Abstract
Ovarian follicular responsiveness to controlled ovarian hyperstimulation (COH) with gonadotropins is extremely variable between individual patients, and even from cycle to cycle for the same patient. High responder patients are characterized by an exaggerated response to gonadotropin administration, accompanied by a higher risk for ovarian hyperstimulation syndrome (OHSS). In spite of its importance, the literature regarding high responders is characterized by heterogeneous classification methodologies. A clear separation should be drawn between risk factors for a high ovarian response and the actual response exhibited by a patient to stimulation. Similarly, it is important to distinguish between high ovarian response and development of clinically significant OHSS. In this article we: (1) review recent publications pertaining to the identification and clinical management of high responders, (2) propose an integrated clinical model to differentiate sub-groups within this population based on this review, and (3) suggest specific protocols for each sub-group. The model is based on a chronological patient assessment in an effort to target treatment based on the specific clinical circumstances. It is our hope that the algorithm we have developed will assist clinicians to supply targeted and precise treatments in order to achieve a favorable reproductive outcome with minimum complications for each patient.
Introduction
Ovarian follicular responsiveness to controlled ovarian hyperstimulation (COH) with gonadotropins is extremely variable between patients and even from cycle to cycle for the same patient [Tremellen and Savulescu Citation2014]. Achieving an ideal follicular response is critical to the success of assisted reproduction treatment (ART). Since the early years of ART, patients have been classified as ‘poor’, ‘normal’, or ‘high’ responders. Although these terms are widely used in research and in daily clinical practice, their precise definitions are still not fully agreed upon. Distinguishing them has been based on various measures of ovarian reserve [Sills et al. Citation2009]. The first description of a poor responder occurred in 1983 [Garcia et al. Citation1983] while the first international consensus criteria for poor responders (the Bologna Criteria) was published in 2011 [Ferraretti et al. Citation2011]. Poor responders, in general, exhibit an inadequate response to hormonal stimulation [Tarlatzis et al. Citation2003] and diminished reproductive outcome [Lainas et al. Citation2008].
In contrast to poor responders, high responders are characterized by an exaggerated response accompanied with a higher risk for ovarian hyperstimulation syndrome (OHSS) [Datta et al. Citation2014; Huber et al. Citation2013]. OHSS is characterized by the release of excessive vasoactive factors (especially vascular endothelial growth factor – VEGF), increased vascular permeability, and fluid shifts out of the intravascular space, resulting in excessive extravascular third spacing [Gomez et al. Citation2010; Papanikolaou et al. Citation2010]. There are two main clinical forms of OHSS, early OHSS and late OHSS, distinguished by their time of onset [Humaidan et al. Citation2010b]. Early OHSS is an acute consequence of exogenous human chorionic gonadotropin (hCG) administration before oocyte retrieval and is related to excessive ovarian response to gonadotropins stimulation. Late OHSS is induced by endogenous placental hCG, with a higher incidence among multiple pregnancies [Navot et al. Citation1992]. Late OHSS is more likely to be severe with a poorer correlation to pre-ovulatory events, compared to early OHSS which is more directly related to the extent of ovarian response [Mathur et al. Citation2000]. OHSS is classically manifested as multiple clinical symptoms (e.g., nausea, dyspnea), sonographic features (such as ovarian sizes, ascites), and laboratory parameters (hemoconcentration, electrolyte disturbances, etc.) [Zivi et al. Citation2010].
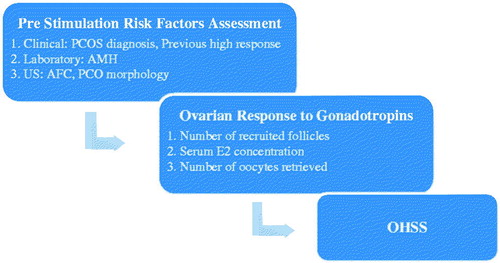
In spite of its importance and relevance for ART, the literature regarding high responders is characterized by heterogeneous classification methodology [Casano et al. Citation2012; Datta et al. Citation2014]. As shown in , variable criteria in the literature relate to: (1) risk factors for high ovarian response, (2) risk factors for OHSS, as well as (3) the definition of a high ovarian response. The objectives of this article are: (1) to review recent developments regarding the identification and clinical management of high responders, (2) to propose an evidence based integrated clinical model to differentiate sub-groups within this population, and (3) to suggest specific protocols for each sub-group.
Table 1. Published criteria for risk for high ovarian response, risk for OHSS, and definition of high ovarian response.
Evaluation of High Ovarian Response and Risk for OHSS
A large ovarian pool of pre-antral follicles is a significant risk factor for high ovarian response and potential for the development of OHSS. Both antral follicle count (AFC) and anti-müllerian hormone (AMH) parameters have been shown to be good predictors of high responsiveness [Broekmans et al. Citation2014; Broer et al. Citation2009; Nastri et al. Citation2015]. Additionally, an individual’s susceptibility to OHSS may result from increased ovarian sensitivity to gonadotropins [Gomez et al. Citation2010].
Since definitions and inclusion criteria for high ovarian response in the literature tend to be inconsistent and overlapping, a clear separation should be made between risk factors for high ovarian response and the actual response to stimulation. While the first are evaluated and identified as predictors of high ovarian response prior to stimulation initiation, the second is measured during and after stimulation by the number of developing follicles, estradiol (E2) serum concentration, and the number of oocytes retrieved [Huber et al. Citation2013]. Similarly, it is important to distinguish between high ovarian response and OHSS development. That separation has become more prominent with novel triggering regimens which have dramatically reduced the incidence of OHSS among patients with high response. It should be noted that pre-stimulation AFC and AMH have been found to be reliable predictors for high ovarian response, but their utility to predict OHSS is limited. However, high ovarian response by itself has been found to be a reliable predictor for OHSS [Nastri et al. Citation2015]. These findings emphasize the importance of chronology in the assessment of high responders as summarized in .
Clinical Management of High Responders
hCG has been administered for decades as a substitute for the LH surge in order to effect final oocyte maturation prior to retrieval [Humaidan et al. Citation2013]. However, a prominent drawback of hCG triggering is the risk for OHSS, ranging from 3% to 30% in normal and high responders, respectively [Humaidan and Polyzos Citation2014], emphasizing the need to establish safer and effective alternative approaches for ovulation induction.
GnRH agonist (GnRHa) triggering was shown to be as effective as hCG at inducing oocyte maturation more than 20 years ago [Gonen et al. Citation1990]. However, due to the use of GnRHa for pituitary down-regulation, its use to trigger ovulation was not possible until the introduction of GnRH antagonists for pituitary suppression [Humaidan and Polyzos Citation2014]. In contrast to hCG triggering, GnRHa triggering is characterized by simultaneous LH and FSH surges, similar to natural ovulation [Fauser et al. Citation2002]. Some studies suggest that this approach may lead to better oocyte maturation and fertilization rates [Humaidan et al. Citation2005, Citation2010a]. Early results with GnRHa triggering were disappointing, as reported in several randomized control trials (RCTs) [Fauser et al. Citation2002; Humaidan et al. Citation2005; Kolibianakis et al. Citation2005], where higher pregnancy loss rates and lower ongoing pregnancy rates were observed [Youssef et al. Citation2014]. Multiple studies have targeted corpus luteum dysfunction as the primary etiology for the poor outcome after GnRHa trigger due to significantly reduced estrogen and progesterone secretion, as well as shorter luteal phase compared to hCG trigger [Acevedo et al. Citation2006; Fauser et al. Citation2002]. Subsequently, outcomes were dramatically improved after the adoption of adjusted regimens to enhance luteal support [Humaidan et al. Citation2011]. These regimens included two approaches for luteal phase support (LPS) [Humaidan and Polyzos Citation2014]: (1) modified LPS which includes1,500 units of hCG on retrieval day leading to the maintenance of LH activity to promote endogenous progesterone and estrogen secretion from the corpus luteum in addition to standard exogenous supplementation e.g., as micronized vaginal progesterone 90 mg daily and 4 mg oral oestradiol [Humaidan Citation2009] and (2) intensive LPS with high dose exogenous IM progesterone (up to 75 mg daily with optional addition of micronized vaginal progesterone) and estrogen supplementation (maximum of four 0.1 mg patches every other day and/or addition of oral micronized E2) to maintain serum concentrations of > 20 ng/ml and > 200 pg/ml, respectively [Humaidan et al. Citation2015]. Over the years, these ‘European’ and ‘American’ approaches [Humaidan et al. Citation2015] have been proven equally as effective for significant OHSS reduction among high risk patients while allowing fresh transfer with excellent reproductive outcome [Humaidan and Polyzos Citation2014]. Still the ideal type of LPS after GnRH a trigger is under investigation [Humaidan et al. Citation2015]. A pivotal study by Engmann et al. [Citation2008] included high risk patients diagnosed with PCOS in their first IVF cycle or patients with a history of high response in a previous cycle. The authors reported no cases of any form of OHSS in those patients who underwent GnRHa triggering together with intensive estrogen and progesterone supplementation, while maintaining comparable reproductive outcome to those receiving hCG triggering [Engmann et al. Citation2008]. Another well designed RCT recruited patients with OHSS risk factors (PCOS as well as oligo/amenorrhea) and further differentiated them on the day of triggering into ‘low’ vs. ‘high’ OHSS risk according to their actual ovarian response. Patients with high ovarian response were randomized to receive either GnRHa triggering followed by a single dose of hCG 1500 units on the day of retrieval (modified LPS) vs. standard hCG triggering with 5000 units alone. Patients with a normal response were randomized to receive either GnRHa triggering followed by two doses of 1500U hCG on the retrieval day and in the mid luteal phase for luteal support vs. standard hCG triggering with 5000 units alone [Humaidan et al. Citation2013]. Although the study was discontinued due to technical difficulties and did not demonstrate significant results, it may serve as a model for sub-population evaluation in the high responder continuum and individualization of triggering and luteal support.
A few years ago, the concept of ‘dual triggering’ was introduced, which combines a bolus of GnRHa together with low dose hCG [Shapiro et al. Citation2008]. This regimen combines a dose of GnRHa, to effectively induce ovulation, together with hCG, to extend luteal support. Although RCTs investigating the effectiveness and safety of dual triggering have not been published yet, primary retrospective results are encouraging. GnRHa triggering accompanied by low dose hCG (adjusted to BMI) in high risk patients (≥20 follicles and E2 ≥ 2500 pmol/l before triggering) have yielded similar IVF success results to GnRHa triggering alone followed by intensified luteal support, without significantly higher OHSS rates [Shapiro et al. Citation2011]. Another retrospective study included patients with a previous history of OHSS, previous cycle cancellation due to OHSS risk, or >13 follicles ≥11 mm on the day of triggering. Patients with an extreme response of E2 ≥ 4000 pmol/ml were excluded. In this study, ‘high responders’ and ‘high risk patients’ were grouped without distinguishing their OHSS risk, risk for high response, or an actual high ovarian response. Success rates were impressively better in the patient group that received dual triggering (GnRHa and 1,000U hCG) compared with those that received GnRHa alone [Griffin et al. Citation2012]. In addition to high responders, recent studies have reported potentially promising pregnancy results on the use of dual triggering for patients with a history of poor oocyte maturation [Griffin et al. Citation2014] and low oocyte yield per follicle [Haas et al. Citation2014]. Although these initial observations are encouraging, the potential risk for OHSS using dual triggering cannot be neglected. Since hCG is the main contributor to OHSS development, likely due to induction of VEGF secretion [Gomez et al. Citation2010], it is reasonable that its administration during dual trigger may increase the risk for OHSS compared to GnRHa trigger with intensive LPS. Furthermore, unlike modified and intensive LPS, which have been investigated comprehensively (as described above), no RCT focused on dual trigger and its possible effect on OHSS among high responders has been published. That risk should be calculated in the future and weighed against the potential benefits of hCG, such as in oocyte maturation [Griffin et al. Citation2014].
Integrated Model for Classification of High Responders and Clinical Management
Clinical management of high responders is continuously improving with the adoption of the novel protocols described above. However, in order to effectively treat high responders, a consensus on diagnostic criteria is needed. To help address this issue, we have developed an evidence based model for the classification of high responders. The model is based on a chronological patient assessment in an effort to target treatment based on the specific clinical circumstances ().
Figure 2. Classification and clinical management of the high ovarian response continuum. Positive and negative predictive values as well as level of evidence are presented in Supplemental . 1One of the following: AMH > 3 ng/ml, AFC > 16, PCOS/PCOM, previous high response (OHSS, cycle cancellation or coasting) [Engmann et al. Citation2008; Humaidan et al. Citation2013; Nastri et al. Citation2015]. 2∼14,700 pmol/L. GnRH: Gonadotropin realising hormone; GnRHa: Gonadotropin realising hormone agonist; LPS: luteal phase support; hCG: human chorionic gonadotropin; PCOS: polycystic ovary syndrome; AMH: anti-müllerian hormone; AFC: antral follicle count; PCO: polycistic ovary; US: ultrasound; OHSS: ovarian hyperstimulation syndrome; E2: estradiol.
![Figure 2. Classification and clinical management of the high ovarian response continuum. Positive and negative predictive values as well as level of evidence are presented in Supplemental Table 1. 1One of the following: AMH > 3 ng/ml, AFC > 16, PCOS/PCOM, previous high response (OHSS, cycle cancellation or coasting) [Engmann et al. Citation2008; Humaidan et al. Citation2013; Nastri et al. Citation2015]. 2∼14,700 pmol/L. GnRH: Gonadotropin realising hormone; GnRHa: Gonadotropin realising hormone agonist; LPS: luteal phase support; hCG: human chorionic gonadotropin; PCOS: polycystic ovary syndrome; AMH: anti-müllerian hormone; AFC: antral follicle count; PCO: polycistic ovary; US: ultrasound; OHSS: ovarian hyperstimulation syndrome; E2: estradiol.](/cms/asset/088c7913-6eff-4f42-8d03-b1ff20d5f421/iaan_a_1089607_f0002_c.jpg)
Pre-stimulation assessment
High ovarian response predicted
This group consists of individuals with accepted risk factors for high ovarian response including one or more of the following: AMH > 21.4 nmol/L (3 ng/ml), AFC > 16 [Nastri et al. Citation2015], previous diagnosis of PCOS [Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Citation2004], or previous history of OHSS/cycle cancellation/coasting [Engmann et al. Citation2008; Humaidan et al. Citation2013]. Meeting one of these criteria would be sufficient to classify a patient as an anticipated high responder. Although this group will be somewhat heterogeneous, these risk factors are strongly associated with each other from both pathophysiologic and clinical aspects. We expect that this liberal approach regarding the prediction of a high response would result in a reduced incidence of ‘unanticipated high responders’ (as described below).
Based on the literature, using a treatment protocol for anticipated high responders aimed at down regulation of gonadotropin secretion by the anterior pituitary gland via the use of a GnRH antagonist, in conjunction with reduced FSH stimulation dosing [Humaidan et al. Citation2010b] should minimize the incidence of OHSS. For example, Nelson et al. [Citation2009] demonstrated that, compared to an agonist protocol with 150 IU FSH given per day, an antagonist protocol with same stimulation dose required fewer days of stimulation, was associated with elimination of the need for ‘freeze all’, and reduced the need for hospitalization due to OHSS [Nelson et al. Citation2009].
While GnRH antagonist protocols have gained popularity and dominance among high responders, gonadotropin stimulation dosing remains an important variable to influence ovarian response [Olivennes Citation2010]. The wide variety of published protocols and algorithms (different initial FSH doses, step down protocol, chronic ultra-low FSH dose, individualized gonadotropin dosing [Damario Citation2010; Olivennes et al. Citation2009; Orvieto and Homburg Citation2009; Popovic-Todorovic et al. Citation2003; Popovic-Todorovic et al. Citation2004], and others emphasizes the multiple methods used to achieve the highest pregnancy rate combined with the lowest OHSS risk. A recent dose-response study which compared 5 fixed doses of rhFSH ranging from 5.2–12.1 µg among patients with different AMH concentrations (5–14.9 pmol/L vs. 15.0–44.9 pmol/L), presented a linear relationship between FSH dose and the number of retrieved ovum. However, stimulation doses had no significant effect on the number of blastocysts and pregnancy rate [Arce et al. Citation2014]. Although there is no definite optimal accepted stimulation dose among patients at risk for high ovarian response, 150 units with a GnRH antagonist protocol seems to be a reasonable starting dose, at least during the first cycle. That dose may be adjusted in later cycles according to ovarian response [Popovic-Todorovic et al. Citation2004] and ovarian markers [Arce et al. Citation2014]. It should be noted that since more than half of the patients anticipated to exhibit a high ovarian response will actually have a normal response [Humaidan et al. Citation2013], the final decision regarding the specific triggering regimen should be postponed until trigger day.
Unanticipated high responders
These patients do not have classic risk factors for high ovarian response during the evaluation prior to IVF initiation. Despite this, they exhibit an exaggerated ovarian response to what would be considered a low to medium gonadotropin dose. Their response may be explained by increased individual sensitivity to gonadotropins [Gomez et al. Citation2010], which cannot be measured prior to stimulation by current methods. Since they were not originally classified as patients at risk for high response, these patients may have undergone a long protocol with GnRHa, which eliminates the option of GnRHa triggering. In situations like these, secondary OHSS prevention methods should be considered including lowering the dose, coasting, cycle cancellation, cryopreservation, the use of dopamine agonist, dietary modification, etc. [Humaidan et al. Citation2010b]. Following an unexpected high response, the patient would be reclassified as ‘high ovarian response predicted’ in future cycles.
Pre-triggering assessment
High ovarian response
Since high ovarian response is a strong predictor of OHSS development, a reliable definition of ‘high ovarian response’ is required. A cutoff of 13 follicles ≥11 mm on the day of triggering is widely cited in clinical studies to classify individuals as having a high ovarian response [Griffin et al. Citation2012; Humaidan et al. Citation2013; Iliodromiti et al. Citation2013; Radesic and Tremellen Citation2011]. This criteria is reliable for predicting OHSS, because it has been shown to predict 87% of severe OHSS cases [Papanikolaou et al. Citation2010]. In addition to the number and size of follicles, the E2 level has also been considered as a predictor of OHSS. However, serum E2 concentration at levels greater than 2,560 pmol/l (∼700 pg/ml) predicts less than half of severe OHSS cases with 49% sensitivity and 77% specificity [Papanikolaou et al. Citation2006]. Furthermore, the rate of E2 rise was also demonstrated to be a poor predictor for OHSS [Humaidan et al. Citation2010b]. Although more than 15–20 retrieved oocytes is another often cited marker for high ovarian response [Broekmans et al. Citation2014; Hamdine et al. Citation2015; Lin et al. Citation2013], this marker cannot be used for prediction, since the oocytes are retrieved after the triggering regimen has already been selected and administered.
As mentioned above, the adoption of GnRHa trigger with intensive or modified LPS has almost eliminated OHSS in patients with high ovarian response IVF cycles with fresh embryo transfer, as well as for egg donors [Humaidan et al. Citation2011]. This led to the concept that GnRHa with intensive or modified LPS should become the first line trigger in this population. However, several reports have been published demonstrating severe OHSS after GnRHa triggering with modified LPS of 1,500 hCG units [Fatemi et al. Citation2014; Seyhan et al. Citation2013]. In other words, it seems that there is a specific sub population among the ‘high ovarian response’ group which is prone to OHSS when exposed to a very low dose of hCG on the day of retrieval after a GnRHa trigger. In order to further decrease the risk for OHSS, the sub group ‘extremely high risk for OHSS’ should be differentiated from other patients with high ovarian response who have a ‘high risk for OHSS’. The optional cutoff may be based on E2 serum concentration/number of follicles above a certain size on trigger day (such as the differentiation between high and normal response) and/or the actual number of retrieved oocytes (which is not relevant for trigger regimen selection).
The largest cohort described to date presenting severe OHSS after GnRHa trigger with modified LPS included 23 high responder patients who had mean E2 of 4891 pg/ml and 20.3 follicles >12 mm on trigger day. Five (22%) cases of severe early OHSS were reported, three of them had E2 levels ≥4000 pg/ml while the two other patients had an extremely high number of oocytes (33 and 49) retrieved. Interestingly, 4/5 patients had 11–25 follicles ≥12 mm on trigger day and the fifth patient had 34 follicles ≥12 mm with E2 of 3,011 pg/ml and 33 eggs retrieved. After risk factor analysis the authors recommended avoiding hCG luteal rescue together with freezing of all embryos when there are ≥18 follicles with a 10–14 mm diameter [Seyhan et al. Citation2013]. In comparison, Humaidan and colleagues administered GnRHa trigger with modified LPS and fresh embryo transfer in 12 patients with ≥25 follicles ≥11 mm on trigger day resulting with a high pregnancy and delivery rate, without a single case of early OHSS [Humaidan Citation2009]. In both reports the threshold of 25 follicles ≥12 mm on trigger day was not a reliable predictor for OHSS after exposure to a low hCG dose on the day of retrieval. Additionally, the small sample sizes makes it unlikely to establish a reliable cutoff of the number of follicles and size to differentiate between ‘high’ and suspected ‘extreme’ risk for OHSS.
Another suggested threshold is E2 serum concentration > 4000 pg/ml, as demonstrated in 3/5 early OHSS cases in the Seyhan et al. cohort [Seyhan et al. Citation2013] and others [Fatemi et al. Citation2014]. Moreover, recent data regarding GnRHa triggering showed that an E2 ≥ 4000 pg/ml in addition to elevated LH 3.5 ± 2.5 units on trigger day are reliable predictors for ART success among patients at high risk for OHSS [Kummer et al. Citation2011]. These patients have high pregnancy rates after GnRHa triggering and do not require an adjuvant low hCG dose which may only increase their risk for OHSS [Griffin et al. Citation2012]. This is likely due to adequate luteal support in these patients, compared to those with levels of E2 < 4000 pg/ml. Although E2 > 4000 pg/ml cannot serve as an evidence based marker for extreme OHSS risk due to minimal published data, it may serve as a clinical and practical threshold to refrain from hCG.
Current literature lack a clear cut off criteria which may differentiate between patients at ‘extreme’ risk for OHSS, among which hCG should be avoided completely, to those at ‘high’ risk who may be treated with low dose of hCG. This is highly relevant due to the importance of hCG during ovulation trigger. Consequently, both ≥18 follicles with a 10–14 mm diameter [Seyhan et al. Citation2013] and E2 > 4000 pg/ml can be used as risk factors to differentiate between patients of high ovarian response with a ‘high’ risk for OHSS to those with suspected ‘extreme’ OHSS risk.
In addition to optimizing trigger selection, the clinical management of patients with high ovarian response (both ‘high’ and ‘extreme’ OHSS risk) may necessitate multiple methods to prevent, or at least reduce, OHSS risk and severity. Since each strategy may be useful at specific times during the IVF protocol (pre-trigger interventions, appropriate trigger selection, and post ovum retrieval/pre-embryo transfer strategies), a detailed continuous evaluation allows for personalized treatment and increased safety. The following approaches should be considered in a stepwise assessment ().
Pre-trigger interventions
Cycle cancellation, refers to refraining from triggering when several risk factors for OHSS are present [Delvigne and Rozenberg Citation2002], was predominantly relevant when using long protocols or antagonist protocols with hCG (prior to the advent of GnRHa triggering) due to the high OHSS risk. In spite of the fact this is the only method to eliminate OHSS completely, its drawbacks are obvious. Therefore growing usage of an antagonist trigger has led to almost complete abandonment of that approach. Coasting, the withholding of gonadotropins for interval prior to hCG administration, has been previously adopted as a method to avoid cycle cancellation [Abdallah et al. Citation2010] and to reduce the risk of OHSS [Delvigne and Rozenberg Citation2002]. Through selective regression of the smaller follicle pool [Fluker et al. Citation1999], coasting results in decreased E2 and vasoactive substances (including VEGF) in follicular fluid [Tozer et al. Citation2004]. However, coasting has a negative effect on the number of oocytes retrieved [Delvigne and Rozenberg Citation2002]. Moreover, coasting results in significantly reduced high quality embryos compared to GnRH antagonist with hCG triggering in RCTs [Aboulghar et al. Citation2007]. D’Angelo et al. have concluded in a Cochrane database review that there is no evidence to suggest a benefit of using coasting to prevent OHSS compared with no coasting or other interventions [D'Angelo et al. Citation2011]. Therefore, although coasting may have a negative impact on cycle outcome it should be considered when cancellation is the alternative.
Trigger regimen
As described above, patients with ≥18 follicles with a 10–14 mm diameter or E2 > 4000 pg/ml are suspected as ‘extreme’ OHSS risk with adequate luteal support and should be triggered by GnRHa alone with intensive LPS, while hCG administration should be avoided completely [Engmann and Benadiva Citation2012]. Patients at ‘high (but not extreme) risk for OHSS’ may be triggered by GnRHa with intensive [Humaidan et al. Citation2015] or modified LPS [Humaidan Citation2009]. Alternatively, a retrospective study which included patients with a history of high ovarian response and E2 < 4000 pg/ml on trigger day has demonstrated significantly higher clinical pregnancy and delivery rates using dual triggering with GnRHa and 1000U of hCG compared to GnRHa trigger with intensive LPS [Griffin et al. Citation2012].
Plan post ovum retrieval/pre-embryo transfer strategies
‘Freeze all’ was first used more than two decades ago [Amso et al. Citation1990] and since then many publications showed different and sometimes contradictory results [D'Angelo Citation2010]. The recent concept of IVF cycle segmentation implies separation between stimulation and trigger by GnRHa with cryopreservation of all embryos. The goal is to eliminate OHSS by the absence of endogenous or exogenous hCG, leading to ‘OHSS-free clinic’ [Devroey et al. Citation2011]. In spite of the promising advantages, especially in the era of excellent embryo survival after vitrification [Evans et al. Citation2014], the ‘freeze all’ approach has some limitations. Not every clinic has an optimal cryopreservation program in addition to concerns regarding higher early pregnancy loss, possible epigenetic effects, and others [Humaidan et al. Citation2015]. An additional strategy to reduce the risk for OHSS is prophylactic dopamine agonist administration in cases of high ovarian response [Alvarez et al. Citation2007], likely due to the diminished VEGF activity leading to decreased vascular permeability [Gomez et al. Citation2010]. A less common method which may be considered in subsequent cycles in select patients at extreme OHSS risk is in vitro maturation (IVM), which requires very little gonadotropin supplementation [Huang et al. Citation2010]. In one study, the IVM pregnancy rate was higher among PCOS patients compared to normal responders [Child et al. Citation2001]. However, there appears to be a lower success rate for IVM compared to IVF [Fadini et al. Citation2013] and there is a lack of RCTs among PCOS patients [Siristatidis et al. Citation2013]. Therefore, IVM may only be suitable for specific populations such as severe PCOS or fertility preservation patients [Fadini et al. Citation2013]. Ideally, all options should be considered and discussed with patients, in order to select a rational strategy to prevent or reduce OHSS severity for the specific clinical circumstances.
Normal ovarian response despite risk factors for a high response
These patients will have ≤13 follicles ≥11 mm on the day of triggering. As previously described, Humaidan et al. [Citation2013] distinguished high risk patients based on OHSS risk factors, and further differentiated patients on the trigger day by their actual ovarian response. Out of 384 patients recruited, only 118 (31%) had a high ovarian response. Although it may be surprising, most patients with risk factors for high ovarian response will eventually have a normal response, leading to their classification as low risk for OHSS [Humaidan et al. Citation2013]. These results emphasize the fundamental discrimination between risk factors assessed prior to ovarian stimulation and the actual ovarian response [Huber et al. Citation2013].
Various triggering regimens have been suggested, GnRHa triggering combined with intensified luteal support [Engmann et al. Citation2008; Humaidan et al. Citation2013], dual triggering with varied doses of hCG, from 1,000 units [Griffin et al. Citation2012] to 5,000 units [Lin et al. Citation2013], as well as traditional hCG triggering (5,000–10,000 units alone) [Humaidan et al. Citation2013] in these patients. These studies are characterized by heterogeneous designs (prospective and retrospective) and variable inclusion/exclusion criteria. One large retrospective study on normal responders (n = 378 cycles) demonstrated significantly higher implantation (29.7% vs.18.4%, p < 0.001), clinical pregnancy (50.8% vs. 40.1%, p = 0.047), and live-birth (41.4% vs. 30.5%, p = 0.042) rates among dual triggering compared to hCG alone [Lin et al. Citation2013].
Summary
Ovarian response to gonadotropin stimulation is a wide continuum and often unpredictable. This heterogeneity requires careful stepwise assessment and evaluation. The model we have proposed may perhaps assist clinicians to supply targeted and precise treatments in order to achieve favorable reproductive outcome with minimum risk of OHSS. Our suggested sub-group classification aids to delineate select patient sub-populations with specific characteristics. Additionally, the model is organized by clinical features present before initiation of stimulation, before triggering, and before transfer. It allows for flexibility during ART cycles, since patients may shift in risk classification according to their clinical response. Our model suggests defined treatment adjustment options for each scenario.
Supplemental materials available online
IAAN_1089607_Supplementary_file.zip
Download Zip (26.3 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author contribution
Collected the relevant articles, developed the model, and wrote the manuscript: IG; Revised the manuscript: ES; Participated in manuscript editing: KQ; Contributed substantially to model improvement and wrote the manuscript: CL. All authors approved revisions to the final manuscript.
References
- Abdallah, R., Kligman, I., Davis, O. and Rosenwaks, Z. (2010) Withholding gonadotropins until human chorionic gonadotropin administration. Semin Reprod Med 28:486–492
- Aboulghar, M.A., Mansour, R.T., Amin, Y.M., Al-Inany, H.G., Aboulghar, M.M. and Serour, G.I. (2007) A prospective randomized study comparing coasting with GnRH antagonist administration in patients at risk for severe OHSS. Reprod Biomed Online 15:271–279
- Acevedo, B., Gomez-Palomares, J.L., Ricciarelli, E. and Hernandez, E.R. (2006) Triggering ovulation with gonadotropin-releasing hormone agonists does not compromise embryo implantation rates. Fertil Steril 86:1682–1687
- Alvarez, C., Marti-Bonmati, L., Novella-Maestre, E., Sanz, R., Gomez, R., Fernandez-Sanchez, M., et al. (2007) Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab 92:2931–2937
- Amso, N.N., Ahuja, K.K., Morris, N. and Shaw, R.W. (1990) The management of predicted ovarian hyperstimulation involving gonadotropin-releasing hormone analog with elective cryopreservation of all pre-embryos. Fertil Steril 53:1087–1090
- Arce, J.C., Andersen, A.N., Fernandez-Sanchez, M., Visnova, H., Bosch, E., Garcia-Velasco, J.A., et al. (2014) Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimullerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril 102:1633–1640 e1635
- Broekmans, F.J., Verweij, P.J., Eijkemans, M.J., Mannaerts, B.M. and Witjes, H. (2014) Prognostic models for high and low ovarian responses in controlled ovarian stimulation using a GnRH antagonist protocol. Hum Reprod 29:1688–1697
- Broer, S.L., Mol, B.W., Hendriks, D. and Broekmans, F.J. (2009) The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 91:705–714
- Casano, S., Guidetti, D., Patriarca, A., Pittatore, G., Gennarelli, G. and Revelli, A. (2012) MILD ovarian stimulation with GnRH-antagonist vs. long protocol with low dose FSH for non-PCO high responders undergoing IVF: a prospective, randomized study including thawing cycles. J Assist Reprod Genet 29:1343–1351
- Child, T.J., Abdul-Jalil, A.K., Gulekli, B. and Tan, S.L. (2001) In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil Steril 76:936–942
- D'Angelo, A. (2010) Ovarian hyperstimulation syndrome prevention strategies: cryopreservation of all embryos. Semin Reprod Med 28:513–518
- D'Angelo, A., Brown, J. and Amso, N.N. (2011) Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev 15:CD002811
- Damario, M.A. (2010) Ovarian hyperstimulation syndrome prevention strategies: oral contraceptive pills-dual gonadotropin-releasing hormone agonist suppression with step-down gonadotropin protocols. Semin Reprod Med 28:468–474
- Datta, A.K., Eapen, A., Birch, H., Kurinchi-Selvan, A. and Lockwood, G. (2014) Retrospective comparison of GnRH agonist trigger with HCG trigger in GnRH antagonist cycles in anticipated high-responders. Reprod Biomed Online 29:552–558
- Delvigne, A. and Rozenberg, S. (2002) Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update 8:559–577
- Devroey, P., Polyzos, N.P. and Blockeel, C. (2011) An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod 26:2593–2597
- Engmann, L. and Benadiva, C. (2012) Agonist trigger: what is the best approach? Agonist trigger with aggressive luteal support. Fertil Steril 97:531–533
- Engmann, L., DiLuigi, A., Schmidt, D., Nulsen, J., Maier, D. and Benadiva, C. (2008) The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril 89:84–91
- Evans, J., Hannan, N.J., Edgell, T.A., Vollenhoven, B.J., Lutjen, P.J., Osianlis, T., et al. (2014) Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update 20:808–821
- Fadini, R., Mignini Renzini, M., Dal Canto, M., Epis, A., Crippa, M., Caliari, I., et al. (2013) Oocyte in vitro maturation in normo-ovulatory women. Fertil Steril 99:1162–1169
- Fatemi, H.M., Popovic-Todorovic, B., Humaidan, P., Kol, S., Banker, M., Devroey, P., et al. (2014) Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril 101:1008–1011
- Fauser, B.C., de Jong, D., Olivennes, F., Wramsby, H., Tay, C., Itskovitz-Eldor, J., et al. (2002) Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab 87:709–715
- Ferraretti, A.P., La Marca, A., Fauser, B.C., Tarlatzis, B., Nargund, G., Gianaroli, L., et al. (2011) ESHRE consensus on the definition of ‘poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26:1616–1624
- Fluker, M.R., Hooper, W.M. and Yuzpe, A.A. (1999) Withholding gonadotropins (“coasting”) to minimize the risk of ovarian hyperstimulation during superovulation and in vitro fertilization-embryo transfer cycles. Fertil Steril 71:294–301
- Garcia, J.E., Jones, G.S., Acosta, A.A. and Wright, G Jr. (1983) Human menopausal gonadotropin/human chorionic gonadotropin follicular maturation for oocyte aspiration: phase II, 1981. Fertil Steril 39:174–179
- Gomez, R., Soares, S.R., Busso, C., Garcia-Velasco, J.A., Simon, C. and Pellicer, A. (2010) Physiology and pathology of ovarian hyperstimulation syndrome. Semin Reprod Med 28:448–457
- Gonen, Y., Balakier, H., Powell, W. and Casper, R.F. (1990) Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab 71:918–922
- Griffin, D., Benadiva, C., Kummer, N., Budinetz, T., Nulsen, J. and Engmann, L. (2012) Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril 97:1316–1320
- Griffin, D., Feinn, R., Engmann, L., Nulsen, J., Budinetz, T. and Benadiva, C. (2014) Dual trigger with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin to improve oocyte maturity rates. Fertil Steril 102:405–409
- Haas, J., Zilberberg, E., Dar, S., Kedem, A., Machtinger, R. and Orvieto, R. (2014) Co-administration of GnRH-agonist and hCG for final oocyte maturation (double trigger) in patients with low number of oocytes retrieved per number of preovulatory follicles-a preliminary report. J Ovarian Res 7:77
- Hamdine, O., Eijkemans, M.J., Lentjes, E.W., Torrance, H.L., Macklon, N.S., Fauser, B.C., et al. (2015) Ovarian response prediction in GnRH antagonist treatment for IVF using anti-Mullerian hormone. Hum Reprod 30:170–180
- Huang, J.Y., Chian, R.C. and Tan, S.L. (2010) Ovarian hyperstimulation syndrome prevention strategies: in vitro maturation. Semin Reprod Med 28:519–531
- Huber, M., Hadziosmanovic, N., Berglund, L. and Holte, J. (2013) Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormone-agonist protocol: suggestions for a new principle to solve an old problem. Fertil Steril 100:1270–1276
- Humaidan, P. (2009) Luteal phase rescue in high-risk OHSS patients by GnRHa triggering in combination with low-dose HCG: a pilot study. Reprod Biomed Online 18:630–634
- Humaidan, P., Bredkjaer, H.E., Bungum, L., Bungum, M., Grondahl, M.L., Westergaard, L., et al. (2005) GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod 20:1213–1220
- Humaidan, P., Ejdrup Bredkjaer, H., Westergaard, L.G. and Yding Andersen, C. (2010a) 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril 93:847–854
- Humaidan, P., Engmann, L. and Benadiva, C. (2015) Luteal phase supplementation after gonadotropin-releasing hormone agonist trigger in fresh embryo transfer: the American versus European approaches. Fertil Steril 103:879–885
- Humaidan, P., Kol, S., Papanikolaou, E.G. and Copenhagen GnRH Agonist Triggering Workshop Group (2011) GnRH agonist for triggering of final oocyte maturation: time for a change of practice? Hum Reprod Update 17:510–524
- Humaidan, P. and Polyzos, N.P. (2014) Human chorionic gonadotropin vs. gonadotropin-releasing hormone agonist trigger in assisted reproductive technology- “the king is dead, long live the king!”. Fertil Steril 102:339–341
- Humaidan, P., Polyzos, N.P., Alsbjerg, B., Erb, K., Mikkelsen, A.L., Elbaek, H.O., et al. (2013) GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod 28:2511–2521
- Humaidan, P., Quartarolo, J. and Papanikolaou, E.G. (2010b) Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril 94:389–400
- Iliodromiti, S., Blockeel, C., Tremellen, K.P., Fleming, R., Tournaye, H., Humaidan, P., et al. (2013) Consistent high clinical pregnancy rates and low ovarian hyperstimulation syndrome rates in high-risk patients after GnRH agonist triggering and modified luteal support: a retrospective multicentre study. Hum Reprod 28:2529–2536
- Kolibianakis, E.M., Schultze-Mosgau, A., Schroer, A., van Steirteghem, A., Devroey, P., Diedrich, K., et al. (2005) A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocyte maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Hum Reprod 20:2887–2892
- Kummer, N., Benadiva, C., Feinn, R., Mann, J., Nulsen, J. and Engmann, L. (2011) Factors that predict the probability of a successful clinical outcome after induction of oocyte maturation with a gonadotropin-releasing hormone agonist. Fertil Steril 96:63–68
- Lainas, T.G., Sfontouris, I.A., Papanikolaou, E.G., Zorzovilis, J.Z., Petsas, G.K., Lainas, G.T., et al. (2008) Flexible GnRH antagonist versus flare-up GnRH agonist protocol in poor responders treated by IVF: a randomized controlled trial. Hum Reprod 23:1355–1358
- Lee, T.H., Liu, C.H., Huang, C.C., Wu, Y.L., Shih, Y.T., Ho, H.N., et al. (2008) Serum anti-Mullerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod 23:160–167
- Lin, M.H., Wu, F.S., Lee, R.K., Li, S.H., Lin, S.Y. and Hwu, Y.M. (2013) Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril 100:1296–1302
- Mathur, R.S., Akande, A.V., Keay, S.D., Hunt, L.P. and Jenkins, J.M. (2000) Distinction between early and late ovarian hyperstimulation syndrome. Fertil Steril 73:901–907
- Nastri, C.O., Teixeira, D.M., Moroni, R.M., Leitao, V.M. and Martins, W.P. (2015) Ovarian hyperstimulation syndrome: physiopathology, staging, prediction and prevention. Ultrasound Obstet Gynecol 45:377–393
- Navot, D., Bergh, P.A. and Laufer, N. (1992) Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril 58:249–261
- Nelson, S.M., Yates, R.W., Lyall, H., Jamieson, M., Traynor, I., Gaudoin, M., et al. (2009) Anti-Mullerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod 24:867–875
- Olivennes, F. (2010) Ovarian hyperstimulation syndrome prevention strategies: individualizing gonadotropin dose. Semin Reprod Med 28:463–467
- Olivennes, F., Howles, C.M., Borini, A., Germond, M., Trew, G., Wikland, M., et al. (2009) Individualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT study. Reprod Biomed Online 18:195–204
- Orvieto, R. and Homburg, R. (2009) Chronic ultra-low dose follicle-stimulating hormone regimen for patients with polycystic ovary syndrome: one click, one follicle, one pregnancy. Fertil Steril 91:1533–1535
- Papanikolaou, E.G., Humaidan, P., Polyzos, N.P. and Tarlatzis, B. (2010) Identification of the high-risk patient for ovarian hyperstimulation syndrome. Semin Reprod Med 28:458–462
- Papanikolaou, E.G., Pozzobon, C., Kolibianakis, E.M., Camus, M., Tournaye, H., Fatemi, H.M., et al. (2006) Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril 85:112–120
- Popovic-Todorovic, B., Loft, A., Bredkjaeer, H.E., Bangsboll, S., Nielsen, I.K. and Andersen, A.N. (2003) A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a ‘standard' dose of 150 IU/day in ‘standard' patients undergoing IVF/ICSI treatment. Hum Reprod 18:2275–2282
- Popovic-Todorovic, B., Loft, A., Ziebe, S. and Andersen, A.N. (2004) Impact of recombinant FSH dose adjustments on ovarian response in the second treatment cycle with IVF or ICSI in “standard” patients treated with 150 IU/day during the first cycle. Acta Obstet Gynecol Scand 83:842–849
- Radesic, B. and Tremellen, K. (2011) Oocyte maturation employing a GnRH agonist in combination with low-dose hCG luteal rescue minimizes the severity of ovarian hyperstimulation syndrome while maintaining excellent pregnancy rates. Hum Reprod 26:3437–3442
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25
- Seyhan, A., Ata, B., Polat, M., Son, W.Y., Yarali, H. and Dahan, M.H. (2013) Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod 28:2522–2528
- Shapiro, B.S., Daneshmand, S.T., Garner, F.C., Aguirre, M. and Hudson, C. (2011) Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril 95:2715–2717
- Shapiro, B.S., Daneshmand, S.T., Garner, F.C., Aguirre, M. and Thomas, S. (2008) Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril 90:231–233
- Sills, E.S., Alper, M.M. and Walsh, A.P. (2009) Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol 146:30–36
- Siristatidis, C.S., Vrachnis, N., Creatsa, M., Maheshwari, A. and Bhattacharya, S. (2013) In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst Rev 10:CD006606
- Tarlatzis, B.C., Zepiridis, L., Grimbizis, G. and Bontis, J. (2003) Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update 9:61–76
- Tozer, A.J., Iles, R.K., Iammarrone, E., Gillott, C.M., Al-Shawaf, T. and Grudzinskas, J.G. (2004) The effects of ‘coasting' on follicular fluid concentrations of vascular endothelial growth factor in women at risk of developing ovarian hyperstimulation syndrome. Hum Reprod 19:522–528
- Tremellen, K. and Savulescu, J. (2014) Ovarian reserve screening: a scientific and ethical analysis. Hum Reprod 29:2606–2614
- Wu, K.L., Zhao, H.B., Liu, H., Zhong, W.X., Yu, G.L. and Chen, Z.J. (2013) Elective single blastocyst transfer is more suitable for normal responders than for high responders. Chin Med J (Engl) 126:2125–2128
- Youssef, M.A., Van der Veen, F., Al-Inany, H.G., Mochtar, M.H., Griesinger, G., Nagi Mohesen, M., et al. (2014) Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev 10:CD008046
- Zivi, E., Simon, A. and Laufer, N. (2010) Ovarian hyperstimulation syndrome: definition, incidence, and classification. Semin Reprod Med 28:441–447