
- Rizos, D., Clemente, M., Bermejo-Alvarez, P., de La Fuente, J., Lonergan, P., Gutierrez-Adan, A. (2008) Consequences of in vitro culture conditions on embryo development and quality. Reprod Domest Anim 43:44–50
- Sabatini, A.G., Marcazzan, G., Caboni, M.F., Bogdanov, S., Almeida-Muradian, L.B. (2009) Quality and standardisation of royal jelly. J ApiProd ApiMed Sci 1:16–21
- Shimada, M., Hernandez-Gonzalez, I., Gonzalez-Robayna, I., Richards. J.S. (2006) Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365
- Shimada, M., Yamashita, Y., Ito, J., Okazaki, T., Kawahata, K., Nishibori, M. (2004) Expression of two progesterone receptor isoforms in cumulus cells and their roles during meiotic resumption of porcine oocytes. J Mol Endocrinol 33:209–225
- Silici, S., Ekmekcioglu, O., Kanbur, M., Deniz, K. (2010) The protective effect of royal jelly against cisplatin-induced renal oxidative stress in rats. World J Urol 29:127–132
- Suzuki, K.M., Isohama, Y., Maruyama, H., Yamada, Y., Narita, Y., Ohta, S., et al. (2008) Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid Based Complement Alternat Med 5:295–302
- Takenaka, T., Echigo, T. (1982) Chemical composition of royal jelly. Honeybee Sci 3:69–74
- Tamura, S., Kono, T., Harada, C., Yamaguchi, K., Moriyama, T. (2009) Estimation and characterisation of major royal jelly proteins obtained from the honeybee Apis merifera. Food Chem 114:1491–1497
- Tatemoto, H., Sakurai, N., Muto, N. (2000) Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Bio Reprod 63:805–810
- Thien, F.C., Leung, R., Baldo, B.A., Weiner, J.A., Plomley, R., Czarny, D. (1996) Asthma and anaphylaxis induced by royal jelly. Clin Exp Allergy 26:216–222
- Wang, H.F., Isobe, N., Kumamoto, K., Yamashiro. H., Yamashita, Y., Terada, T. (2006) Studies of the role of steroid hormone in the regulation of oocyte maturation in cattle. Reprod Bio Endocrinol 4:4
- Yang, M.Y., Rajamahendran, R. (2002) Expression of BCL2 and BAX proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim Reprod Sci 70:159–169
- Zhang, G-M., Gu, C-H., Zhan, Y-L., Sun, H-Y., Qian, W-P., Zhou, Z-R., et al. (2013) Age-associated changes in gene expression of goat oocytes. Theriogenology 80:328–336
